Trans. Nonferrous Met. Soc. China 25(2015) 824-831
Effects of chitosan coating on biocompatibility of Mg-6%Zn-10%Ca3(PO4)2 implant
Jun ZHAO1, Liang-jian CHEN2,3, Kun YU1,2, Chang CHEN3,
Yi-long DAI1, Xue-yan QIAO1, Yang YAN1, Zhi-ming YU1
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
3. The Third Xiangya Hospital, Central South University, Changsha 410083, China
Received 16 April 2014; accepted 31 October 2014
Abstract:
A Mg-6%Zn-10%Ca3(PO4)2 composite with a chitosan coating was prepared to study its in vivo biodegradation properties. The chitosan dissolved in a 0.2% acetic acid solution was applied on the surface of Mg-6%Zn-10%Ca3(PO4)2 composite specimens and solidified at 60 °C for 30 min to form the coating. The cytotoxicity evaluation of chitosan coated specimens is at level 0, which indicates that such coating is safe for cellular applications. The in vivo tests of chitosan coated composite show that the concentration of metal ions from the composite measured in the venous blood of Zelanian rabbits is less than that from the uncoated composite specimens. The chitosan coating impedes the in vivo degradation of the composite after surgery. The in vivo testing also indicates that the chitosan coated composite is harmless to important visceral organs, including the heart, kidneys and liver of the rabbits. The new bone formation surrounding the chitosan coated composite implant shows that the composite improves the concrescence of the bone tissues. And the chitosan coating is an effective corrosion resistant layer that reduces the hydrogen release of the implant composite, thereby decreasing the subcutaneous gas bubbles formed.
Key words:
biocompatibility; magnesium composite; chitosan; cytotoxicity;
1 Introduction
Magnesium alloys have the potential to serve as biomedical implants because their elastic modulus matches that of cortical bone tissue, allowing them to avoid the stress shielding effect induced by a serious mismatch between the modulus of the natural bone and implants [1,2]. Mg-based implants corrode and degradate with the body fluid in the electrolytic physiological environment [3,4]. Therefore, Mg alloys are candidates for biodegradable implants for repairing bone fractures [5]. Biodegradable Mg alloys can provide good mechanical properties and produce non-toxic corrosion products in physiological systems [6,7]. However, some disadvantages to the biodegradation of Mg alloys are perceived, including a rapid corrosion rate, hydrogen gas emissions and a high environmental pH value of corrosion products [5,8]. Since rapid corrosion is an intrinsic response of magnesium to the human body’s fluid or plasma, controlling or decreasing the corrosion rate of magnesium in body fluid is a significant issue in the development of magnesium implants [9]. Alloying can significantly slow down the corrosion rate of magnesium in body fluids. For example, magnesium alloyed with Al can reduce dissolution and hydrogen evolution rates [10]. But the presence of Al ions in the human body is undesirable for human health. Some other alloying elements in the magnesium matrix such as cadmium, manganese or rare earth metals have also been proposed in order to improve the alloy’s corrosion resistance, but these elements can barely be tolerated because they are poisonous to the human body [11-13]. Therefore, there are a limited numbers of elements that can be employed in magnesium alloys used for biomaterials.
One of the alternatives for adjustment of the corrosion rates of Mg alloys to meet the requirement of bone repair is the application of Mg-based metal matrix composites (MMCs) and their surface coating treatments [14,15]. Previous studies showed that Mg-6%Zn- 10%Ca3(PO4)2 exhibits good synthetic properties as a biomaterial [16]. A suitable addition of hydroxyapatite or tricalcium phosphate can decrease the corrosion rate of the magnesium matrix and increase its biocompatibility. An anodizing or micro-arc oxidizing surface treatment of Mg alloys has been reported to improve their corrosion resistance. Such surface treatments of magnesium alloys have been employed for industrial applications but have seldom been explored for using in a bio-environment because the surface of the implanted magnesium must be non-toxic to the human body [17-19]. The surface treatment of the implanted magnesium must be non-toxic to the human body. Chitosan has been proven to be biocompatible with the human body [20]. Therefore, in the present investigation, a chitosan coating is applied to the surface of l Mg-6%Zn-10%Ca3(PO4)2 composite in order to adjust its biodegradable behavior and to study the effects of the chitosan coating on the in vivo degradation characteristics of the magnesium implant.
2 Experimental
2.1 Material production and measurement
The composite specimens were prepared with Mg, Zn and Ca3(PO4)2 powders and sintered at 620-640 °C for 1 h in a vacuum furnace under argon gas protection. The average particle diameter of the Mg and Zn powders was 23.0 μm, and the average particle diameter of the Ca3(PO4)2 powder was 7.85 μm. The specimens for testing were cut from the sintered composite billets. The specimens for chitosan coating were firstly abraded using sand paper and treated with a 40%H3PO4+H2O solution as a pre-treatment. Chitosan, with a relative molecular mass of 300 k, was mixed in a 0.2% acetic acid solution to form the coating mixture. The coating mixture was then smeared on the surface of the composite specimens and solidified at 60 °C for 30 min. All of the experimental results for in vitro corrosion testing and in vivo measurements were obtained from the chitosan coated composite specimens. The uncoated Mg-6%Zn- 10%Ca3(PO4)2 composite was utilized experimentally as the control specimen. The microstructures of the experimental MMCs were observed with a JEOL JSM-5600Lv scanning electron microscope (SEM).
2.2 Cytocompatibility assessments
The cytotoxicity of the experimental composites was measured via indirect contact testing according to ISO 10993-5:1999. The composite specimens were immersed in a 10% fetal bovine serum in a humidified incubator with 95% relative humidity and 5% CO2 at 37 °C. L-929 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM). The culture medium was replaced by 100% extraction media or by 50% or 10% dilutions. The DMEM acted as a negative control, and a sample of the DMEM medium containing phenol acted as a positive control. 3-(4,5-Dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) was then dissolved in a phosphate-buffered saline (PBS) solution at a concentration of 5 mg/mL. The samples were incubated for 4 h after adding 10 μL of the MTT solution. Subsequently, 100 mL of the formazan solution was added to each sample, and the optical density (OD) was measured using a spectrophotometer. The cell relative growth rate (R) was calculated as follows:
R=(Dtest/Dnegative)×100%
where R is the cell relative growth rate; Dtest and Dnegative are the test and negative optical density, respectively.
2.3 In vivo biodegradation testing
All animal experiments, including anesthetic, surgical and post-operative treatments, were performed by ISO 10993-5:1999 and approved by and fulfilled the requirements of the Ethics Committee of the Xiangya Third Hospital and complied with the animal welfare legislation of the Chinese government. Eight adult male Zelanian rabbits weighing between 2.0 and 2.5 kg were randomly assigned to two groups. One group received uncoated Mg-6%Zn-10%Ca3(PO4)2 composite implants, while the others received the chitosan coated specimens. The rabbits in both groups were measured at the times of 2 weeks, 4 weeks, 8 weeks and 12 weeks. In the animal experiments, a 12 mm × 5 mm × 2 mm sized splint from the two types of experimental composites was fastened to the animal’s pre-broken femoral shaft. A 5 mm×5 mm × 2 mm size composite sample was implanted into the dorsal muscle of the animals. All rabbits were anaesthetized with amyl-barbiturate (30 mg/kg) for the surgery. Venous blood samples from the rabbits were phlebotomized at different times from 1 d to 12 weeks to detect the variations in the concentration of Mg2+, Zn2+, and Ca2+ in the blood with the LT-I Biochemical Analyzer. The animals were euthanized 12 weeks after the surgery. The muscle tissue around the implanted composite and the tissue from the heart, liver and kidneys of the rabbit were also stained with hematoxylin and eosin (HE) for histological analysis and in order to detect whether the degradation of the composites harmed these important visceral organs. Micro-computed tomography devices were used to observe the in vivo degradation process of the composite and the pre-broken bone healing process after the implant fixation.
3 Results
3.1 Morphology and microstructure characterization
The surface morphology of the Mg-6%Zn- 10%Ca3(PO4)2 composite without chitosan is shown in Fig. 1(a), with the polished scratches from the sand paper clearly exhibited. The 40%H3PO4+H2O solution pretreatment before applying the chitosan produces a dispersed porous surface morphology, which is beneficial for the adherence of chitosan to the specimens (Fig. 1(b)). The specimen’s surface with the chitosan is shown in Fig. 1(c). The chitosan coating is integral and dense on the surface of the specimen with a thickness of about 3.5 μm (Fig. 1(d)).
3.2 In vitro test
The biodegradation characteristic of Mg-6%Zn- 10%Ca3(PO4)2 composite is due to the corrosion performance of the magnesium matrix in the body fluid or plasma, which is a chloride-containing solution. In such environment, magnesium will corrode rapidly, resulting in corrosion products, such as gas bubbles, which would influence the healing process after surgery. With the reaction of Mg and H2O in the body fluid, the dissolution of the Mg matrix would lead to Mg2+. Mg2+ can be easily absorbed or consumed by the human body. Even if the amount of dissolved Mg2+ is in excess of the daily intake limit (300-400 mg/d), no side effects of a Mg2+ overdose can be found in the body [21,22]. Therefore, the dissolution of the Mg matrix in the body fluid is acceptable from a physiological point of view.
However, other corrosion products of the Mg implant, such as gas bubbles, would harm the body [23]. Hydrogen gas bubbles, which are one of the reaction products of Mg with H2O in the body fluid, will lead to subcutaneous bubbles and delay the healing of the surgical region. Such phenomenon is observed with the in vivo experiment with the rabbits. Since such hydrogen gas bubbles are inevitable, the effective method to mitigate such negative effects is to reduce the release of the gas bubbles.
As is well known, the low corrosion rate of Mg implant means low rates of hydrogen release. This can allow the body to gradually absorb or consume such corrosion by-products and avoid the formation of subcutaneous bubbles. One of the possible solutions to such a problem is adding alloying elements into the Mg matrix. Both zinc and Ca3(PO4)2 are effective additions in the Mg matrix to improve the corrosion resistance and have been discussed in the literature [16]. However, such additions are not sufficient to impede the corrosion process. Therefore, another approach to improve the corrosion resistance of Mg is surface treatment or coating. SONG et al [5] provided a possible method of anodizing on the surface of Mg alloys to slow down the corrosion rate of Mg alloy. In this work, the chitosan coating is selected as another surface treatment method to prevent the corrosion of the Mg matrix because the chitosan has good biocompatibility with bone. The comparison of the hydrogen gas release of Mg-6%Zn-10%Ca3(PO4)2 composite specimens in the SBF with or without chitosan coating is shown in Fig. 2. The results show that the hydrogen gas release volume increases quickly during the first 6 d in the immersion test for the uncoated Mg-6%Zn-10%Ca3(PO4)2 composite specimen. The chitosan coating is an effective corrosion resistant layer to reduce the corrosion rate of the composite during the beginning period. During the first 6 d, hydrogen release of the coated specimen is slowed down significantly compared to the uncoated specimen. This is helpful for the body to adjust to the composite implant and decreases the likelihood of forming subcutaneous gas bubbles.
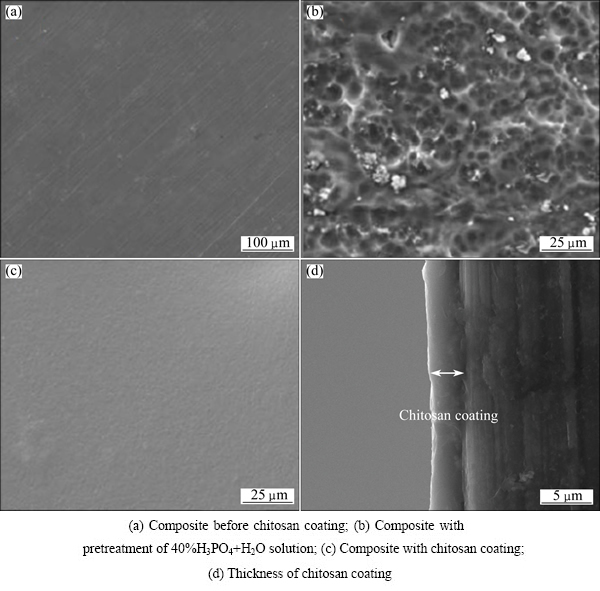
Fig. 1 Surface morphologies of Mg-6%Zn-10%Ca3(PO4)2 composite
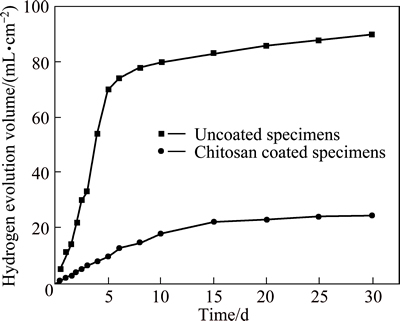
Fig. 2 Hydrogen evolution of chitosan coated and uncoated specimens
Chitosan has the property of rapidly clotting blood and has recently gained approval in the United States and Europe for use in hemostatic agents [23]. The research found that chitosan with an average relative molecular mass of 700000 can stimulate bone formation in an animal model. It is also found that a chitosan scaffold leads to significant bone formation. Therefore, using chitosan to coat the surface of a Mg-6%Zn-10%Ca3(PO4)2 composite can both improve its biocompatibility and impede the corrosion process.
3.3 Cytocompatibility test
The MTT assay is always used to determine the cytotoxicity of metal, polymer or composite materials toward mammalian cells [13]. In this work, L-929 cells are cultured in different immersion extracts with concentrations of 100%, 50% and 10%. The L929 cells can adhere, survive and proliferate in all three of these extracts in a cell culture system. The relative growth rates (RGRs) of the L-929 cells are shown in Fig. 3. The cells in different extracts of the coated specimens are normal and healthy and are similar to the results of the negative control. According to the ISO 10993-5:1999 standard [24], which focuses on cytocompatibility and quantifies the toxicity level of implants, the RGRs of the cells in the extracts of the coated specimens all exceed 100%, with values of 128.3, 109.6 and 105.9, which means that the cytotoxicity of these extracts is no toxicity (level 0). Similarly, the RGRs of the cells in the extracts of the uncoated specimens display no toxicity, with RGR values of 112.8, 96.9 and 90.9. But the extracts with 50% and 100% concentrations of composites reach the level of 1 (RGR range from 75 to 100). Obviously, the chitosan coating improves the cytocompatibility of the Mg-6%Zn-10%Ca3(PO4)2 composite.
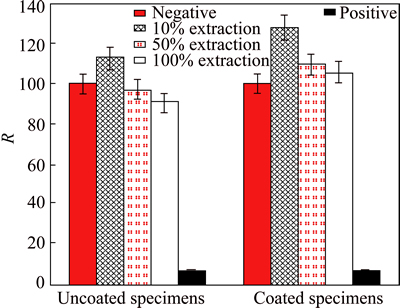
Fig. 3 Relative growth rates of L-929 cells cultured in different extraction of uncoated and chitosan coated composites (P>0.05)
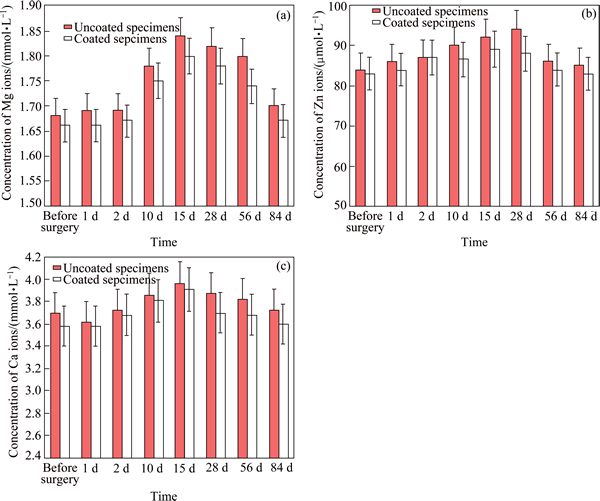
Fig. 4 Concentrations of magnesium ions (a), zinc ions (b) and calcium ions (c) from uncoated and chitosan coated specimens in venous blood of Zelanian rabbits
3.4 In vivo test
The concentrations of different metal ions of the uncoated and chitosan coated Mg-6%Zn-10%Ca3(PO4)2 composites in the venous blood of Zelanian rabbits are measured and the results are compared in Fig. 4. It has been found that the concentrations of Mg2+, Zn2+ and Ca2+ ions change during the experimental time. They reach the maximum values between 15 d and 4 weeks, then decrease to preoperative levels after 12 weeks. Although the released metal ions of uncoated and chitosan coated specimens are both in a normal and acceptable range, the concentration of three metal ions of the chitosan coated Mg-6%Zn-10%Ca3(PO4)2 composite are less than that of the chitosan uncoated composite specimens during the whole experimental period. This result indicates that the chitosan coating impedes the in vivo degradation of the composite after the surgery.
Figure 5 shows the different tissues responses to the chitosan coating composite after 12 weeks of implantation. The heart, liver, kidney and muscle tissues are normal, indicating that the chitosan coating composite is harmless to these important visceral organs.
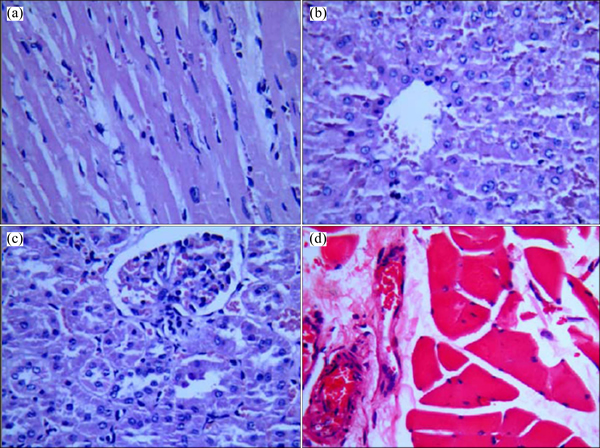
Fig. 5 Hematoxylin and eosin (HE) stained slices of heart (a), liver (b), kidney (c) and muscle tissues (d)
The implanted composite with the chitosan coating is shown in Fig. 6(a), the micro-CT graph of the bone fracture and the implanted composite after surgery 2 weeks is shown in Fig. 6(b). The chitosan coating stimulates the new bone tissues, which are obviously depositing and surrounding the implant. The edges of the composite implant still remain distinct, which means that the degradation of the composite is impeded by the chitosan coating. After 12 weeks, the pre-fabricated fracture on the bone has healed and new bone tissues around the implant can be observed. The new bone formation surrounding the implant shows that the composite improves the concrescence of the bone tissues. The composite implant visibly shows degradation, and the residual implant becomes smaller (Fig. 6(c)). Throughout the entire degradation process, no obvious inflammation response is observed, despite the appearance of subcutaneous bubbles, which are the product of the degradation of the composite with the body fluid.
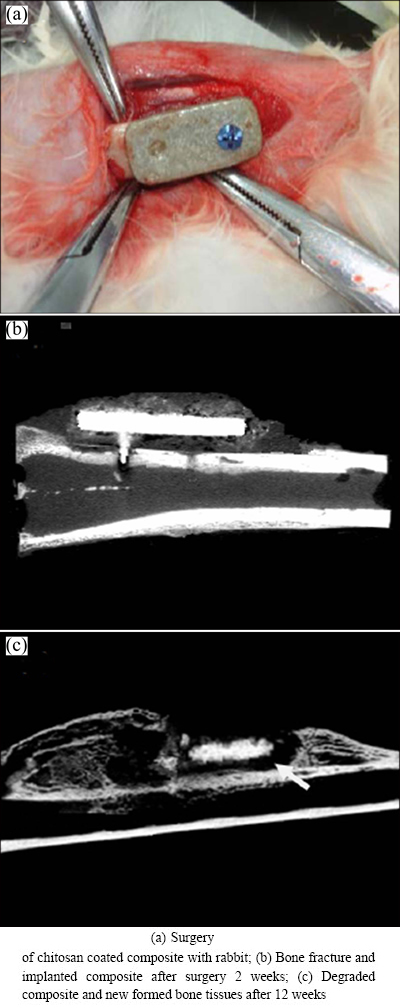
Fig. 6 Implanted composite with chitosan coating
In this in vivo experiment of Zelanian rabbit broken bones, the coated chitosan stimulates bone formation during the beginning recovery period after surgery. The bone tissue slice after 4 weeks shows the capillary vessel formed in the fiber connective tissue around the implanted composite (Fig. 7(a)). Some of the bone tissues formed are obvious after 8 weeks. Compared to the bone tissues with the uncoated chitosan composites [16], the chitosan coated composites stimulate the formation of bone tissues. Therefore, a suitable surface treatment with chitosan for the Mg-6%Zn- 10%Ca3(PO4)2 composite is beneficial for improved biocompatibility. Surface treatment is a shortcut to controlling the corrosion rate of the implanted Mg alloy or composite by slowing down the hydrogen release. It is therefore a most promising approach that will lead to the practical application of Mg composite implants.
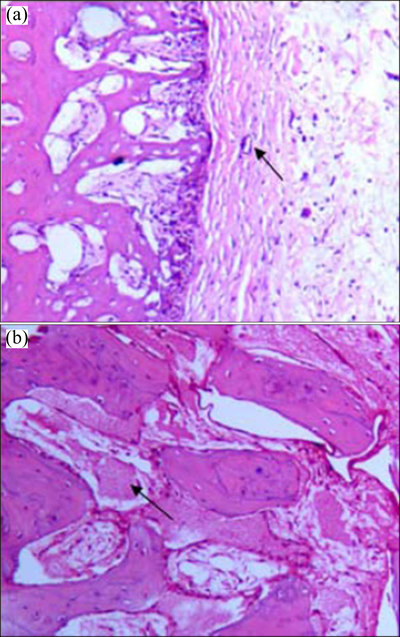
Fig. 7 Bone tissue slices after 4 weeks (a) and 8 weeks (b) around implanted composite
4 Conclusions
1) Chitosan in a 0.2% acetic acid solution can be smeared on the surface of the Mg-6%Zn-10%Ca3(PO4)2 composite and solidified at 60 °C for 30 min. Such chitosan coating is integral and dense on the surface of the composite specimen.
2) A total grade of 0 for the chitosan coated specimens with an cytotoxicity towards L-929 cells compared with a parameter grade of 1 for the uncoated specimen indicates that such coating is safe for cellular applications.
3) The concentration of the metal ions of chitosan coated Mg-6%Zn-10%Ca3(PO4)2 composite is less than that of the uncoated composite specimens in the venous blood of Zelanian rabbits during the experimental period of 12 weeks. This indicates that the chitosan coating is beneficial for slowing down the in vivo degradation of the composite after surgery. In vivo testing also indicates that the chitosan coated composite is harmless to the important visceral organs, including the heart, kidneys and liver of the rabbits.
4) The new bone formation surrounding the chitosan coated Mg-6%Zn-10%Ca3(PO4)2 composite implant shows that the composite improves the concrescence of the bone tissues. Importantly, the chitosan coating is an effective corrosion resistant layer that reduces the hydrogen released by the Mg matrix corrosion. This is helpful for reducing the formation of subcutaneous gas bubbles.
References
[1] BASU B, KATTI D, KUMAR A. Advanced biomaterials fundamentals, processing and applications [M]. New Jersey, USA: John Wiley & Sons Inc, 2009: 12-45.
[2] NIINOMI M. Recent metallic materials for biomedical applications [J]. Met Mater Trans A, 2002, 33: 477-486.
[3] HEUBLEIN B, ROHDE R, KAESE V, NIEMEYER M, HARTUNG W, HAVERICH A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? [J] Heart, 2003, 89: 651-656.
[4] WITTE F, KAESE V, HAFERKAMP H, SWITZER E, MEYER LINDENBERG A, WIRTH C J. In vivo corrosion of four magnesium alloys and the associated bone response [J]. Biomaterials, 2005, 26: 3557-3563.
[5] SONG G L, SONG S Z. A possible biodegradable magnesium implant material [J]. Adv Eng Mater, 2007, 9: 298-302.
[6] MULLER W D, NASCIMENTO M L, de MELE M F L. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications [J]. Acta Biomaterialia, 2010, 6: 1749-1755.
[7] STAIGER M P, PIETAK A M, HUADMAI J, DIAS G. Magnesium and its alloys as orthopedic biomaterials: A review [J]. Biomaterials, 2006, 27: 1728-1734.
[8] KIRKLAND N T, BIRBILIS N, STAIGER M P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations [J]. Acta Biomaterialia, 2012, 8: 925-936.
[9] WITTEA F, FISCHER J, NELLESEN J, CROSTACK H A, KAESE V, PISCH A K. In vitro and in vivo corrosion measurements of magnesium alloys [J]. Biomaterials, 2006, 27: 1013-1018.
[10] ALVAREZ-LOPEZ M, PEREDA M D, del VALLE J A, FERNANDEZ-LORENZO M, GARCIA-ALONSO M C, RUANO O A. Corrosion behaviour of AZ31 magnesium alloy with different grain sizes in simulated biological fluids [J]. Acta Biomaterialia, 2010, 6: 1763-1771.
[11] BOURG H, LISLE A. Biomaterials developments and applications [M]. New York: Nova Science Publishers Inc, 2010: 224-284.
[12] GUELCHER S A, HOLLINGER J O. An introduction to biomaterials [M]. Boca Raton, FL, USA: Taylor & Francis Group CRC Press, 2006: 11-74.
[13] CIAPETTI G, CENNI E, PRATELLI L, PIZZOFERRATO A. In vitro evaluation of cell/biomaterial interaction by MTT assay [J]. Biomaterials, 1993, 14: 359-364.
[14] WITTE F, FEYERABEND F, MAIER P, FISCHER J, STORMER M, BLAWERT C. Biodegradable magnesium-hydroxyapatite metal matrix composites [J]. Biomaterials, 2007, 28: 2163-2174.
[15] XU L P, PAN F, YU G N, YANG L, ZHANG E L, YANG K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy [J]. Biomaterials, 2009, 30: 1512-1523.
[16] YU K, CHEN L J, ZHAO J, LI S J, DAI Y L, HUANG Q, YU Z M. In vitro corrosion behavior and in vivo biodegradation of biomedical b-Ca3(PO4)2/Mg-Zn composites [J]. Acta Biomaterialia, 2012, 8: 2845-2855.
[17] HWANG I J, HWANG D Y, KO Y G, SHIN D H. Correlation between current frequency and electrochemical properties of Mg alloy coated by micro arc oxidation [J]. Surface and Coatings Technology, 2012, 206: 3360-3365.
[18] ZHANG Y, BAI K F, FU Z Y, ZHANG C L, ZHOU H, WANG L G. Composite coating prepared by micro-arc oxidation followed by sol–gel process and in vitro degradation properties [J]. Applied Surface Science, 2012, 258: 2939-2943.
[19] ALLEN B, CHEN Z J. Effect of electrolyte additives on anti-corrosion ability of micro-arc oxide coatings formed on magnesium alloy AZ91D [J]. Surface and Coatings Technology, 2009, 203: 1956-1963.
[20] KIM S W, GABER M W, ZAWASKI J A, ZHANG F, RICHARDSON M, ZHANG X A. Inhibition of glioma growth in vitro and in vivo by a chitosan/ellagic acid composite biomaterial [J]. Biomaterials, 2009, 27: 4743-4751.
[21] ZENG R, DIETZEL W, WITTE F, HORT N, BLAWERT C. Progress and challenge for magnesium alloys as biomaterials [J]. Advanced Engineering Materials, 2008, 10: B3-B14.
[22] SONG G L. Control of biodegradation of biocompatible magnesium alloys [J]. Corrosion Science, 2007, 49: 1696-1701.
[23] DASH M, CHIELLINI F, OTTENBRITE R M, CHIELLINI E. Chitosan—A versatile semi-synthetic polymer in biomedical applications [J]. Progress in Polymer Science, 2011, 36: 981-1014.
[24] DAVIS J R. Handbook of materials for medical devices [M]. OH: ASM International, 2006: 136-181.
壳聚糖涂层对Mg-6%Zn-10%Ca3(PO4)2植入材料生物相容性的影响
赵 俊1,陈良建2,3,余 琨1,2,陈 畅3,戴翌龙1,乔雪岩1,颜 阳1,余志明1
1. 中南大学 材料科学与工程学院,长沙410083;
2. 中南大学 粉末冶金国家重点实验室,长沙 410083;
3. 中南大学 湘雅三医院,长沙 410013
摘 要:研究壳聚糖包覆的Mg-6%Zn-10%Ca3(PO4)2植入材料生物体内的降解特性。采用0.2%的醋酸溶液溶解壳聚糖,将其在60 °C下涂覆在复合材料样品表面,固化30 min得到壳聚糖的涂层。壳聚糖涂覆后的样品细胞毒性为0级,说明该涂层完全没有毒性,可以用于生物实验。生物体内实验表明:与无涂层的样品相比,植入涂覆有壳聚糖的复合材料后,在新西兰兔静脉血中的离子浓度较低。壳聚糖涂层可以减缓复合材料在生物体内的降解速率。生物体内实验表明,涂覆壳聚糖的复合材料对新西兰兔的重要器官,如心、肾、肝、肌肉都没有损伤,而新生骨组织会聚集在壳聚糖涂覆的复合材料周围,有利于骨组织的生长。壳聚糖涂层有利于减缓植入材料在生物体中的降解过程,减少氢气析出造成的皮下气泡产生,有利于提高复合材料的生物相容性。
关键词:生物相容性;镁基复合材料;壳聚糖;细胞毒性
(Edited by Yun-bin HE)
Foundation item: Project (2014) supported by the Open Fund of the State Key Laboratory of Powder Metallurgy, China
Corresponding author: Kun YU; Tel: +86-731-88879341; E-mail: yukun2010@csu.edu.cn
DOI: 10.1016/S1003-6326(15)63669-X
Abstract: A Mg-6%Zn-10%Ca3(PO4)2 composite with a chitosan coating was prepared to study its in vivo biodegradation properties. The chitosan dissolved in a 0.2% acetic acid solution was applied on the surface of Mg-6%Zn-10%Ca3(PO4)2 composite specimens and solidified at 60 °C for 30 min to form the coating. The cytotoxicity evaluation of chitosan coated specimens is at level 0, which indicates that such coating is safe for cellular applications. The in vivo tests of chitosan coated composite show that the concentration of metal ions from the composite measured in the venous blood of Zelanian rabbits is less than that from the uncoated composite specimens. The chitosan coating impedes the in vivo degradation of the composite after surgery. The in vivo testing also indicates that the chitosan coated composite is harmless to important visceral organs, including the heart, kidneys and liver of the rabbits. The new bone formation surrounding the chitosan coated composite implant shows that the composite improves the concrescence of the bone tissues. And the chitosan coating is an effective corrosion resistant layer that reduces the hydrogen release of the implant composite, thereby decreasing the subcutaneous gas bubbles formed.


