
J. Cent. South Univ. (2018) 25: 783-794
DOI: https://doi.org/10.1007/s11771-018-3783-y
Properties of boron-rich slag separated from boron-bearing iron concentrate
WANG Guang(王广), WANG Jing-song(王静松), XUE Qing-guo(薛庆国)
State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing,Beijing 100083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
In the present paper, the fundamental research on the properties of boron-rich slag melting separated from boron-bearing iron concentrate was performed. The melting and fluidity of B2O3–MgO–SiO2–FeO slag system, crystallization of separated boron-rich slag and factors on the extraction efficiency of boron-rich slag were systematically investigated. B2O3 content would heavily affect the melting and fluidity property of boron-rich slag. Generally, FeO could improve the melting and fluidity property of boron-rich slag. Boron-containing crystalline phase mainly precipitated in temperature range from 1200 °C to 1100 °C. Higher smelting temperature and B2O3 reduction ratio were negative for the extraction of boron. The cooling rate of 10–20 °C/min was better for the crystallization of boron-containing crystalline phase. Based on the obtained experimental results, the optimum operating parameters for the development of pyrometallurgical boron and iron separation process and further boron-rich slag cooling process were proposed.
Key words:
boron-bearing iron concentrate; boron-rich slag; melting; fluidity; crystallization;
Cite this article as:
WANG Guang, WANG Jing-song, XUE Qing-guo. Properties of boron-rich slag separated from boron-bearing iron concentrate [J]. Journal of Central South University, 2018, 25(4): 783–794.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3783-y1 Introduction
Boron compounds, chiefly borates, are widely used in more than 300 applications and more than three quarters of the world’s supply is consumed in ceramics, detergents, fertilizer and glass [1]. Boron is also a significant alloying element for high strength steel production [2]. The Liaoning and Jilin Provinces of China reserve 0.28 billion tons of low-grade ludwigite deposit, which accounts for 58% of the boron reserves of China. The average composition of the ore is approximately 7 wt% B2O3 and 30 wt% TFe, and it can not be directly utilized as single iron or boron ore resource by traditional processing methods because of the much lower grades of total iron (TFe) and B2O3 than the value of industrial demand [3, 4]. In China, the comprehensive utilization of ludwigite is becoming increasingly urgent because of the increasing consumption demand for boron compounds and the depletion of the traditional high-grade szaibelyite. Boron-bearing iron concentrate and boron concentrate can be effectively obtained through ore dressing method from the low grade crude ludwigite. Boron concentrate can be directly used as raw material for boron industry and the beneficiation of boron from boron-bearing iron concentrate should be realized in order to improve the total yield of boron. The boron and iron separation of boron-bearing iron concentrate can be realized through selective reduction and melting separation method, which has been proposed for many years by Chinese researchers [5–9]. The separated boron-rich slag is a kind of new man-made boron resource of high grade, which can be used as the raw materials for borax or boric acid production [10, 11].
In the pyrometallurgical utilization process of boron-bearing iron concentrate, the property (such as melting and fluidity properties) of the boron-rich slag at high temperature will affect the smelting separation result. Correspondingly, the smelting operation parameters of the pyrometallurgical process will also affect the crystallization and extraction property of the boron-rich slag in the next borax production stage. Generally, the key factor in pyrometallurgical utilization of boron- bearing iron concentrate is the high extraction efficiency of boron (EEB) of the slag. The EEB of the boron-rich slag is closely related to its mineral phase composition and microstructure, which depend on the slag chemical composition, initial molten state and cooling condition. This is a very complicated process. On the premise of iron and slag melting separation, the optimum operating parameters to guarantee the high EEB in the pyrometallurgical process should be systematically investigated, which can help to determine the appropriate flow sheet. Many researchers have studied the properties of boron-rich slag separated by blast furnace, such as slag composition on the EEB, mineral phase composition of cooled boron- rich slag, viscosity and surface tension of molten boron-rich slag [12–14]. However, they have not investigated the crystallization process and the effect of operating parameters on the EEB of boron-rich slag to guide the operation. In the present paper, the fundamental research on the melting and fluidity of B2O3–MgO–SiO2–FeO slag system, crystallization of separated boron-rich slag and factors on the extraction efficiency of boron-rich slag have been performed at laboratory scale to provide referencing data for the development of the rotary hearth furnace reduction and electric furnace melting separation utilization process.
2 Experimental
2.1 Raw materials
In the experiment, separated slag from boron- bearing iron concentrate/coal composite pellet and chemical oxides of analytical reagent (AR) grade (B2O3, MgO, SiO2, Fe3O4) were used. FeO was used to adjust the composition of the slag and was obtained by the reduction of Fe3O4 at 900 °C for 3 h under the atmosphere of 50%CO–50%CO2 with a flow rate of 5 L/min. The XRD analysis showed that all the Fe3O4 had been reduced into FeO.
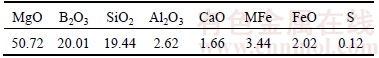
The boron-bearing iron concentrate used in this study was obtained from Liaoning Province, China. Pulverized anthracite coal was used as the reducing agent. The boron-bearing iron concentrate and pulverized coal were mixed together with mole ratio of “fixed carbon” in the coal to “reducible oxygen” in the iron oxide (C/O) of 1.2. The ore/coal mixture was completely mixed and was pelletized in a horizontal twin roller machine. The pellet was reduced at 1400 °C for 15 min in graphite crucible in a MoSi2 resistance furnace to realize iron and slag melting separation. The chemical composition of the boron-rich slag was listed in Table 1. The separated slag was used to perform fundamental research on the properties of boron-rich slag.
Table 1 Chemical composition of boron-rich slag (mass fraction, %)
2.2 Experimental procedure
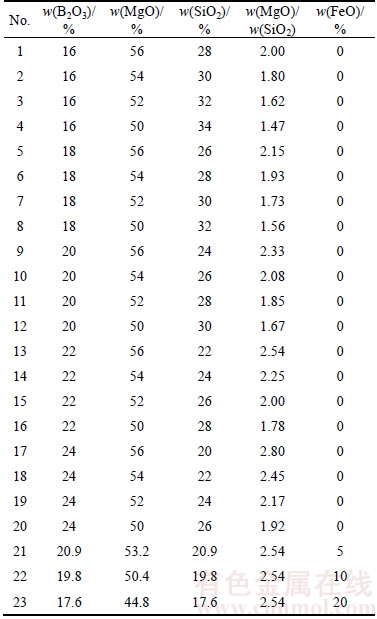
The melting property of the boron-rich slag was tested by differential scanning calorimetry (DSC, TA Q600) method and the temperature of endothermic peak of the DSC curve was set as the melting temperature. During the test, the sample was under the protection of high purity N2 with a flow rate of 100 mL/min. The heating rate was set as 20 °C/min and the slag sample powder was put into a Al2O3 crucible with the size of d 5.2×5.0 mm. The slag samples were prepared using the B2O3, MgO, SiO2 and FeO chemicals, and the composition was adjusted based on the mineral phase composition of the boron-bearing iron concentrate and the chemical composition of the minerals. The compositions of slag samples were listed in Table 2. These oxides were crushed into fine power and well mixed according to the data in the table.
The fluidity of the slag samples was also tested. Traditionally, the fluidity of molten slag was characterized by the viscosity value. In the experiment, the fluidity was characterized by a projection area method [15]. The fluidity index of slag was defined as the increasing of vertical projection area after molten slag flowed under a certain experimental condition. The calculation formula was as follows:
IF is the fluidity,
IF=(Aafter–Abefore)/Abefore (1)
where Aafter is the vertical projection area of slag after melting, Abefore is the vertical projection area of primary-slags before melting. The test was performed in a muffle furnace at 1450 °C for 5 min under air atmosphere.
Table 2 Chemical composition of slag samples
The separated boron-rich slag was smelted again in six graphite crucibles at 1400 °C for 30 min in the MoSi2 furnace. The mass of slag in each graphite crucible was approximately 20 g. After the six crucibles were heated, the furnace was powered off and allowed to slowly cool. One of the crucibles was removed from the furnace at each temperature of 1400, 1300, 1200, 1100, 1000 and 900 °C and was subsequently quenched in water. The mineral composition of each quenched slag was characterized by X-ray diffraction (XRD, MAX-RB) and electron probe microanalyzer (EPMA, JXA-8230) to determine the microstructural evolution during the cooling process.
Some influencing factors related to the future practical operation process, such as smelting temperature and reduction of B2O3 in slag, on the crystallization and extractive efficiency of boron were simulated in a muffle furnace. Once the heating finished, the furnace was turned off and the slag samples were cooled to room temperature with the furnace. All the samples were characterized by XRD, scanning electron microscope with energy dispersive spectrometer (SEM-EDS, MLA250) and EEB test. The normal pressure alkaline leaching method was applied to evaluate the EEB value [6]. The parameters of the method was described as follows: amount of boron-rich slag: 4.000 g, 98% particle size of slag: <0.074 mm, concentration of lye: 20 wt% NaOH, volume of lye: 40 mL, leaching time: 4 h. The boron content of primary and residual slag was analyzed by ICP-AES method. The EEB was defined by Eq.(2).
EEB=(Aps–Ars)/Aps×100% (2)
where Aps is the boron amount of primary slag; Ars is the boron amount of residual slag.
The effect of cooling rate on the crystallization of boron-rich slag was performed using the single hot thermocouple technology (SHTT), which had been widely used in the study on the crystallization of different kinds of slags [16, 17]. The separated boron-rich slag was firstly heated to 1400 °C and then was cooled continuously from 1400 °C to 1100 °C at the cooling rate of 5, 10, 20, 40, 60 and 100 °C/min. The continuous cooling test aims to investigate the effect of cooling rate on the crystallization of boron-rich slag.
3 Experimental results and analysis
3.1 Melting and fluidity of boron-rich slag
3.1.1 Melting property
The variation of melting temperature of synthesized boron-rich slag with different chemical compositions is shown in Figure 1. It can be seen that the melting temperature gradually decreases from about 1290 °C to 1190 °C with the decreasing of MgO content (i.e., increase of SiO2) when the B2O3 content is fixed at a certain value, such as 16%, 18%, 20%, 22% and 24%. When the MgO content is fixed at a certain value, the effect of B2O3 content on the melting temperature is different. When the MgO content is 56 wt%, the melting temperature slightly increases with the increasing of B2O3 from 16 wt% to 24 wt%. At the meanwhile, the SiO2 content gradually decreases with the increasing of B2O3 in the same interval value. When the MgO content is in the range of 50 wt%–54 wt%, the melting temperature firstly increases with the B2O3 content increasing from 16 wt% to 22 wt% and then decrease with the B2O3 content increasing from 22 wt% to 24 wt%. It indicates that the effect of B2O3 on the melting temperature gradually increase with the decreasing of MgO and is heavier than SiO2 when the B2O3 content is as much as 24 wt%.
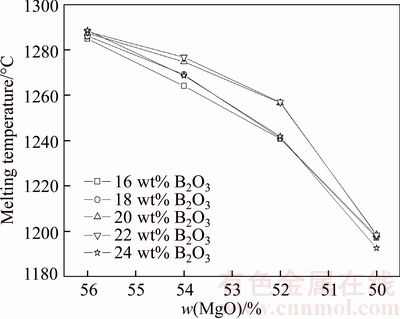
Figure 1 Variation of melting temperature of synthesized boron-rich slag with different chemical compositions
The variation trend of B2O3 on melting temperature is different when the content of SiO2 and B2O3 is in different range. It is known that the melting point of B2O3 is only 450 °C and it can theoretically reduce the slag melting temperature by common sense. However, the experimental result indicates that the B2O3 can obviously reduce the melting temperature only when the B2O3 content is 24 wt%. The reason may be the increase of SiO2 will make the composition of the slag more close to the eutectic point of MgO–SiO2 binary slag system, which will reduce the slag melting point [18]. The effect of eutectic reaction on the decrease of melting temperature is heavier than the addition of low melting point B2O3 when the MgO content is high and the B2O3 content is low.
During the reduction and melting separation of boron-bearing iron concentrate/coal composite pellet, there must be a certain amount of FeO in the separated boron-rich slag. Therefore, the effect of FeO on the melting of synthesized boron-rich slag is studied and the result is shown in Figure 2. The melting point of FeO is 1371 °C. The eutectic reaction in the FeO–SiO2 binary slag system can reduce the melting temperature. Therefore, the slag melting temperature gradually decreases with the increasing of FeO from 0 to 10 wt%. However, the slag melting temperature increases when the FeO content increases from 10 wt% to 20 wt%. The further increase of FeO making the slag system far from the eutectic point may be the main reason for the increasing of melting temperature.
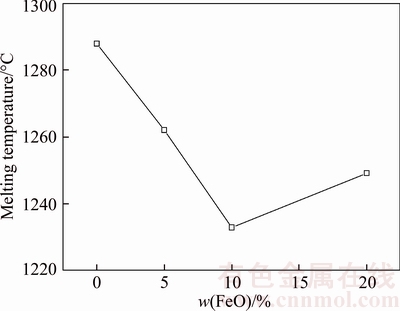
Figure 2 Effect of FeO on melting temperature
3.1.2 Fluidity property
The fluidity index of the synthesized boron-rich slag (No. 1–No. 20) is given in Figure 3, which shows that the fluidity index increases with the increasing of B2O3 content. The fluidity of the slag is very poor when the B2O3 and SiO2 contents are low. When the B2O3 content is 16 wt%–18 wt% and SiO2 content is lower than 30 wt%, the slag sample only melts but nearly does not flow. When the B2O3 content is higher than 22 wt%, the fluidity of the slag obviously increases. When the B2O3 content is 24 wt%, the slag sample completely melts and wets the surface of substrate Fe–Cr–Al alloy plate. All the results above indicate that the B2O3 can improve the fluidity of the slag at high temperature although it is a network forming acidic oxide. Many researchers obtain the same result [19–21]. B2O3 is easy to form a eutectic which is even more effective to reduce the viscosity of molten slag. At high temperature, some tetrahedral [BO4]5– changes into triangle [BO3]3– which will disintegrate the chains/molecules of molten slag and makes the structure of molten matrix become loose, so the viscosity will further decrease.
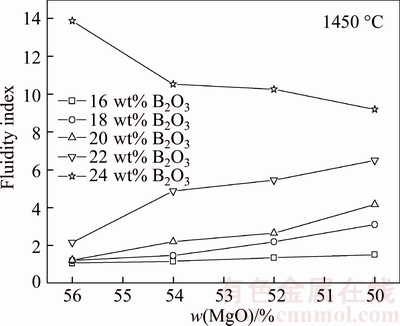
Figure 3 Variation of fluidity index of synthesized boron-rich slag with different chemical compositions
On the other hand, the fluidity index increases with the decreasing of MgO content (i.e. increasing of SiO2) when the B2O3 content is in the range of 16 wt%–22 wt%, and the increasing rate is bigger when the B2O3 content is high. However, the fluidity index decreases with the decreasing of MgO content (i.e. increasing of SiO2) when the B2O3 content is 24 wt%. The reason may be that the SiO2 behaves as a stronger network forming acidic oxide and it can result the increasing of slag viscosity through improving the bonding of [SiO4]4– tetrahedral structural units [22]. The boron-bearing iron concentrate/coal composite in the present experiment can melt separation at 1400 °C and the chemical composition of the separated boron-rich slag is similar as the No. 13 slag, whose fluidity index is 2.16. Therefore, the fluidity index of boron-rich slag should be higher than 2.16 to guarantee the melting separation of the composite pellet.
The effect of FeO on the fluidity of the synthesized boron-rich slag is shown in Figure 4. It can be seen that the fluidity index gradually increase when the FeO in the slag increases from 0 to 20 wt%. It is well known that FeO is a network modifier (basic oxide) and it supplies free Fe2+ and O2– ions to the silicate melts resulting depolymerization of the silicate network [23]. Therefore, the addition of FeO can cause continuous decreasing of slag viscosity at high temperature with the increasing FeO content.
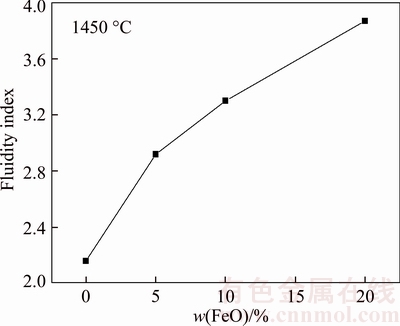
Figure 4 Effect of FeO on fluidity index of synthesized slag
3.2 Crystallization and phase characterization of separated boron-rich slag
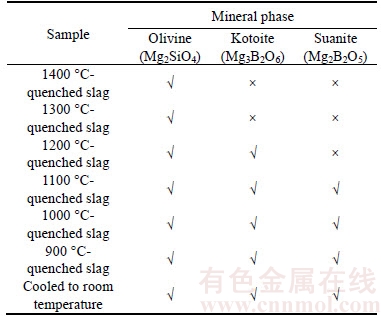
The main mineral phase species of the quenched separated boron-rich slag at different temperatures during slow cooling are shown in Table 3. The obtained results can help us to understand the detail information of crystallization process of boron-rich slag. It can be seen that the phase composition of the slag changes obviously during slow cooling. For the slag quenched at 1400 °C, the main crystalline phase is olivine. The main crystalline phase of the slag quenched at 1300 °C is still olivine. At 1200 °C, a new phase appears and it should be kotoite according to the XRD results.At 1100 °C, a new suanite phase begins to appear and the olivine and kotoite phases exist as well. If the slag is further cooled, the phase species of the slag change little. The results indicate that the temperature range from 1300 °C to 1100 °C is the most important range for the crystallization of the boron-containing phases.
Table 3 Main mineral phases of quenched separated boron-rich slag during slow cooling
The EPMA is further utilized to characterize mineral phase and study element migration during the crystallization process. The 1400 °C, 1300 °C, 1200 °C and 1100 °C quenched samples are investigated and the results are shown in Figure 5. For the 1400 °C quenched sample, the acicular phase and matrix phase account for the main volume ratio and consist of B2O3, MgO and SiO2 in relatively large quantity as well as Al2O3, CaO, FeO and MnO in small quantity. The MgO/B2O3 (molar ratio) of the acicular phase is 1.72, which is close to the composition of suanite, and it may be the precursor of boron-containing crystalline phase. What is more, the acicular phase contains 6.35 wt% SiO2. The matrix phase among the acicular phase contains high of B2O3 as well as Al2O3, CaO, FeO and MnO, which results low melting point. The original and precipitated olivine both contains a little amount of B2O3. For the 1300 °C quenched sample, the MgO/B2O3 (molar ratio) of the acicular phase increases a little and the B2O3 content of the matrix phase slight decreases. For the 1200 °C quenched sample, the size of acicular phase increases and the MgO/B2O3 (molar ratio) continues increasing to 1.92. The concentration of B2O3 and other elements in the matrix phase continue to increase. For the 1100 °C quenched sample (i.e., Figure 5(d)), the Area 1 is kotoite and Area 2 is suanite. It can be seen that the amount of suanite is more than kotoite. Both of the two crystalline phases contain a certain amount of SiO2 and the SiO2 content in suanite is lower than that of kotoite. B2O3 and other elements still form an amorphous phase. Based on above results, the following conclusion can be made. During the slow cooling process of molten boron-rich slag, a precursor phase mainly containing B2O3, MgO and SiO2 will first precipitate and the residual B2O3, MgO, SiO2 and other elements will enrich in the liquid phase. With the cooling going on, the B2O3 and MgO in the liquid phase continually migrate into the precursor phase to make it grow bigger, and the impurity elements in the precursor phase will be discharged into the residual liquid phase until the formation of suanite and kotoite. The low melting temperature liquid phase containing high of B2O3 and other elements will solidify at final stage and forms an amorphous phase distributed among the other crystalline phases.
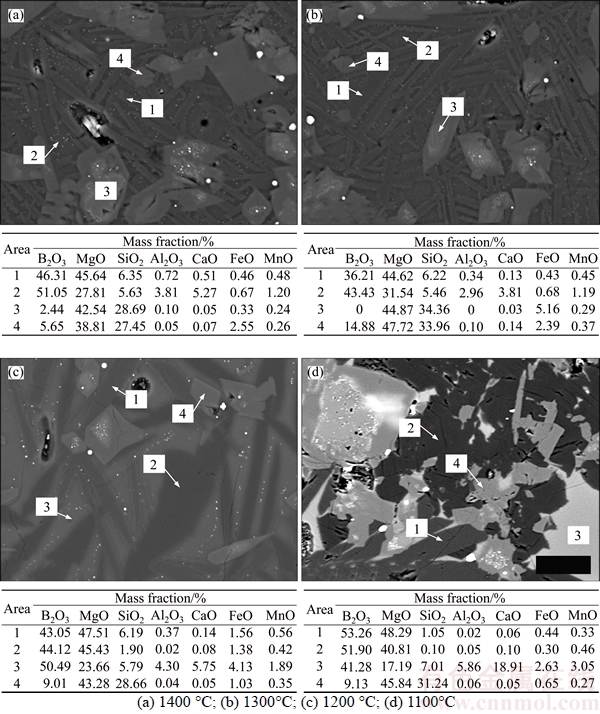
Figure 5 EPMA analysis of quenched slags:
3.3 Influencing factors on crystallization and extractive efficiency of separated boron-rich slag
3.3.1 Smelting temperature
When using different pyrometallurgical process to realize boron and iron separation from boron-bearing iron concentrate, the smelting temperature is different and the initial structure of boron-rich slag melt will surely be different. In the experiment, the separated boron-rich slag samples are smelted at 1400 °C, 1450 °C, 1500 °C and 1550 °C for 15 min and cooled slowly to room temperature, respectively. The XRD, SEM-EDS and EEB tests are then performed.
The XRD analysis shows that the mineral phase composition of the four slag samples smelted at different temperatures are nearly the same. The SEM images of the four slag samples are shown in Figure 6. It can be seen that the smelting temperature has great influence on the microstructure and the morphology of crystalline phases. For the 1400 °C smelted sample, there are many pores in the slag and the size of boron- containing crystalline phase and olivine is relatively smaller. Most of the olivine is the original type and is mainly in granular or lath shape, whose length is smaller than 200 μm. For the 1450 °C smelted sample, the slag becomes much denser, and the boron-containing crystalline phase and olivine aggregate into larger size. For the 1500 °C and 1550 °C smelted samples, the size of olivine obviously increases and the big columnar crystal appears. Some of the olivine crystals are larger than 2 mm. The size of boron-containing crystalline phase also becomes much larger. On the other hand, the amount of amorphous phase obviously increases when the temperature is higher than 1450 °C. From the morphology of the big columnar olivine, it can be concluded that this kind of olivine precipitates from the melt during the cooling process and most of the original olivine has dissolved into the slag phase at the temperature above 1500 °C.
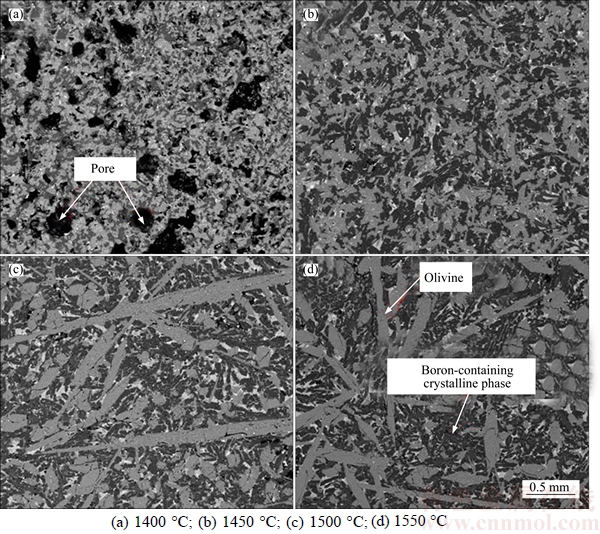
Figure 6 Effect of smelting temperature on microstructure of boron-rich slag:
During the slow cooling process, the olivine will precipitate first and it can act as the crystallization core of the boron-containing crystalline phase. If the smelting temperature is higher than 1500 °C, there will be wider temperature range for the growth of olivine phase before the precipitation of the boron-containing crystalline phase and most of the precipitated olivine will grow much bigger. Big olivine is not suitable for acting as the crystallization core. On the other hand, the bigger size of olivine at early stage will restrain the element migration, which may be bad for the crystallization of boron-containing crystalline phase.
The effect of smelting temperature on the EEB of boron-rich slag is shown in Figure 7. It can be seen that the EEB value gradually decreases with the increasing of initial smelting temperature. The reasons may be as follows: a) The density of boron-rich slag increases with the increasing of smelting temperature, which results in the worse leaching efficiency. b) Because the amount of amorphous phase increases and the amorphous phase contains high of B2O3, resulting in more B2O3 migrating into the amorphous phase with the increasing of smelting temperature. However, only the B2O3 in crystalline phase can be easily leached out, so the extraction property of the boron-rich slag becomes worse.
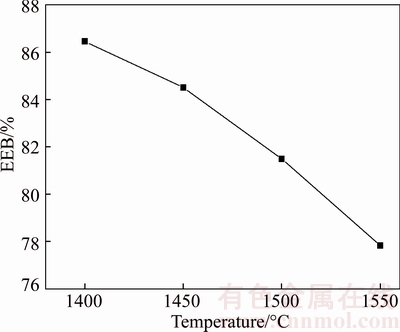
Figure 7 Effect of smelting temperature on EEB of boron-rich slag
3.3.2 Reduction of B2O3 in slag
If the solid carbon exists during the pyrometallurgical boron and iron separation process at high temperature, the B2O3 in the boron-rich slag will be reduced into molten iron by carbon. In the case, the B2O3 content will decrease and the MgO and SiO2 content will increase, which may affect the crystallization and extraction of boron-rich slag. The effect of B2O3 reduction on the mineral phase composition of boron-rich slag is shown in Figure 8. It can be seen that the peak of suanite becomes gradually weak and the peak of kotoite becomes gradually strong with the reduction of B2O3. When the B2O3 content in the slag is 20 wt%, the main boron-containing crystalline phase is suanite. However, the main boron-containing crystalline phase changes into kotoite if the B2O3 content in the slag decreases to 14 wt%. Figure 9 shows that the size and volume ratio of boron-containing crystalline phase decreases due to the B2O3 reduction. The melting temperature of the boron- rich slag will also increase, which results the dissolution of olivine into the slag phase becoming difficult. Therefore, it is difficult for olivine to grow bigger by secondary crystallization and most of the olivine formed in the slowly cooled slag is in the granular shape. The effect of B2O3 reduction on the EEB of boron-rich slag is shown in Figure 10. Generally speaking, the extraction property of the boron-rich slag gradually becomes worse with the reduction of B2O3. When the B2O3 content decreases from 20 wt% to 18 wt%, the EEB value increases a little. However, EEB value obviously decreases with the further decreasing of B2O3 content. The reason is that the boron-containing crystalline phase transforms from suanite to kotoite and the extraction property of kotoite is worse than suanite.
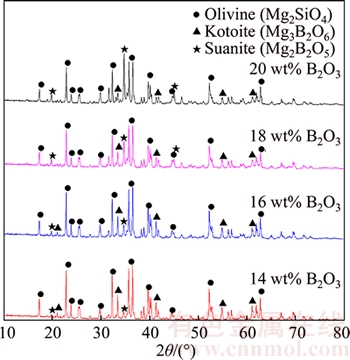
Figure 8 Effect of B2O3 reduction on mineral phase composition of boron-rich slag
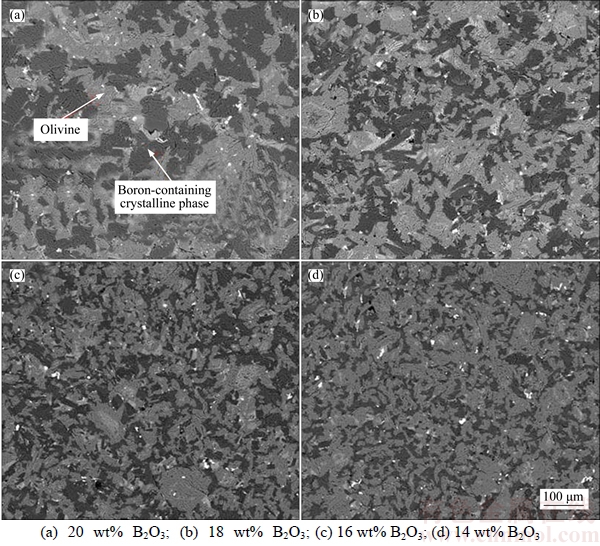
Figure 9 Effect of B2O3 reduction on boron-rich slag microstructure:
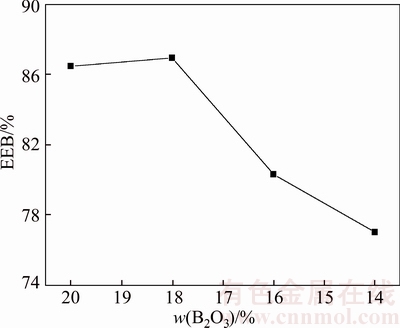
Figure 10 Effect of B2O3 reduction on EEB of boron- rich slag
3.3.3 Cooling rate
The microstructure SEM images of boron-rich slag under different continuous cooling rates are shown in Figure 11. It can be seen that the cooling rate has great influence on the microstructure and crystalline phase composition. When cooled by 5 °C/min, the olivine crystalizes well and its crystal size is very big. However, the amount and size of boron-containing crystalline phase is small. The other components, such as Al2O3, CaO, FeO and MnO, are gradually enriched and will form low melting temperature amorphous phase with B2O3, MgO and SiO2 distributed among the olivine crystals. It can be concluded that too small cooling rate is not good for the crystallization of boron- containing crystalline phase. When cooled by 10 °C/min, the amount and size of boron-containing crystalline phase increases and the amorphous phase disappears. When cooled by 20 °C/min, the amount and size of boron-containing crystalline phase continue to increase. The amount and size of boron-containing crystalline phase obviously decrease when the cooling rate increases to 40 °C/min. When cooled by 60 °C/min and 100 °C/min, the mineral phases in the slag cannot crystallize well and the crystal size is small. Therefore, the appropriate cooling rate for the boron-rich slag is about 10–20 °C/min.
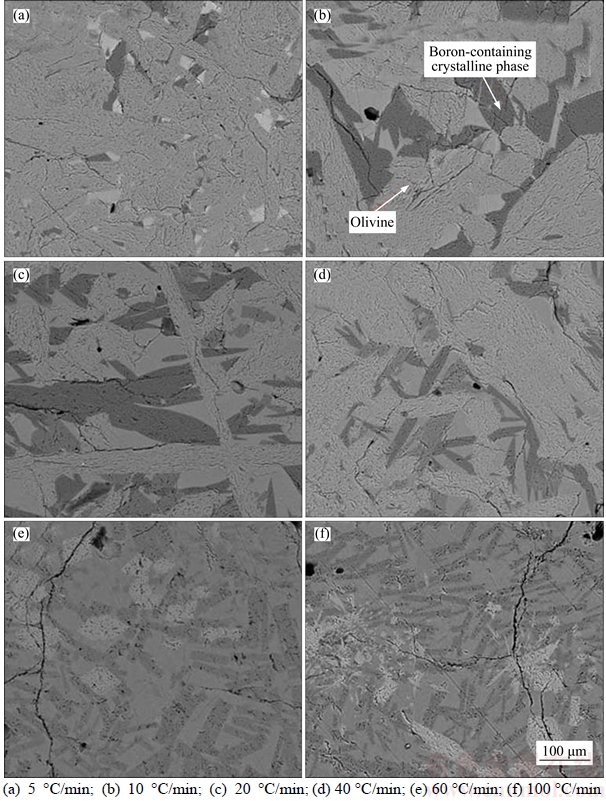
Figure 11 Effect of cooling rate on boron-rich slag microstructure:
Based on above experimental results, the optimum operating parameters for pyrometallurgical boron and iron separation process and further boron-rich slag cooling process are suggested as follows: the B2O3 content in the B2O3–MgO–SiO2 system is no smaller than 22 wt%, a certain content of FeO (<10 wt%) in the slag is favorite, smelting separation temperature is around 1400 °C, inhibition the reduction of B2O3 in the slag, fast cooling the molten slag from initial smelting temperature to 1200 °C and keeping the cooling rate as 10–20 °C/min from 1200 °C to 1100 °C.
4 Conclusions
1) The melting temperature of synthesized boron-rich slag gradually decreases with the decreasing of MgO content when the B2O3 content is fixed. B2O3 will dominate the melting property of the boron-rich slag when its content is higher than 24 wt%. The slag melting temperature first decreases when the FeO increasing from 0 to 10 wt% and then increases when the FeO content further increasing to 20 wt%.
2) The fluidity of the slag increases with the increasing of B2O3 content. The fluidity increases with the decreasing of MgO content when the B2O3 content is in the range of 16 wt%–22 wt%, and then decreases when B2O3 content increasing to 24 wt%. The fluidity property gradually increases with the FeO in the slag increasing from 0 to 20 wt%.
3) The microstructure and phase composition of boron-rich slag change obviously during the slow cooling process. Original olivine exists in the initial molten slag. Kotoite will firstly precipitate in little amount at 1200 °C and suanite will precipitate in large quantity at 1100 °C. Suanite is the main boron-containing crystalline phase in the slow cooled boron-rich slag.
4) The size of boron-containing crystalline phase and olivine increases with the increasing of smelting temperature, however, the EEB value gradually decreases on the contrary. The size and volume ratio of boron-containing crystalline phase and EEB decrease if the B2O3 reduced. The appropriate cooling rate is 10–20 °C/min.
Acknowledgments
The authors would like to express their gratitude for the financial support of the Fundamental Research Funds for the Central Universities (FRF-TP-16-019A1) and National Natural Science Foundation of China (Grant No. 51274033).
References
[1] KEN S, MARCIA K M. Mineral commodity summaries [M]. Reston: US Geological Survey, 2015.
[2] LIU Jia-ning, SONG Yan-li, LU Jue, GUO Wei. Effect laws and mechanisms of different temperatures on isothermal tensile fracture morphologies of high-strength boron steel [J]. Journal of Central South University, 2015, 22(4): 1191–1202.
[3] LIU Ran, XUE Xiang-xin, JIANG Tao, ZHANG Shu-hui, HUANG Da-wei. Comprehensive utilization of ludwigite and its prospect [J]. Multipurpose Utilization of Mineral Resources, 2006, 2: 33–37. (in Chinese)
[4] ZHANG Xian-peng, LANG Jian-feng, CUI Chuan-meng, LIU Su-lan. Comprehensive utilization of low grade ludwigite ore with blast furnace smelting [J]. Iron and Steel, 1995, 30(12): 9–11. (in Chinese)
[5] LIU Su-lan, CUI Chuan-meng, ZHANG Xian-peng. Pyrometallurgical separation of boron from iron in ludwigite ore [J]. ISIJ International, 1998, 38(10): 1077–1079.
[6] CHU Man-sheng, ZHAO Jia-qi, Fu Xiao-jiao, LIU Zheng-gen. New efficient process utilizing ludwigite on gas-based shaft furnace direct reduction and electric furnace smelting separation [J]. Journal of Northeastern University, 2016, 37(6): 805–809. (in Chinese)
[7] WANG Guang, WANG Jing-song, DING Yin-gui, MA Sai, XUE Qing-guo. New separation method of boron and iron from ludwigite based on carbon bearing pellet reduction and melting technology [J]. ISIJ International, 2012, 52(1): 45–51.
[8] WANG Guang, XUE Qing-guo, SHE Xue-feng, WANG Jing-song. Carbothermal reduction of boron-bearing iron concentrate and melting separation of the reduced pellet [J]. ISIJ International, 2015, 55(4): 751–757.
[9] WANG Guang, WANG Jing-song, YU Xin-yun, SHEN Ying-feng, XUE Qing-guo. Innovative method for boron extraction from the iron ore containing boron [J]. International Journal of Minerals, Metallurgy and Materials, 2016, 23(3): 247–256.
[10] XUE Xiang-xin, DONG Meng-ge, YANG He, SONG Li. Purification of the leaching liquid from the boron-rich slags by the alkaline leaching method under ordinary pressure and the preparation of borax [J]. Journal of Northeastern University, 2015, 36(6): 786–789. (in Chinese)
[11] HU Wei, YANG Xiao-jun, FU Han-guang. Research on crystallization of boric acid in leaching solution hydrochloric acid decompose boron rich slag [J]. Industrial Minerals and Processing, 2016(8): 31–36. (in Chinese)
[12] LANG Jian-feng, ZHANG Xian-peng, LIU Su-lan. Activity of B-rich slag in MgO–B2O3–SiO2 system [J]. Journal of Northeast University of Technology, 1993, 14(1): 32–35. (in Chinese)
[13] SUI Zhi-tong, ZHANG Pei-xin, YAMAUCHI C. Precipitation selectivity of boron compounds from slags [J]. Acta Materialia, 1999, 47(4): 1337–1344.
[14] CUI Chuan-meng, XU Xiu-guang, ZHANG Guo-fan, LIU Su-lan, HAN Wei-ru, WANG Kui-han. Determination of physical properties of melting boron-rich slags [J]. Journal of Iron and Steel Research, 1996, 8(2): 54–58. (in Chinese)
[15] WU Sheng-li, DU Jian-xin, MA Hong-bin, TIAN Yun-qing, XU Hai-fa. Fluidity of liquid phase of iron ores during sintering [J]. Journal of University of Science and Technology Beijing, 2005, 27(3): 291–294. (in Chinese)
[16] LI Jing, WANG Xi-dong, ZHANG Zuo-tai. Crystallization behavior of rutile in the synthesized Ti-bearing blast furnace slag using single hot thermocouple technique [J]. ISIJ International, 2011, 51(9): 1396–1402.
[17] WEI Juan, WANG Wan-lin, ZHOU Le-jun, HUANG Dao-yuan, ZHAO Huan, MA Fan-jun. Effect of Na2O and B2O3 on the crystallization behavior of low fluorine mold fluxes for casting medium carbon steels [J]. Metallurgical and Materials Transactions B, 2014, 45(2): 643–652.
[18] KEENE B J, MILLS K C, SUSA M. Slag atlas (2nd Ed) [M]. Düsseldorf: Verlag Stahleisen GmbH, 1995.
[19] REN Shan, ZHANG Jian-liang, WU Liu-shun, LIU Wei-jian, BAI Ya-nan, XING Xiang-dong, SU Bu-xin, KONG De-wen. Influence of B2O3 on viscosity of high Ti-bearing blast furnace slag [J]. ISIJ International, 2012, 52(6): 984–991.
[20] WANG Hong-ming, ZHANG Ting-wang, ZHU Hua, LI Gui-rong, YAN Yong-qi, WANG Jian-hua. Effect of B2O3 on melting temperature, viscosity and desulfurization capacity of CaO-based refining flux [J]. ISIJ International, 2011, 51(5): 702–706.
[21] QI Xin, WEN Guang-hua, TANG Ping. Viscosity and viscosity estimate model of fluoride-free and titanium- bearing mold fluxes [J]. Journal of Iron and Steel Research, International, 2010, 17(6): 6–10.
[22] NAKAMOTO M, LEE J, TANAKA T. A model for estimation of viscosity of molten silicate slag [J]. ISIJ International, 2005, 45(5): 651–656.
[23] LEE Y S, MIN D J, JUNG S M, YI S H. Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO [J]. ISIJ International, 2004, 44(8): 1283–1290.
(Edited by HE Yun-bin)
中文导读
含硼铁精矿熔分富硼渣基础特性研究
摘要:本文针对含硼铁精矿熔分所得富硼渣的基础特性进行了基础研究,具体包括:B2O3–MgO– SiO2–FeO渣系的熔化和流动特性、熔分富硼渣的结晶行为和不同因素对富硼渣浸出率的影响。B2O3含量对富硼渣的熔化和流动特性有显著影响。总体上,FeO可以提高富硼渣的熔化和流动性;缓冷过程中含硼晶相主要在1200 °C–1100 °C这一温度区间析出;过高的熔炼温度和B2O3还原率对富硼渣中硼的提取不利;适宜的富硼渣缓冷速率为10 °C/min–20 °C/min。基于上述试验结果,可以为硼铁精矿中硼–铁火法分离及后续富硼渣缓冷工艺的开发提供优化参数。
关键词:含硼铁精矿;富硼渣;熔化性;流动性;结晶
Foundation item: Project(FRF-TP-16-019A1) supported by the Fundamental Research Funds for the Central Universities, China; Project(51274033) supported by the National Natural Science Foundation of China
Received date: 2016-12-02; Accepted date: 2017-01-23
Corresponding author: WANG Guang, PhD, Research Assistant; Tel: +86–10–82376018; E-mail: wangguang@ustb.edu.cn; ORCID: 0000-0002-0982-3447
Abstract: In the present paper, the fundamental research on the properties of boron-rich slag melting separated from boron-bearing iron concentrate was performed. The melting and fluidity of B2O3–MgO–SiO2–FeO slag system, crystallization of separated boron-rich slag and factors on the extraction efficiency of boron-rich slag were systematically investigated. B2O3 content would heavily affect the melting and fluidity property of boron-rich slag. Generally, FeO could improve the melting and fluidity property of boron-rich slag. Boron-containing crystalline phase mainly precipitated in temperature range from 1200 °C to 1100 °C. Higher smelting temperature and B2O3 reduction ratio were negative for the extraction of boron. The cooling rate of 10–20 °C/min was better for the crystallization of boron-containing crystalline phase. Based on the obtained experimental results, the optimum operating parameters for the development of pyrometallurgical boron and iron separation process and further boron-rich slag cooling process were proposed.


