
Fabrication of TiO2 nanotube arrays on biologic titanium alloy and properties
ZhANG Wen-yan (张文彦), LI Guang-zhong (李广忠), LI Ya-ning(李亚宁),
YU Zheng-tao(于振涛), XI Zheng-ping(奚正平)
Northwest Institute for Non-ferrous Metal Research, Xi’an 710016, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The vertically aligned highly ordered TiO2 nanotube arrays were fabricated by potentiostatic anodization of biologic Ti alloys(TLM) and pure Ti substrates, followed by annealing at 480 and 550 ℃ for 6 h. High-resolution scanning electron microscopy (SEM) was applied to characterize the original films. The phase of the film was characterized by XRD. The interfacial adhesion and bond strength between thin films coating and substrate were tested by scratch method. The results show that the films on the TLM alloy have high adhesion strength compared with them on pure Ti.
Key words:
TiO2; nanotube arrays; titanium alloys; adhesion strength; properties;
1 Introduction
Titanium and titanium alloys, such as Ti6Al4V, or other new kind of biological titanium alloys (TLM, a b-type, with nominal composition of Ti-Zr-Mo-Nb) are widely applied for orthopedic and dental implantation because of their superior mechanical properties, low modulus, excellent corrosion resistance and good biocompatibility[1-3]. However, titanium and its alloys, being bioinert, cannot directly bond with bone. So surface treatments, such as hydroxyapatite coating, formation of anatase phase TiO2 or other physical treatments, have been used to further improve the bioactivity of Ti alloys[4-7]. Studies have demonstrated that bone tissue could form on the titanium surface with a very thin TiO2 oxide layer[2-3]. Recently, TiO2 bioactive layer with nanostructures has been suggested as a potential alternative to HA coatings[8-10].
Titanium oxide nanotubes can be fabricated by hydrothermal reaction or anodization. JIN et al[11] have reported that the arrays of vertically aligned titanium oxide nanotubes on Ti implant surface can be useful for accelerated bone growth. The typical nanotube arrays consist of single tube possessing a uniform morphologywith diameter of 100-150 nm and length up to 0.5- 20 μm, and their physical and chemical properties can be controlled by changing the fabrication conditions. Another advantage of using this kind of TiO2 nanotube arrays as bioactive layer is that it can be fabricated directly on the Ti/ titanium alloys surface by cost-effective techniques.
As we know that the stability and the durability are obviously necessary for all the implant tissues materials,because of the long-term staying in vivo living body. On the view of the applications, the most important attribute of titanium and titanium alloys is good adhesion strength property for bioactive layer on its surface. Thus, it is significant to improve the adhesion strength of these implant materials and characterize the adhesion strength relationship between bioactive layer and implant materials. However, to our knowledge, a systematic investigation of the adhesion strength between self-assembled, vertically aligned nanotubes and Ti/ titanium alloys substrate has not yet been carried out. In this paper, the anodization process for fabrication the TiO2 nanotube arrays on the pure Ti and biological Ti alloy in an organic electrolyte solution were reported, the adhesion strength between the layer of TiO2 nanotubes arrays and pure Ti/Ti alloys substrate was also investigated.
2 Experimental
The vertically aligned TiO2 nanotube arrays were fabricated on the titanium and biological titanium alloys (TLM) sheets using anodization technique. Both the pure titanium (0.50 mm thick, 99.9% purity) and TLM alloy (about 0.6 mm thick, β-type Ti-Nb-Zr-Mo alloy) sheets were produced by Northwest Institute for Non-ferrous Metal Research, Xi’an, China. These sheets were cut into small pieces with dimensions of 2.0 cm×3.0 cm, then chemically etched to remove oxide layer for 5 min in 1.0 mol/L of HNO3 with a few drops of HF (ACS grade, Xi’an Chemicals Co, China), cleaned in distilled water, ethanol and dried with N2 stream. After treated, the Ti and TLM alloy were anodized at 40 V for 8 h with a two electrodes system, respectively. In order to reduce the interfacial stress between layer of TiO2 nanotube arrays and substrates, the electrolyte solution for anodization process was prepared as ethylene glycol containing 0.55% (mass fraction) NH4F, 1.0% H2O. A platinum electrode (thickness of 0.1 mm, area of 6.0 cm2, purity of 99.99%, Xi’an Chemicals Co, China) was used as the cathode. After anodized, samples were rinsed with deionized water and dried with N2 stream. Then the samples were annealed at elevated temperature.
The morphology of the surfaces was characterized using a field emission scanning electron microscope (FESEM) JSM-6700F. The chemical composition of the nanostructures was determined by energy dispersive X-ray spectroscope (EDS) analysis. XRD of D/max- 2550VB/PC type was used to carry out the crystal structure characterization of the as-prepared samples and annealed samples. The adhesion strength between the layer of nanotube arrays and Ti/Ti alloys substrate was evaluated by a WS-2005 Scratch Tester.
3 Results and discussion3.1 Nanotube arrays on substrate
Two kinds of metallic sheets were anodized in an ethylene glycol solution containing 0.55% NH4F and 1.0% H2O for 8 h. Fig.1 shows FESEM images of TiO2 nanostructures on pure titanium surface. The typical dimensions of the nanotubes grown in this study are about 100 nm in outer diameter, 70 nm in inner diameter, 15 nm in wall thickness, and 9 μm in length. The whole surface of TiO2 layer is very plane and the dimensions of nanotubes are uniform. However, as Figs.2(a), (b), (c) and (d) show, the nanostructures on the TLM alloy surface are completely different compared with those on Ti surface. The results indicate that some microscale cracks exist on the oxides surface, though the oxide layer still consists of nanoporous structures. So a very interesting micro- and nanoscale hierachical rough surface is obtained, which might be useful to the cell’s growth. It is found from Fig.2(b) that the inner diameter of the tube is approximately 80 nm and like the diameters, as previously reported, of zirconium oxides nanotube arrays or porous niobium oxides [12–15]. These FESEM images demonstrate that a highly regular self-organized nanotube arrays layer can be fabricated by anodization of the new biological titanium alloy (TLM) in a fluoride-containing organic electrolyte. From the EDS spectrum, as Fig.3 shows, it is concluded that the oxide layer on the TLM alloy consists of titanium, niobium, molybdenum and zirconium oxides.
3.2 Annealing treatment
The oxide layer of nanotube arrays by anodization was proved to be amorphous by the presence of a diffuse diffraction pattern in thin film X-ray diffraction analysis, which carried out at a glancing angle of 2?, as Fig.4(a) shows. As we know, the anatase phase TiO2 is much more efficient for nucleation and growth of osteal cell than the rutile phase TiO2 because of the better bioactive.
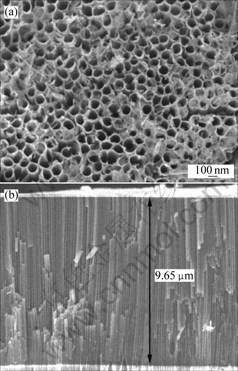
Fig.1 FESEM images of nanotube arrays sample grown at 40 V in ethylene glycol solution containing 0.55% NH4F and 1.0% H2O on titanium for 8 h: (a) Views of top surface; (b) Cross- sectional view
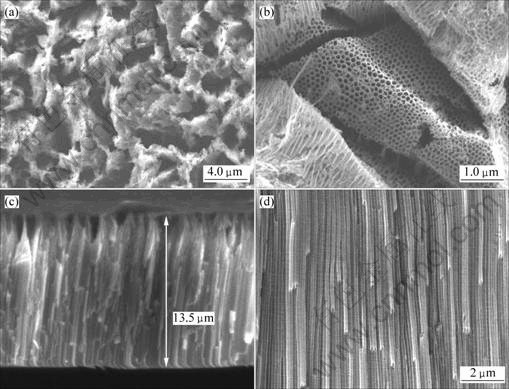
Fig.2 FESEM images of nanotube arrays sample grown at 40 V in ethylene glycol solution containing 0.55% NH4F and 1.0% H2O on titanium for 8 h: (a), (b) Views of top surface; (c), (d) Cross-sectional views
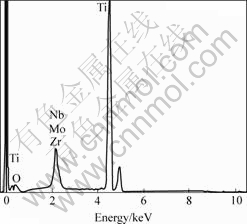
Fig.3 EDS analysis for oxide layer on TLM
And crystal lattice of anatase phase also matches well with hydroxyapatite phase[16]. Therefore, these samples after anodized were annealed, with heating and cooling rates of 1 ℃/min, in order to obtain more bioactive nanostructure TiO2 with anatase phase. Figs.4(b) and (c) show the XRD patterns of oxide layer of nanotube arrays prepared on titanium surface, which were annealed at 480 and 550 ℃ for 6 h, respectively. The patterns indicate that TiO2 layer of nanotube arrays on the substrate are anatase phase and the sample annealed at 550 ℃ is crystallite completely.
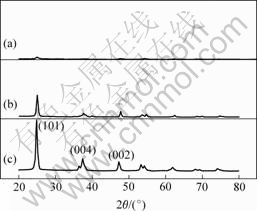
Fig.4 XRD patterns of nanotube arrays sample grown on titanium surface: (a) As-formed without annealing treatment; (b) Annealed at 480 ℃; (c) Annealed at 550 ℃
3.3 Adhesion strength
Adhesion strength is an important target in evaluating the quality of bioactive coating layers on orthopedic and dental implantation materials. The traditional way of adhesion strength test for coating layers is bonding pull-off method and scratching test for the surface. In this case, The Ws-2000 scratch tester, which has an autoloaders system for adding load on the scratch needle, moving scratch needle at synchronization that through the coating surface, was used to valuate the adhesion strength of oxide layers with nanostructures and the Ti & TLM alloy substrates.
The scratching test results are shown in Table 1. The experimental data are the valuations of critical load of oxide layer breaking away from substrate, which were obtained by methods of acoustic emission signals recording and observation of scratching tracks. From the experimental data we can conclude that adhesion strength of crystallized nanotube arrays and substrates are relatively low, the average critical load are just about 7.89 N and 6.45 N for the Ti and TLM alloy. However the adhesion strength of amorphous nanotube arrays and substrates are much higher. And the average critical load measured on the TLM alloy is up to 38.73 N, which is nearly twice as that on titanium.
Table 1 Critical load of oxide layer breaking away from substrate by acoustic emission signals recording and observation of scratching tracks by scratch tester

4 Conclusions
1) Nanotube arrays were fabricated using anodic oxidation method in ethylene glycol solution electrolyte containing 0.55% NH4F and 1.0% H2O on titanium and TLM alloy. After treatment, micro- and nano-scale hierachical rough oxide surface was obtained on the TLM alloy substrate.
2) When these samples after anodized were annealed at 480 and 550 ℃ for 6 h, TiO2 layer of nanotube arrays on the substrates transfer into anatase phase completely. However, the adhesion strength between the TiO2 layer of nanotube arrays and the substrate reduces.
3) The oxide layer with micro- and nano-scale hierachical structures on TLM alloy sheet shows better adhesion strength than those on the pure titanium sheet.
References[1] NISHIGUCHI S, NAKAMURA T, KOBAYASHI M, KIM HM, MIYAJI F, KOKUBO T. The effect of heat treatment on bone-bonding ability of alkali-treated titanium [J]. Biomaterials, 1999, 20: 491-500.
[2] SENNERBY L, THOMSEN P, ERICSON LE. Ultrastructure of the bone-titanium interface in rabbits [J]. Mater Med, 1992, 3: 262-271.
[3] BRANEMARK P I, HANSSON B O, ADELL R, BRENINE U, LINDSTRO M U,HALLEN O, OHMAN A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10 year period. [J] Scand J Plast Reconstruct Surg, 1977, 11: 1-132.
[4] DEGROOT K, GEESINK R, KLEIN CPAT, SEREKIAN P. Plasma sprayed coatings of hydroxyapatite[J].Biomed Mater Res, 1987, 21: 1375-1381.
[5] MCPHERSON R, GANE N, BASTOW T J. Structural characterization of plasma-sprayed hydroxylapatite coatings[J]. J Mater Sci—Mater M, 1995, 6: 327-334.
[6] KURZWEG H, HEIMANN R B, TROCZYNSKI T. Adhesion of thermally sprayed hydroxyapatite-bond-coat systems measured by a novel peel test[J]. J Mater Sci—Mater M, 1998, 9: 9-16.
[7] LIN C M, YEN SK. Characterization and bond strength of electrolytic HA/TiO2 double layers for orthopedic applications[J]. J Mater Sci—Mater M, 2004, 15: 1237-1246.
[8] KIM H M, KANEKO H, KAWASHITA M, KOKUBO T, NAKAMURA T. Mechanism of apatite formation on anodically oxidized titanium metal in simulated body fluid[J]. Key Eng Mater, 2004, 254/256: 741-744.
[9] KIM H M, HIMENO T, KAWASHITA M, LEE J H, KOKUBO T, NAKAMURA T. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid[J]. J Biomed Mater Res, 2003, 67A: 1305-1309.
[10] YANG B, UCHIDA M, KIM H M, ZHANG X, KOKUBO T. Preparation of bioactive titanium metal via anodic oxidation treatment[J]. Biomaterials, 2004, 25: 1003-1010.
[11] SEUNGHAN OH, SUNGHO JIN, Titanium oxide nanotubes with controlled morphology for enhanced bone growth[J]. Mater Sci Eng C, 2006, 26:1301-1306.
[12] SIEBER I, KANNAN B, SCHMUKI P. Self-assembled porous tantalum oxide prepared in H2SO4/HF electrolytes[J]. Electrochemical and Solid-State Letters, 2005, 8: J10-J12.
[13] SIEBER I V, SCHMUKI P. Porous tantalum oxide prepared by electrochemical anodic oxidation[J]. Electrochem Soc, 2005, 152: C639-C644.
[14] SIEBER I, HILDEBRAND H, FRIEDRICH A, SCHMUKI P. Formation of self-organized niobium porous oxide on niobium[J]. Electrochem Commun, 2005, 7: 97-100.
[15] TSUCHIYA H, MACAK J M, GHICOV A, TANG Y C, SCHMUKI P. Self-organized high-aspect-ratio nanoporous zirconium oxides prepared by electrochemical anodization[J]. Electrochimica Acta, 2006, 52: 722-725.
[16] UCHIDA M, KIM H M, KOKUBO T, FUJIBAYASHI S, NAKAMURA T. Structural dependence of apatite formation on titania gels in a simulated body fluid[J]. Biomed Mater Res, 2003, 64: 164.
(Edited by LONG Huai-zhong)
Foundation item: Projects supported by the National West Development Foundation of China
Corresponding author: XI Zheng-ping; Tel: +86-29-86264926; E-mail: amzhwy@c-nin.com


