砷锑价态对铜电解液中砷锑铋脱除率的影响
郑雅杰1,周文科1,彭映林1,马玉天2
(1. 中南大学 冶金科学与工程学院,湖南 长沙,410083;
2. 金川集团有限公司 精炼厂,甘肃 金昌,737100)
摘 要:
b>2还原,使铜电解液中As(Ⅴ)还原为As(Ⅲ),Sb(Ⅴ)还原为Sb(Ⅲ),并调节电解液中As(Ⅴ)与As(T),Sb(Ⅴ)与Sb(T)的物质的量比,蒸发浓缩电解液,冷却结晶后,能有效脱除铜电解液中As,Sb和Bi等杂质。研究结果表明:当铜电解液体积为3 L,Cu的质量浓度为32 g/L,As(T)的质量浓度为9.36 g/L,Sb(T)的质量浓度为0.65 g/L,Bi的质量浓度为0.45 g/L,n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4时,蒸发浓缩电解液,使浓缩前电解液与浓缩后电解液体积比为2.5,冷却至10 ℃结晶,过滤,铜电解液中Cu,As,Sb和Bi脱除率分别为82%,62%,55%和85%。蒸发浓缩结晶产物中含CuSO4·5H2O和As2O3。其结晶产物中Cu为29%,As为5%,Sb为0.37%,Bi为0.2%。
关键词:
中图分类号:TF803.25 文献标志码:A 文章编号:1672-7207(2012)03-0821-06
Effect of valences of arsenic, antimony on removal rates of arsenic, antimony and bismuth in copper electrolyte
ZHENG Ya-jie1, ZHOU Wen-ke1, PENG Ying-lin1, MA Yu-tian2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Refinery, Jinchuan Group Corporation Limited, Jinchang 737100, China)
Abstract: Arsenic(Ⅴ) and antimony(Ⅴ) in copper electrolyte were reduced to arsenic(Ⅲ) and antimony(Ⅲ) with the aid of sulfur dioxide, and n(As(Ⅴ))/n(As(T)) and n(Sb(Ⅴ))/n(Sb(T)) were adjusted, then the copper electrolyte was concentrated to crystallize, As, Sb, Bi in the copper electrolyte could be removed effectively. The results indicate that when the volume of the copper electrolyte is 3 L, Cu concentration is 32 g/L, As(T) concentration is 9.36 g/L, Sb(T) concentration is 0.65 g/L, Bi concentration is 0.45 g/L, both n(As(Ⅴ))/n(As(T)) and n(Sb(Ⅴ))/n(Sb(T)) are 0.4, the removal rates of Cu, As, Sb and Bi are 82%, 62%, 55% and 85% respectively after copper electrolyte is concentrated and the ratio of initial volume and final volume is 2.5 time to crystallized at 10 ℃. XRD analysis shows that the crystallized product includes CuSO4·5H2O and As2O3. XRF analysis shows that the content of Cu, As, Sb and Bi in the crystallized product are 29%, 5%, 0.37% and 0.2% respectively.
Key words: copper electrolyte; reducing by sulfur dioxide; arsenic trioxide; purification; evaporation
铜电解精炼过程中,阳极铜中的As,Sb和Bi杂质,以一定分配比进入铜电解液并逐渐积累。它们不仅会在阴极上沉积,而且会形成漂浮阳极泥,影响阴极铜质量。因此,As,Sb和Bi杂质的脱除是铜电解液净化的主要目标。生产中多采用电积法[1]脱除As,Sb和Bi等杂质,但电积法能耗高,产生黑铜渣,并有剧毒AsH3气体放出[2-3]。萃取法[4]、离子交换法[5]和共沉淀法等[6-7]也用于铜电解液净化,但因其处理成本高,效果单一,只能作为铜电解液净化的辅助工艺。研究表明:As,Sb和Bi在铜电解液中主要以H3AsO4,HAsO2,AsO+,HSb(OH)6,SbO+和Bi3+等形式存在。本文作者在研究As(Ⅲ)净化铜电解液基础上[8-10],利用SO2还原铜电解液,并调整电解液中As(Ⅴ)与As(T),Sb(Ⅴ)与Sb(T)的物质的量的比,蒸发浓缩使电解液中As,Sb和Bi杂质生成相应大分子沉淀,可实现电解液中As,Sb和Bi杂质的良好脱除[11]。
1 实验
1.1 实验步骤
取500 mL铜电解液(国内某冶炼厂提供)于三颈瓶中,化学组成如表1所示。根据实验需要,加入As2O5(AR)和Sb2O3(AR),加热搅拌溶解后, 通入SO2还原,然后加入As2O5(AR)以及采用Sb2O5与硫酸反应制得的HSb(OH)6溶液调整电解液中As(Ⅴ)与As(T),Sb(Ⅴ)与Sb(T)的物质的量的比,加热浓缩电解液至一定体积后,冷却至10 ℃下结晶,过滤,并对滤液及结晶产物进行分析。其工艺流程如图1所示。
表1 铜电解液化学组成(质量浓度)
Table 1 Compositions of copper electrolyte g/L

1.2 分析与检测
实验采用硫酸铈-溴酸钾滴定法分析As(Ⅲ)和Sb(Ⅲ)的质量浓度,电感耦合等离子光谱仪(Intrepid II XSP)分析Cu,As,Sb和Bi的质量浓度。用X线衍射仪(XRD)(日本理学,Cu Kα,50 kV,300 Ma)分析结晶产物物相,用X荧光分析(XRF)仪(菲利浦24)分析结晶产物成分。
铜电解液中Cu,As,Sb和Bi的脱除率(r)按如下式计算:
![]()
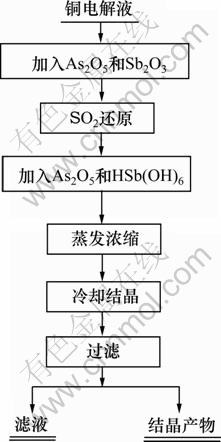
图1 铜电解液净化工艺流程图
Fig.1 Process of copper electrolyte purification
式中:![]() 为电解液中Cu,As,Sb和Bi的质量浓度,g/L;
为电解液中Cu,As,Sb和Bi的质量浓度,g/L;![]() 为电解液浓缩结晶后Cu,As,Sb和Bi的质量浓度,g/L;V0为电解液体积,L;V1为电解液浓缩结晶后体积,L。
为电解液浓缩结晶后Cu,As,Sb和Bi的质量浓度,g/L;V0为电解液体积,L;V1为电解液浓缩结晶后体积,L。
2 结果与讨论
2.1 浓缩体积比对铜电解液中As,Sb和Bi杂质脱除率的影响
实验取铜电解液500 mL,化学组成如表1所示,加入Sb2O3调整电解液中Sb(T)的质量浓度为0.75 g/L,通入SO2还原,反应温度为25 ℃,反应时间为2 h,电解液中n(Sb(Ⅲ))/n(Sb(T))和n(As(Ⅲ))/n(As(T))分别为0.95。加热蒸发铜电解液至一定体积,浓缩体积比对铜电解液中As,Sb和Bi脱除率的影响如图2所示,浓缩体积比为蒸发浓缩前电解液体积与浓缩后电解液体积比。
由图2可知:铜电解液中As,Sb和Bi杂质脱除率随浓缩体积比增加而增加。当浓缩体积比从1.0增加至3.3时,As,Sb和Bi的脱除率分别从0增加至77%,26%和87%。当浓缩体积比为2.5时,电解液中As,Sb和Bi的脱除率分别为75%,20%和78%。
由表2可知:当浓缩体积比从1.0增加至3.3时,H2SO4的质量浓度从208 g/L增加至645 g/L,Cu的脱除率从0增加到88%。当浓缩体积比为3.3时,结晶产物黏度增大,其中H2SO4和Ni的含量增加[12]。因此,适宜的浓缩体积比为2.5。
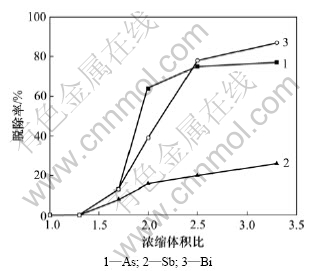
图2 浓缩体积比对铜电解液中As,Sb和Bi脱除率的影响
Fig.2 Influence of ratio of initial volume to final volume on removal rates of As, Sb and Bi in copper electrolyte
表2 浓缩体积比对铜电解液中H2SO4质量浓度和Cu脱除率的影响
Table 2 Influence of ratio of initial volume to final volume on concentration of H2SO4 and removal rate of Cu in copper electrolyte

电解液经SO2还原后,As(Ⅴ)还原成As(Ⅲ),As(Ⅲ)在酸性溶液中的溶解度远低于As(Ⅴ)[13],且随H2SO4质量浓度增加而降低[14]。电解液蒸发浓缩过程中,硫酸质量浓度的增加及CuSO4·5H2O晶体的形成,有利于HAsO2脱水生成As2O3,使As得以脱除。其反应如下所示:
SO2+H3AsO4=HAsO2+HSO4-+H+ (1)
2HAsO2=As2O3↓+H2O (2)
As2O3结晶过程,晶核的形成是控制步骤,CuSO4·5H2O等结晶物的形成能显著降低As2O3的晶核形成能,促使As2O3结晶析出。在SO2还原作用下,Bi3+生成BiO和(Bi2O3)4·3SO3[15],在由于浓缩过程中CuSO4·5H2O及As2O3的吸附及共沉淀作用,使得电解液中以各种形态存在的Sb和Bi杂质得以脱除。
2.2 As(T)质量浓度对铜电解液中As,Sb和Bi杂质脱除率的影响
上述其他实验条件不变,加入As2O5调节电解液中As(T)的质量浓度,通入SO2充分还原, n(As(Ⅲ))/n(As(T))为0.95,浓缩体积比为2.5时,电解液中As(T)的质量浓度对As,Sb和Bi脱除率的影响如图3所示。

图3 As(T)的质量浓度对铜电解液中As,Sb和Bi脱除率的影响
Fig.3 Influence of As(T) concentration on removal rates of As, Sb and Bi in copper electrolyte
由图3可知:As脱除率随铜电解液中As(T)质量浓度的增加而增加,当总砷质量浓度达到10 g/L时,As脱除率达到85%。As(T)质量浓度对电解液中Sb和Bi的脱除率影响不大。
在一定温度和硫酸质量浓度条件下,As(Ⅲ)在电解液中溶解度一定。As(Ⅲ)含量升高,As脱除率升高。
2.3 n(As(Ⅴ))/ n(As(T))对铜电解液中As,Sb和Bi杂质脱除率的影响
上述其他条件不变,电解液浓缩体积比为2.5,当电解液中As(T)质量浓度为10 g/L时,n(As(Ⅴ))/ n(As(T))对电解液中As,Sb和Bi脱除率的影响如图4所示。
由图4可知:As脱除率随n(As(Ⅴ))/n(As(T))升高而降低,Sb脱除率随n(As(Ⅴ))/n(As(T))升高而升高。

图4 n(As(Ⅴ))/n(As(T))对铜电解液中As,Sb和Bi脱除率的影响
Fig.4 Influence of n(As(Ⅴ))/n(As(T)) on removal rates of As, Sb and Bi in copper electrolyte
当电解液中n(As(Ⅴ))/n(As(T))从0.05升高至0.8时,As的脱除率由85%降低至34%,Sb的脱除率由20%升高至54%,Bi脱除率均达到80%。
电解液中As(Ⅲ)减少,产生的As2O3结晶减少,As脱除率降低。而在As(T)质量浓度不变的情况下,As(Ⅲ)含量减少,As(Ⅴ)含量增多,电解液中As(Ⅴ)增多促使SbO+与AsO43-反应[16],使Sb脱除率升高,相关反应如下:
H3AsO4+SbO+→SbAsO4↓+H++H2O (3)
综合考虑电解液中As,Sb和Bi脱除情况,适宜的n(As(Ⅴ))/n(As(T))为0.4。
2.4 n(Sb(Ⅴ))/n(Sb(T))对铜电解液中As,Sb和Bi杂质脱除率的影响
以上其他条件不变,保持铜电解液中n(As(Ⅴ))/ n(As(T))为0.4,n(Sb(Ⅴ))/n(Sb(T))对电解液中As,Sb和Bi脱除率的影响如图5所示。
由图5可知:As脱除率不随n(Sb(Ⅴ))/n(Sb(T))变化,Sb脱除率随电解液中n(Sb(Ⅴ))/n(Sb(T))增大而增大。当n(Sb(Ⅴ))/n(Sb(T))从0增加到0.6时,Sb脱除率从41%增加至70%,n(Sb(Ⅴ))/n(Sb(T))达到0.2时,Bi脱除率达到90%以上。
电解液中Sb和Bi脱除率升高,主要是因为As(Ⅲ),As(Ⅴ),Sb(Ⅲ),Sb(Ⅴ)和Bi(Ⅲ)相互作用生成了锑酸盐、砷锑酸盐及亚砷锑酸锑等大分子沉淀物质。浓缩过程中,CuSO4·5H2O和As2O3结晶的形成会
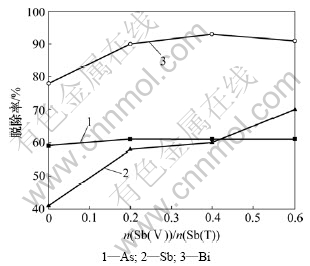
图5 n(Sb(Ⅴ))/n(Sb(T))对铜电解液中As,Sb和Bi脱除率的影响
Fig.5 Influence of n(Sb(Ⅴ))/n(Sb(T)) on removal rates of As, Sb and Bi in copper electrolyte
促使这些物质吸附共沉淀,有利于As,Sb和Bi杂质的脱除。其反应机理为[17-18]:
HSb(OH)6+AsO+→AsSbO4↓+3H2O+H+ (4)
aH3AsO4+bH[Sb(OH)6]+cMeO+→
MecAsaSbbO(3a+5b+c/2+1)H(a+5b-2c+2)·xH2O+cH++
(a+b+c/2-1-x)H2O (Me=As(Ⅲ), Sb(Ⅲ), Bi(Ⅲ);
a≥1; b≥1; c≤(3a+b)) (5)
26H++6HAsO2+4SbO++8HSb(OH)6→
3H2O+H30(As2O3)3·(Sb2O3)2·(Sb2O5)4·26H2O (6)
综合考虑电解液中As,Sb和Bi的脱除情况,调节铜电解液中n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4,浓缩结晶,已经能够有效地脱除电解液中As,Sb和Bi等杂质。
根据上述实验结果,取3 L铜电解液进行实验室放大实验,加入As2O5和Sb2O3,加热搅拌溶解后,通入SO2还原,然后加入As2O5及HSb(OH)6溶液调整铜电解液中n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4,蒸发浓缩,控制浓缩体积比为2.5,冷却至10 ℃结晶,过滤后用少量去离子水清洗杯壁,得到滤液为1.2 L。电解液净化前后化学成分如表3所示,放大实验产物XRD结果如图6所示,结晶产物XRF分析结果如表4所示。
由表3实验结果计算可知:铜电解液中As和Sb价态调整后,蒸发浓缩结晶,其中Cu,As,Sb和Bi脱除率可分别达到82%,62%,55%和85%。
表3 铜电解液净化前后的化学组成
Table 3 Compositions of copper electrolyte before and after purification g/L
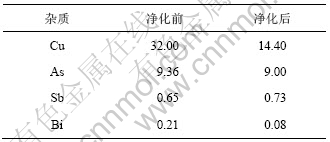

图6 结晶产物的XRD谱
Fig.6 XRD pattern of crystal product
表4 结晶产物的化学成分(质量分数)
Table 4 Compositions of crystal product %

由图6可知:还原后蒸发浓缩结晶产物中含有CuSO4·5H2O和As2O3。
由表4可知:还原后蒸发浓缩结晶产物成分含Cu为29%,As为5%,Sb为0.37%,Bi为0.2%。
3 结论
(1) 采用SO2还原铜电解液,经蒸发浓缩,冷却结晶,铜电解液中As,Sb和Bi脱除率随浓缩体积比增加而增加;浓缩体积比一定时,As脱除率随铜电解液中As(T)质量浓度增加而增加,总砷质量浓度对Sb和Bi脱除率影响不大。
(2) 当铜电解液中As(T),Sb(T)质量浓度一定时,调节电解液中n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/ n(Sb(T)),As脱除率随n(As(Ⅴ))/n(As(T))增加而降低,Sb脱除率随n(As(Ⅴ))/n(As(T))增加而增加;n(As(Ⅴ))/ n(As(T))一定时,Sb脱除率随n(Sb(Ⅴ))/n(Sb(T))增加而降低;适宜的n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4。
(3) 当铜电解液体积为3 L,H2SO4的质量浓度为208 g/L,Cu的质量浓度为32 g/L,总As的质量浓度为9.36 g/L,总Sb的质量浓度为0.65 g/L,Bi的质量浓度0.21 g/L,电解液中n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4时,蒸发浓缩使浓缩体积比为2.5,然后冷却至10 ℃结晶、过滤,Cu,As,Sb和Bi脱除率分别达到82%,62%,55%和85%。
(4) 蒸发浓缩结晶产物为CuSO4·5H2O和As2O3,结晶产物中Cu为29%,As为5%,Sb为0.37%,Bi为0.2%。
参考文献:
[1] Panda B, Das S C. Electrowinning of copper from sulfate electrolyte in presence of sulfurous acid[J]. Hydrometallurgy, 2001, 59(1): 55-67.
[2] 仇勇海, 陈白珍, 梅显芝, 等. 控制阴极电势电积法新工艺及其应用[J]. 中南工业大学学报: 自然科学版, 1999, 30(5): 501-504.
QIU Yong-hai, CHEN Bai-zhen, MEI Xian-zhi, et al. Study and application of new technology of controlling cathodic potential electrowinning[J]. Journal of Central South University of Technology: Natural Science, 1999, 30(5): 501-504.
[3] 仇勇海, 唐仁衡, 陈白珍. 砷化氢析出电势探讨[J]. 中国有色金属学报, 2002, 10(1): 101-104.
QIU Yon-hai, TANG Ren-heng, CHEN Bai-zhen. Evolution potential of arsine[J]. The Chinese Journal of Nonferrous Metals, 2002, 10(1): 101-104.
[4] Iberhan L, Wi?niewski M. Extraction of arsenic(Ⅲ) and arsenic(Ⅴ) with Cyanex 925, Cyanex 301 and their mixtures[J]. Hydrometallurgy, 2002, 63(1): 23-30.
[5] Raghavan R, Bhatt C V. Comparative study of certain ion-exchange resins for application in copper-bearing process solutions[J]. Hydrometallurgy, 1998, 50(2): 169-183.
[6] 王学文, 肖炳瑞, 张帆. 铜电解液碳酸钡脱铋新工艺[J]. 中国有色金属学报, 2006, 16(7): 1295-1299.
WANG Xue-wen, XIAO Bing-rui, ZHANG Fan. New process of bismuth removal from copper electrolyte with barium carbonate[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1295-1299.
[7] Navarro P, Alguacil F J. Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon[J]. Hydrometallurgy, 2002, 66(1/2/3): 101-105.
[8] XIAO Fa-xin, ZHENG Ya-jie, WANG Yong, et al. Purification mechanism of copper electrolyte by As(Ⅲ)[J]. Transaction of Nonferrous Metals Society of China, 2008, 18(5): 1275-1279.
[9] XIAO Fa-xin, ZHENG Ya-jie, WANG Yong, et al. Novel technology of purification of copper electrolyte[J]. Transaction of Nonferrous Metals Society of China, 2007, 17(5): 1069-1074.
[10] 郑雅杰, 赵攀峰, 王勇, 等. 高电流密度电解对阴极铜质量的影响[J]. 中南大学学报: 自然科学版, 2009, 40(2): 311-316.
ZHENG Ya-jie, ZHAO Pan-feng, WANG Yong, et al. Effect of electrorefining with high current density on cathode copper[J]. Journal of Central South University: Science and Technology, 2009, 40(2): 311-316.
[11] 郑雅杰, 彭映林, 周文科. 价态调控净化铜电解液的方法: CN, 102181882A[P]. 2011-04-09.
ZHENG Ya-jie, PENG Ying-lin, ZHOU Wen-ke. Method of purifying copper electrolyte by valence state-controllable means: CN, 102181882A[P]. 2011-04-09.
[12] 朱祖泽, 贺家齐. 现代铜冶金学[M]. 北京: 科学出版社, 2003: 500-506.
ZHU Zu-ze, HE Jia-qi. Modern metallurgy of copper[M]. Beijing: Science Press, 2003: 500-506.
[13] 迪安J A. 兰氏化学手册[M]. 北京: 科学出版社, 1991: 300-544.
Dean J A. Lange’s handbook of chemistry[M]. Beijing: Science Press, 1991: 300-544.
[14] 郑雅杰, 罗园, 王勇. 采用含砷废水沉淀还原法制备三氧化二砷[J]. 中南大学学报: 自然科学版, 2009, 40(1): 48-54.
ZHENG Ya-jie, LUO Yuan, WANG Yong. Arsenic trioxide made by precipitation-reduction method from As-containing waste water[J]. Journal of Central South University: Science and Technology, 2009, 40(1): 48-54.
[15] 张传福, 谭鹏夫. 第VA族元素物理化学数据手册[M]. 长沙: 中南大学出版社, 1995: 151-151.
ZHANG Chuan-fu, TAN Peng-fu. Physical chemistry data manual of the VA group element[M]. Changsha: Central South University Press, 1995: 151-151.
[16] 文燕, 张源, 张胜树. 铜电解过程中杂质分配的控制[C]//全国铜镍钴生产技术、装备、材料及市场研讨会. 北京: 中国有色金属学会, 2003: 93-97.
WEN Yan, ZHANG Yuan, ZHANG Sheng-shu. Control of impurities in copper electrolysis[C]//Proceedings of Symposium of Production Technology, Equipment, Material and Market of Copper, Nickel and Cobalt in China. Beijing: Chinese Nonferrous Metal Society, 2003: 93-97.
[17] ZHENG Ya-jie, XIAO Fa-xin, WANG Yong, et al. Industrial experiment of copper electrolyte purification by copper arsenite[J]. Journal of Central South University: Science and Technology, 2008, 15(2): 204-208.
[18] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, et al. Identification of arsenato antimonates in copper anode slimes[J]. Hydrometallurgy, 2006, 84(3/4): 211-217.
(编辑 陈爱华)
收稿日期:2010-04-08;修回日期:2011-06-20
基金项目:广东省教育部产学研重大项目(2009B090200053)
通信作者:郑雅杰(1959-),男,湖南常德人,教授,博士生导师,从事有色金属冶金,水污染治理和资源综合利用研究;电话:0731-88836285;E-mail: zzyyjj01@yahoo.com.cn
摘要:采用SO2还原,使铜电解液中As(Ⅴ)还原为As(Ⅲ),Sb(Ⅴ)还原为Sb(Ⅲ),并调节电解液中As(Ⅴ)与As(T),Sb(Ⅴ)与Sb(T)的物质的量比,蒸发浓缩电解液,冷却结晶后,能有效脱除铜电解液中As,Sb和Bi等杂质。研究结果表明:当铜电解液体积为3 L,Cu的质量浓度为32 g/L,As(T)的质量浓度为9.36 g/L,Sb(T)的质量浓度为0.65 g/L,Bi的质量浓度为0.45 g/L,n(As(Ⅴ))/n(As(T))和n(Sb(Ⅴ))/n(Sb(T))分别为0.4时,蒸发浓缩电解液,使浓缩前电解液与浓缩后电解液体积比为2.5,冷却至10 ℃结晶,过滤,铜电解液中Cu,As,Sb和Bi脱除率分别为82%,62%,55%和85%。蒸发浓缩结晶产物中含CuSO4·5H2O和As2O3。其结晶产物中Cu为29%,As为5%,Sb为0.37%,Bi为0.2%。


