
Aluminum production by carbothermo-chlorination reduction of
alumina in vacuum
YUAN Hai-bin(袁海滨)1, 2, 3, YANG Bin(杨 斌) 1, 2, 3, XU Bao-qiang(徐宝强) 1, 2, 3,
YU Qing-chun(郁青春) 1, 2, 3, FENG Yue-bin(冯月斌) 1, 2, 3, DAI Yong-nian(戴永年) 1, 2, 3
1. National Engineering Laboratory of Vacuum Metallurgy,
Kunming University of Science and Technology, Kunming 650093, China;
2. Key Laboratory of Nonferrous Metals Vacuum Metallurgy of Yunnan Province,
Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Materials and Metallurgy Engineering,
Kunming University of Science and Technology, Kunming 650093, China
Received 3 August 2009; accepted 20 January 2010
Abstract:
Aluminum production by carbothermo-chlorination reduction of alumina in vacuum was investigated by XRD, SEM, EDS and thermodynamic analysis. Thermodynamic calculations indicate that AlCl(g) generated by carbothermo-chlorination process among Al2O3-C-AlCl3 system should be at 1 377-1 900 K (100 Pa) and AlCl (g) will disproportionate into aluminum and AlCl3(g) below 950-1 050 K at 10-102 Pa. Experimental results demonstrate that Al4O4C and Al4C3 begin to be formed by Al2O3-C system over 1 698 K (40-150 Pa). It is Al4O4C and Al4C3 but not Al2O3-C that participate in the carbothermic-chlorination reaction. Temperature for AlCl(g) generated by Al4O4C-AlCl3-C, Al4C3-Al2O3-AlCl3 and Al4O4C-Al4C3-Al2O3-AlCl3-C system is 1 703-1 853 K (40-150 Pa). Aluminum metal is produced by AlCl(g) disproportionation process below 933 K. The average purity of aluminum metal reaches 95.32%, which has perfect crystallization and uniform grain size.
Key words:
alumina; aluminum; carbothermic-chlorination reduction; AlCl;
1 Introduction
During the past 50 years, there were numerous attempts to produce aluminum from chemical reduction to electrolytic reduction[1-4]. The production of aluminum was a chemical reduction process in the early periods, which was given up by expensive reductant and low production. Till 1886, the appearance of electrolytic reduction made aluminum production obtain great development which was used widely. Although currently aluminum is produced industrially via the Hall-Héroult process by dissolving Al2O3 in fused NaF-AlF3 followed by direct current electrolysis, the main drawbacks of the electrolytic production are of very high energy consumption (0.186 GJ/kg Al), the release of perfluorocarbons, and the high specific CO2 emissions (7.42 kg CO2/kg Al)[5-6]. Therefore, much effort has been spent to achieve the carbothermic reduction of Al2O3 to metallic Al[2-4]. At the ALCOA Corporation, a stack-type reactor was developed in which a charge of Al2O3 and C was inserted in a high-temperature upper reaction zone to form a liquid mixture of Al2O3 and Al4C3 which was then transferred to a lower reaction zone for the extraction of liquid Al. The total energy demand of 0.121 GJ/kg Al by this process for both electric energy and carbon consumption was thus significantly lower than that by Hall-Héroult process. Replacement of the electrochemical process by carbothermic reduction of Al2O3 would decrease the total greenhouse gas emissions by at least 30%[7-8]. In spite of considerable effort, the carbothermic reduction of Al2O3 to Al remains a formidable technical challenge, due to the high temperature required, and the formation of aluminum carbide and oxycarbide byproducts[9].
As the oxycarbide products are not easy to be separated in the carbothermic reduction of alumina processes, a new method of aluminum production by carbothermic-chlorination processes in vacuum was investigated. AlCl(g) will be generated at high temperature by the carbothermic-chlorination processes and it will be decomposed at low temperature, and the decomposition products of Al and AlCl3 (g) are easy to be separated. WANG et al[10-14] used bauxite as raw material of alumina, coal as reducing agent, and anhydrous-AlCl3 as chloridizing agent, and the overall reactions can be represented by
Al2O3+3C+AlCl3=3AlCl+3CO,
![]() =1 774.3 kJ/mol (1)
=1 774.3 kJ/mol (1)
3AlCl=2Al+AlCl3, ![]() =-430.2 kJ/mol (2)
=-430.2 kJ/mol (2)
WANG et al[10] aimed at investigating the practicality of the experiments, designing vacuum furnace suitable for aluminum metallurgy[11] and exploring optimum processes about it[12-14]. However, both reactions are complicated by the formation of aluminum carbide, Al4C3, and the oxycarbides Al2OC, Al4O4C and Al2O in the carbothermic process, also impurities have uncertain effects on the carbothermic- chlorination processes which exist in the raw materials of bauxite and coal.
The main purpose of this work was to investigate the thermodynamic analysis of carbothermic process and carbothermic-chlorination process for aluminum production in vacuum. To avoid impurities effect, experiments have been carried out by using analytical chemical agents under that condition. The resultant samples were investigated by X-ray diffractometry, scanning electron microscopy with EDS, and thermodynamic calculation analysis.
2 Methods
2.1 Thermodynamic analysis
Reaction enthalphies were calculated using the data of the NIST-JANAF chemistry web-book[15] and corresponding data from Refs.[16-17]. As the working system pressure of vacuum furnace is 10-200 Pa and the system pressure is 100 Pa, relationships between Gibbs free energy and temperature were calculated.
2.2 Characterization
X-ray diffraction (XRD) analysis was performed on a Japan diffractometer(D/max-3B)using Cu Kα radiation with a scanning rate of 2 (?)/min. The morphology of the condensation product was observed by scanning electron microscope (SEM: XL30ESEM-TMP, Phillips, Holland). The contents of metal elements were determined with EDS pattern (EDS: EDAX, PHOENIXTM, USA).
2.3 Experimental
The raw materials for experiments include alumina
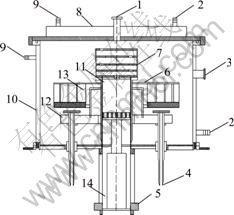
Fig.1 Schematic diagram of vacuum furnace: 1 Vacuum pump; 2 Cooling water outlet; 3 Thermocouple; 4 Water cooled anode; 5 Heating jacket; 6 Thermal insulating layer; 7 Graphite condensation tower; 8 Vacuum furnace top; 9 Cooling water inlet; 10 Vacuum furnace body; 11 Carbothermic-chlorination reaction crucible; 12 Exothermic body base; 13 Graphite exothermic body; 14 AlCl3 sublimation crucible
(analytical grade), graphite (carbon content of above 99.85 %) and anhydrous-AlCl3 (analytical grade).
Experiments were carried out in a vacuum furnace (see Fig.1), which were designed by National Engineering Lab for Vacuum Metallurgy of Kunming University of Science and Technology, China.
Carbon and alumina powders with a certain molar ratio were mixed evenly and performed to be blocks of d20 mm×10 mm under 2-8 MPa. Those blocks were put into crucible of vacuum furnace after being dried at 150 °C for 180 min. The blocks were heated to a certain temperature at 50-100 Pa before AlCl3 was heated to be AlCl3 vapor and entered the crucible filled with perform blocks. Reaction temperature, AlCl3 sublimation temperature and system pressure were kept stable. Then, stop heating and keep vacuum system working, cool the furnace to room temperature during the above process. At last, reduction products and slag were obtained.
3 Results and discussion
3.1 Thermodynamic analysis in vacuum
The thermodynamic analysis aims at carbothermic process, carbothermic-chlorination process and AlCl(g) disproportionation process by calculating the Gibbs free energy and vapor pressure of gaseous species at different temperatures, and determining system pressure and gas composition. Substantial reduction of Al2O3 to Al was found to occur only above the melting point of Al, 933.5 K, and to be almost complete only close to the boiling point, 2 767 K [1].
3.1.1 Reaction of alumina with carbon
The carbothermic reduction process of Al2O3 is simulated with an initial reaction mixture of Al2O3+3C. The equilibrium compositions in the temperature range of 298-2 100 K and the potential reactions in Al2O3-C are listed in Table 1.
Table 1 Reactions for Al2O3-C system at 298-2 100 K
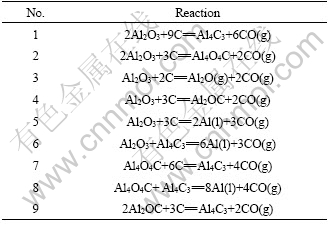
The relationships between Gibbs free energy change and temperature of the reactions were calculated at 100 Pa and the results are shown in Fig.2. From Fig.2, the temperatures of reactions (1)-(9) that can occur by Al2O3-C system should be 1 712, 1 689, 1 744, 1 717, 1 773, 1 872, 1 724, 1 882, 1 704 K (100 Pa), respec- tively. The preferential order of products is Al4O4C> Al4C3>Al2OC>Al2O>Al while direct carbothermal reduction of alumina. Another product is Al4C3 when reactants Al2OC and Al4O4C further react with carbon at 1 704-1 724 K (100 Pa). Aluminum generated by Al2O3- Al4C3 and Al4O4C-Al4C3 should be at higher temperature.
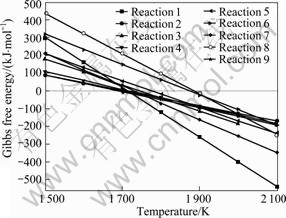
Fig.2 Relationships between ΔGT and T for Al2O3-C system at 100 Pa
3.1.2 Reaction of carbothermic-chlorination
With AlCl3(g) entering into high temperature carbothermic zone, it will participate in carbothermic- chlorination reactions with preheated mixture of Al2O3-C. Based on the thermodynamic analysis of alumina reacting with carbon, the potential reactions in Al2O3-C-AlCl3 system are listed in Table 2.
Table 2 Reactions for Al2O3-C-AlCl3 system at 298-2 100 K

The relationships between Gibbs free energy and temperature of the reactions were calculated at 100 Pa and the results are shown in Fig.3. As can be seen from Fig.3, the Gibbs free energy of above reactions is all negative and declined downwards in the temperature ranges of 1 380-1 900 K. It is concluded the gaseous AlCl can be made by preheated mixture of Al2O3-C reacting with AlCl3(g), the preferential order for the reaction is reaction 11>reaction 13>reaction 12>reaction 10>reaction 14, and the temperatures of reactions (10)-(14) that can occur among Al2O3-C-AlCl3 should be 1 500, 1 377, 1 459, 1 433, 1 900 K (100 Pa), respectively.
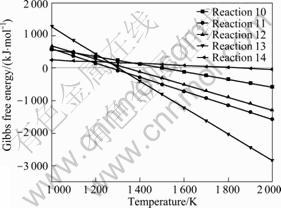
Fig.3 Relationships between ΔGT and T for Al2O3-C-AlCl3 system at 100 Pa
3.1.3 Reaction of AlCl(g) disproportionation
AlCl(g) should be generated by reactions (10)-(14) among Al2O3-C-AlCl3 in the temperature range of 1 377-1 900 K, which will disproportionate into alumi- num and AlCl3(g) at low temperature zone. The reaction can be described by
3AlCl(g)=2Al+AlCl3(g, s) (15)
The relationship between Gibbs free energy and temperature of the reaction at different system pressures is shown in Fig.4. As can be seen, the initial reaction temperature for reaction (15) is gradually decreased with system pressure down from 105 to 10 Pa. AlCl(g) disproportionation process is a capacity reduction reaction, and the larger the system pressure is, the more beneficial for AlCl(g) disproportionation process to carry out. The temperature for reaction (15) should be 950- 1 050 K at 10-102 Pa.
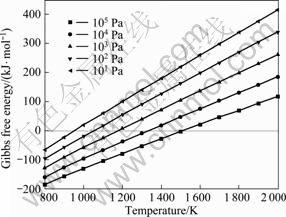
Fig.4 Relationship between Gibbs free energy and temperature at different system pressures
3.2 Experiments on carbothermic and carbothermic- chlorination processes
3.2.1 Process of carbothermic reaction
Carbothermic reactions were carried out under the following conditions: Al2O3+3C (molar ratio), temperature 1 693-1 853 K, 60 min, system pressure 40-150 Pa. The XRD patterns of slag are shown in Fig.5.

Fig.5 XRD patterns of carbothermic reduction slag: (a) 1 693- 1 698 K; (b)1 698-1 703 K; (c)1 753 K; (d)1 853 K
From Fig.5, there is no any Al2OC found by XRD analysis in the slag of carbothermic process and no aluminum is collected in condensation tower of vacuum furnace. Additionally, Al2O(g) would disproportionate into aluminum and alumina at low temperature. According to the carbothermic reduction analysis, we can deduce that reactions (3), (4), (5), (6), (8) and (9) did not occur between alumina and carbon at 40-150 Pa. However, the characteristic peaks of Al4O4C and Al4C3 can be detected clearly over 1 698 K. The intensities of the XRD peaks of Al4O4C and Al4C3 increase while the intensities of the XRD peaks of carbon and alumina decrease with temperature rising. The initial temperatures of reactions (1), (2) and (7) are 1 712, 1 689 and 1 724 K (100 Pa), respectively. That is to say, Al4O4C was firstly formed in the carbothermic reaction, and then Al4C3 was formed at higher temperature. Thus, the diffraction intensity of Al4O4C is stronger than that of Al4C3 (Fig.5, from 1 698 K to 1 853 K), which can be explained according to the above reasons with increasing temperature. This analysis and experimental results are well agreed with the study of Ref.[18], who pointed out that the first product is Al4O4C which is a stable compound in thermodynamics and another product is Al4C3 when reactants further react with carbon. Additionally, Al4C3 could also be formed by reaction (7) at higher temperature.
3.2.2 Process of carbothermic-chlorination
Carbothermic-chlorination reactions were carried out under the conditions of Al2O3+3C+4AlCl3 (molar ratio), AlCl3 sublimation temperature set at 403 K, the temperature of carbothermic-chlorination reaction 1 703-1 853 K, 60-90 min, 40-150 Pa. The XRD patterns of slag are shown in Fig.6.
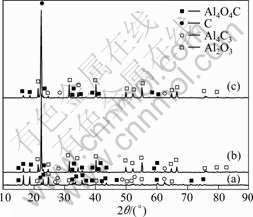
Fig.6 XRD patterns of slag of carbothermic-chlorination reaction process at 1 703-1 853 K: (a) Before chlorination at 1 703-1 853 K; (b) After chlorination at 1 703-1 803 K; (c) After chlorination at 1 803-1 853 K
Based on the analysis of reaction (10), which shows the temperature of AlCl(g) generated among Al2O3-C-AlCl3 system is 1 500 K at 100 Pa. According to Fig.5(b), the slag in the crucible identified by XRD mainly consists of alumina and carbon at 1 693-1 698 K. The slag were chlorided at 1 693 K for 60 min (40-150 Pa) and no aluminum was collected in condensation tower, which indicates that reaction (10) among Al2O3-C-AlCl3 system did not occur under that conditions.
From Fig.6, the diffraction intensity of Al4O4C after chlorination is weak compared with that before chlorination, the phase of Al4C3 almost disappeared completely and the diffracted intensities of carbon and alumina are strengthened obviously at 1 703-1 853 K (40-150 Pa) after chlorination. Therefore, we can deduce that it is Al4O4C and Al4C3 that participate in the reaction of carbothermic-chlorination by inference. Based on the thermodynamic analysis of carbothermic-chlorination process, the initial temperatures of reactions (11), (12) and (13) are 1 377, 1 459 and 1 433 K (100 Pa), respec- tively, which indicates that the temperature of Al4C3 participated in the process of carbothermic-chlorination is lower than that of Al4O4C, that is why the phase of Al4C3 almost disappeared completely after chlorination and the diffraction intensity of Al4O4C after chlorination is only weaker than that before chlorination.
Condensation products were collected in the condensation tower of vacuum furnace below 933 K, which were examined by XRD and SEM as shown in Figs.7(a) and (b), respectively. The XRD pattern of the condensation products demonstrates that all peaks are sharp and well-defined, which suggesting that product is well crystallized. The XRD peaks appearing at 2θ of 38.48?, 44.74?, 65.10?, 78.24?, and 82.42?, which are close to JCPDS standard aluminum (No.04-0787).

Fig.7 XRD pattern (a) and SEM (b) for condensation products
Therefore, the final product is aluminum metal. This indicates that the high purity of the final product Al metal with no other phase is detected. It can be supposed clearly that AlCl(g) is generated by reactions (11), (12) and (13), and Al metal is formed by AlCl(g) disproportionation below 933 K in vacuum. Furthermore, the average purity of aluminum metal attains 95.32%, which was examined by EDS. The SEM image shows that aluminum metal is in polyhedral shape with particle size of about 10 μm, and the whole dispersion performance is well.
4 Conclusions
1) The thermodynamics analysis of carbothermic reaction indicates that Al4C3, Al4O4C, Al2O, Al2OC and Al generated by Al2O3-C system should be in the temperature range of 1 704-1 882 K at 100 Pa. The temperature of AlCl(g) generated by carbothermic- chlorination process among Al2O3-C-AlCl3 system should be 1 377-1 900 K and AlCl(g) will dispropor- tionate into aluminum and AlCl3(g) below 950-1 050 K at 10-102 Pa.
2) Experimental results show that only Al4O4C and Al4C3 are formed in the carbothermic process at 1 698 - 1 853 K (40-150 Pa), Al4O4C is firstly formed, and then Al4C3 is formed at higher temperature. It is Al4O4C and Al4C3 but not Al2O3-C that participate in the carbothermic-chlorination process. AlCl(g) is generated by Al4O4C-AlCl3-C, Al4C3-Al2O3-AlCl3, and Al4O4C- Al4C3-Al2O3-AlCl3-C system at 1 703-1 853 K (40-150 Pa), and which disproportionate into Al and AlCl3(g) below 933 K. The average purity of Al metal reaches 95.32%, which has perfect crystallization and uniform grain size.
3) As the initial temperature of AlCl(g) generated by Al2O3-C-AlCl3 system in vacuum is lower than that at 105 Pa, and it will be decomposed at low temperature, the decomposition products of Al and AlCl3(g) are easy to be separated. The mechanism and processes of aluminum production by carbothermic-chlorination process are well worth further studying.
References
[1] HALMANN M, FREI A, STEINFELD A. Carbothermal reduction of alumina: Thermochemical equilibrium calculations and experimental investigation [J]. Energy, 2007, 32: 2420-2427.
[2] MURRAY J P. Aluminum production using high-temperature solar process heat [J]. Solar Energy, 1999(2): 133-142.
[3] FRUEHAN R J, CARKIN G. The pressure of Al2O and Al in equilibrium with a Al4C3- Al2O3 (saturated) slag at 1 950 °C to 2 020 °C [J]. Metallurgical and Materials Transactions, 2004, 35B(5): 1011-1013.
[4] FRUEHAN R J, LI Y, CARKIN G. Mechanism and rate of reaction of Al2O, Al, and CO vapors with carbon [J]. Metallurgy and Materials Transactions, 2004(8): 617-623.
[5] CHOATE W, GREEN J A S. U.S. Energy Requirements for Aluminum Production: Historical Perspective, Theoretical Limits and Current Practices [M]. US: U.S. Department of Energy: 2007.
[6] STEINFELD A, THOMPSON G. Solar combined thermochemical processes for CO2 mitigation in the iron, cement, and syngas industries [J]. Energy, 1994, 19(10): 1077-1081.
[7] COCHRAN C N, FITXGERALD N M. Energy efficient production of aluminum by carbothermic reduction of alumina. US, 80-125644 [P]. 1980-02-28.
[8] MYKLEBUST H, RUNDE P. Greenhouse gas emission from aluminum carbothermic technology compared to Hall-Héroult technology [J]. Light Metals, 2005: 519-522.
[9] CHOATE W, GREEN J. Technoeconomic assessment of the carbothermic reduction process for aluminum production [J]. Light Metals, 2006: 445-450.
[10] WANG Ping-yan, DAI Yong-nian, JIANG Shi-xin, ZHONG Hong-quan. Study on production of aluminum by carbothermic reduction and subhalide decomposition under vacuum [J].Nonferrous Metals (Metallurgy), 2005(2): 11-13. (in Chinese)
[11] WANG Ping-yan, YANG Bu-zheng, DAI Yong-nian, LIU Yong-sheng. Test equipment for the production of aluminum by carbothermic reduction of aluminum-bearing materials with the aid of subhalide decomposition under vacuum [J]. Mining and Metallurgical Engineering, 2005(4): 43-45. (in Chinese)
[12] WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of Al from bauxite by carbothermic reduction-chlorination [J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377-380. (in Chinese)
[13] WANG Ping-yan, DAI Yong-nian. Experimental investigation on carbothemic reduction chloride to produce aluminum [D]. Kunming: Kunming University of Science and Technology, 2005: 51-86. (in Chinese)
[14] WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Thermodynamic study on the reaction of producing liquid aluminum and gas aluminum trichloride from subchloride [J]. Light Metals, 2008(1): 13-16. (in Chinese)
[15] CHASE M W. NIST-JANAF thermochemical tables [M]. 4th ed. New York: American Chemical Society and the American Institute of Physics for the National Institute of Standards and Technology, 1998: 98-143.
[16] LIHRMANN J M. Thermodynamic of the Al2O3-Al4C3 system I. Thermochemical functions of Al oxide, carbide and oxycarbides between 298 and 2 100 K [J]. Journal of the European Ceramic Society, 2008(28): 633-642.
[17] [Turkey] Ihsan·Barin. Thermochemical date of pure substances, 3rd edition [M]. CHENG Nai-liang, NIU Si-tong, XU Gui-ying. Beijing: Science Press, 2003. (in Chinese)
[18] QIU Zhu-xian. Physical chemistry of aluminum metallurgy [M]. Shanghai: Shanghai Science and Technology Press, 1985: 351. (in Chinese)
Foundation item: Project(u0837604) supported by the Joint Funds of the National Natural Science Foundation of China and Yunnan Province; Project(20095314110003) supported by the special Research Funds of the Docter Subject of Higher School, China
Corresponding author: YANG Bin; Tel: +86-871-5161583; E-mial:kgyb2005@126.com
DOI: 10.1016/S1003-6326(09)60329-0


