Trans. Nonferrous Met. Soc. China 30(2020) 509-517
Preparation of spherical cobalt carbonate from cobalt sulfate solution
Zhi-hong PENG, Fang HE, Hao JIA, Xiao-bin LI, Qiu-sheng ZHOU, Tian-gui QI, Gui-hua LIU
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 27 February 2019; accepted 26 December 2019
Abstract:
A precursor of cobaltous dihydroxycarbonate was firstly prepared by precipitation reaction of cobalt sulfate solution and ammonium carbonate solution, and then a hydrothermal process for the precursor was conducted to obtain the spherical cobalt carbonate with low sulfur content. The experimental results show that the feeding method, final pH value of the precipitation reaction slurry and the concentration of the cobalt sulfate solution have obvious effects on the sulfur content, morphology and particle size distribution of the precursor. The sulfur content of the precursor is 0.0115 wt.% under the optimized conditions. The hydrothermal treatment with temperatures of 125-150 °C can transform the precursor of cobaltous dihydroxycarbonate into spherical cobalt carbonate and decrease the sulfur content to 0.0030 wt.% in the obtained product.
Key words:
cobalt sulfate solution; cobaltous dihydroxycarbonate; ammonium carbonate; spherical cobalt carbonate; hydrothermal treatment;
1 Introduction
Cobalt carbonate is an important raw material for producing battery-grade cobalt oxides [1,2]. The impurity and morphology of cobalt carbonate make a great impact on the electrochemical performance of the obtained cobalt oxides [3,4]. So, the production of high quality cobalt carbonate with low impurity and proper morphology is the inevitable choice to meet the market requirements. To prepare the cobalt carbonate with low impurity, aqueous cobalt chloride or nitrate solution is usually used as cobalt source to react with ammonium carbonate or bicarbonate solution [5-10]. In this process, the wastewater of ammonium chloride or nitrate solution is inevitably generated and the evaporation crystallization process is required to dispose the unrecyclable ammonium-containing wastewater, resulting in high energy consumption, high cost and serious equipment corrosion [11]. Additionally, vast volume high-salinity wastewater generated from the cobalt chloride or cobalt nitrate preparation process is another hard issue for the commercial cobalt carbonate production process, because a solvent extraction process should be employed to covert the cobalt sulfate solution obtained by sulfuric acid leaching of cobalt concentrate or cobalt-bearing materials to cobalt chloride or cobalt nitrate [12]. If cobalt carbonate can precipitate from cobalt sulfate solution directly, the discharge of high-salinity wastewater may be avoided. Furthermore, the simultaneously generated ammonium sulfate in the spent liquor can react with calcium carbonate at elevated temperatures and generate NH3, CO2 and CaSO4, and thus the ammonium carbonate consumed in the cobalt carbonate precipitation process may be regenerated by absorbing NH3 and CO2 and recycles in the precipitation process [13]. However, when directly preparing cobalt carbonate from cobalt sulfate solution, the sulfur species are readily adsorbed or incorporated into cobalt carbonate precipitates, which is difficult to remove and causes the sulfur content exceeding in the product [14]. So, it is extremely urgent to find an efficient method to control sulfur content in cobalt carbonate product.
Besides its impurity, the morphology of cobalt carbonate has also attracted much attention from the researchers due to its importance in the lithium-ion battery industry to manufacture high-quality product. SANKAR et al [15] adopted a simple surfactant-free method to prepare Co2(CO3)(OH)2 nanoflakes and polyhedron flowers. XING et al [16] used urea as a precipitant to prepare loose spherical basic cobalt carbonate from cobaltous chloride hexahydrate and sodium dodecyl carbonate(SDS) solutions. XIE et al [17] have successfully prepared basic cobalt carbonate with different shapes such as flower-like, fun-shaped (sheaf-like) or cubic products by using additives. NASSAR and AHMED [3] obtained squared-shape nano particles of cobaltosic oxide, with an average size from 30 to 39 nm by calcining the cobalt carbonate precursor. Considering the fact that spherical cobalt carbonate is easy to form porous and high tap density Co3O4 [1], more researches are focused on the preparation of spherical cobalt carbonate [18]. Hence, how to effectively regulate the morphology of cobalt carbonate is also an important issue when using the cobalt sulfate solution as cobalt source.
In this study, a novel two-stage process for directly preparing spherical cobalt carbonate with low sulfur from cobalt sulfate solution was proposed. In the first stage, the cobaltous dihydroxycarbonate with small amount of sulfur content precipitated from cobalt sulfate solution by using ammonium carbonate as the precipitant. In the second stage, a hydrothermal process was employed to convert the obtained cobaltous dihydroxycarbonate into spherical cobalt carbonate and the sulfur in the product was removed simultaneously. The factors influencing the sulfur content and morphology of cobalt carbonate, such as feeding method of raw solutions, pH value, and solution concentration, were systemically studied, and also the optimized processing conditions for preparing spherical cobalt carbonate with low sulfur content were determined. This work is beneficial to developing a cleaner and sustainable technology of directly manufacturing cobalt carbonate from cobalt sulfate solution.
2 Experimental
2.1 Materials
The cobalt sulfate solution used in the experiment was prepared from industrial grade cobalt sulfate heptahydrate (Jiangxi Tungsten and Cobalt Industry Company, China). The X-ray diffraction (XRD) pattern of cobalt sulfate heptahydrate is shown in Fig. 1, indicating that there is no detectable impurity phase in the raw material. The distilled water was used through this work. All the other reagents were of analytical grade.
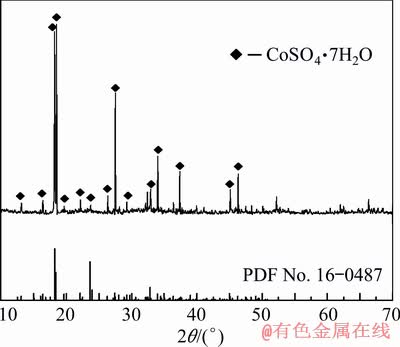
Fig. 1 XRD pattern of solid cobalt sulfate heptahydrate
2.2 Experimental procedure
In the first stage, the precursor of cobaltous dihydroxycarbonate was prepared by the reaction of cobalt sulfate solution and ammonium carbonate solution. Cobalt sulfate and ammonium carbonate solutions with certain concentration were prepared by dissolving corresponding reagents into distilled water, respectively. The cobalt sulfate solution and ammonium carbonate solution were mixed with the feeding methods to adjust the pH value of the mixture solution and the cobaltous dihydroxy- carbonate precipitated. A beaker fixed in water bath was used as reactor, and the pH value of the solution was measured by a pH meter. The obtained slurry was separated by vacuum filtration and the filter cake was washed twice by hot distilled water to obtain the precursor of cobaltous dihydroxycarbonate.
The feeding methods used in this work when mixing cobalt sulfate and ammonium carbonate solutions can be described as follows.
Positive adding method: The ammonium carbonate solution is dropped into the cobalt sulfate solution. In this process, the precipitate of cobaltous dihydroxycarbonate is formed in a weakly acidic solution, and the pH value of the bulk solution will shift from the cobalt sulfate solution of about 5.4 to the final controlled value of 7.0-7.3.
Reverse adding method: Cobalt sulfate solution is dropped into the ammonium carbonate solution. In this process, the precursor is formed in a week alkaline solution, and the pH value of the solution will change from the ammonium carbonate solution with the pH value of about 8.6 to the final controlled pH value of 7.0-7.3.
Parallel adding method: Cobalt sulfate solution and ammonium carbonate solution are simultaneously added into the reaction vessel, and the pH value of the mixture solution was maintained at a constant value by controlling the dropping rate of the solutions. So, the precursor will precipitate from the slurry with a fixed pH value.
In the second stage, the hydrothermal transformation reactions were carried out at 150 °C by mixing the cobaltous dihydroxycarbonate precursor and distilled water with liquor-to-solid ratio of 15:1. The obtained slurry was filtered, and the filter cake was washed by distilled water. The cobalt carbonate product was finally obtained by drying the filter cake at 100 °C.
2.3 Analysis methods
The particle size distribution of the samples was analyzed by laser particle size analyzer (MPT-2, Malvern Instruments, UK). Surface microscopic morphology was observed by SEM (JSM-6360LV, JEOL, Japan). The mineral phases were identified by XRD (TTR-III, Rigaku Corporation, Japan) with Cu Kα monochromatic X-ray at a scan rate of 10 (°)/min. The sulfur content of the product was detected by inductively coupled plasma spectrometer (Intrepid II XSP, Thermo Electron, America).
3 Results and discussion
3.1 Precipitation of cobaltous dihydroxycarbonate
3.1.1 Effect of feeding method
The feeding methods make the acid-base conditions for cobaltous dihydroxycarbonate precipitation different, and thus significantly affect the nucleation and the growth process of the solid particles [19,20]. In order to control the precipitation process and reduce the sulfur content in the precipitate product, it is necessary to select an appropriate feeding method. Consequently, three feeding methods were used to verify their effect on the sulfur content of the synthesized cobaltous dihydroxycarbonate precursor.
The influences of feeding method on sulfur content in synthetic products are shown in Table 1, with controlling final pH values of 7.1 and 7.3, respectively. The results show that the sulfur content in the precursor prepared with the reverse adding method is obviously lower than that prepared with the positive adding method, and the sulfur content in the precursors obtained with the parallel adding method is between that of the other two adding methods.
Table 1 Effect of feeding method on sulfur content in synthetic products (60 °C, washing with boiling water)
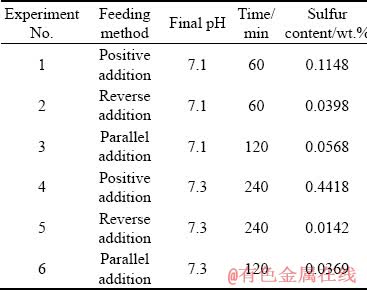
The high sulfate ion concentration in the precipitation reaction process may be contributed to the high sulfur content in the precursors synthesized with the positive adding method. The reverse adding method is opposite to the positive adding method, there exists less sulfate ion in the reaction system when the precursor begins to precipitate. Moreover, the parallel adding method has the best solid-liquid separation effect, and the system is relatively stable because it keeps the pH value of the solution constant all the time. To obtain low sulfur content precursor of cobaltous dihydroxy- carbonate precipitation, the reverse adding method and parallel adding method were selected in the subsequent experiments.
3.1.2 Effect of final pH value
The results in Table 1 indicate that the final pH value may affect the precipitation process and the sulfur content in the obtained cobaltous dihydroxycarbonate. So, the sulfur content in the cobaltous dihydroxycarbonate precipitated with different pH values was investigated. The results are shown in Fig. 2.
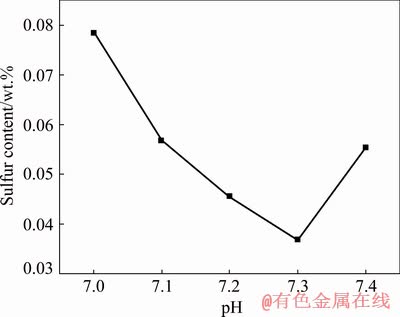
Fig. 2 Effect of final pH value on sulfur content of cobaltous dihydroxycarbonate precursor (60 °C, 120 min, parallel adding method, cobalt sulfate concentration of 84 g/L, ammonium carbonate concentration of 100 g/L)
The results in Fig. 2 demonstrate that the final pH value has an obvious influence on the sulfur content in the cobaltous dihydroxycarbonate precipitation process. When the final pH value varies from 7.0 to 7.4, the sulfur content in the obtained cobaltous dihydroxycarbonate precursor firstly shows a downward trend and reaches a minimum value of 0.0369 wt.% at pH value of 7.3, and then the sulfur content of the precursor rebounds with continuously increasing the pH value. The probable reason for the sulfur content increasing with the pH in high pH range is the formation of colloid cobalt hydroxide. The colloid cobalt hydroxide may be formed easily at a higher pH value of the system, which has high adsorption capacity of the impurity ion in the solution [21]. Therefore, the pH value of the system should be controlled reasonably, the optimum system pH value was determined to be 7.3.
3.1.3 Effect of cobalt sulfate concentration
According to the homogeneous nucleation theory and reaction kinetics [22], the reaction material concentration has a great influence on the formation and growth rate of precipitation grains. To verify the effect of the initial concentration of raw reagent on the sulfur content in cobaltous dihydroxycarbonate precursor, cobalt sulfate solutions with various concentrations were added into the ammonium carbonate solution at a constant rate, and the sulfur content in the obtained cobaltous dihydroxycarbonate precursor was detected. The effect of cobalt sulfate concentration on the sulfur content in the precursor is shown in Fig. 3.
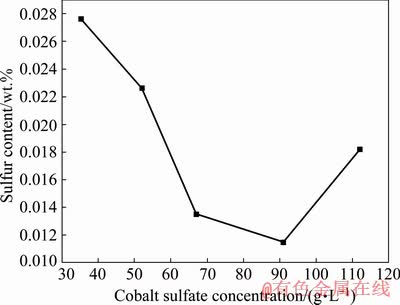
Fig. 3 Effect of cobalt sulfate concentration on sulfur content of cobaltous dihydroxycarbonate precursor (60 °C, 60 min, pH 7.3, reverse adding method, ammonium carbonate concentration of 100 g/L, feeding rate of 3.50 mL/min)
It can be seen from Fig. 3 that the content of impurity sulfur in the obtained cobaltous dihydroxycarbonate precursor gradually decreases with the increase of cobalt sulfate concentration when the cobalt sulfate concentration is in the range of 37-91 g/L, and the sulfur content shows an upward trend with the increase of cobalt sulfate concentration higher than 91 g/L. The sulfur content can be as low as 0.0115% with the cobalt sulfate concentration of ~90 g/L.
The reason is that when the concentration of cobalt sulfate solution is too low, the particle size is large because the crystal grows mainly, and the probability of the impurity sulfur entering into the cobaltous dihydroxycarbonate increases. When the concentration of the cobalt sulfate solution is too high, the high-density particles grow up due to the collision of Browning motion, which leads to the aggravation of agglomeration phenomenon [23], thus increasing the sulfur content of the cobaltous dihydroxycarbonate precursor. The results indicate that both the high and low concentrations of cobalt sulfate concentration are not conductive to synthesize the precursor with low sulfur content. Consequently, in the subsequent experiments, 90 g/L of cobalt sulfate and 100 g/L of ammonium carbonate solutions were selected as raw materials to obtain high-quality products with low sulfur content.
3.1.4 Effect of feeding rate
Besides the impurity sulfur content, the morphology and particle size distribution of cobalt oxides should meet the limit value for battery-grade materials, while the morphology and particle size of the final products of cobalt oxides to a large extent depend on those of the precursors of cobalt carbonate and cobaltous dihydroxycarbonate. According to the principle of crystallization kinetics, the precipitation reaction rate and the supersaturation of the solution have an obvious influence on the nucleation and agglomeration of the crystallization and thus affect the morphology and particle size of the products [24]. RAHIMI- NASRABADI et al [4] showed that flow rate of Co2+ addition to carbonate ion solution has a significant effect on particle size of produced cobalt carbonate nanoparticles. When the cobalt sulfate solution is dropped into ammonium carbonate solution, the reaction rate of the formation of cobaltous dihydroxycarbonate is determined by the dropping speed of cobalt sulfate solution. Therefore, the experiments with various dropping speeds by reverse adding method were carried out and the morphology and particle size of the obtained product were analyzed by scanning electron microscope and particle size analysis, as shown in Fig. 4 and Fig. 5, respectively.
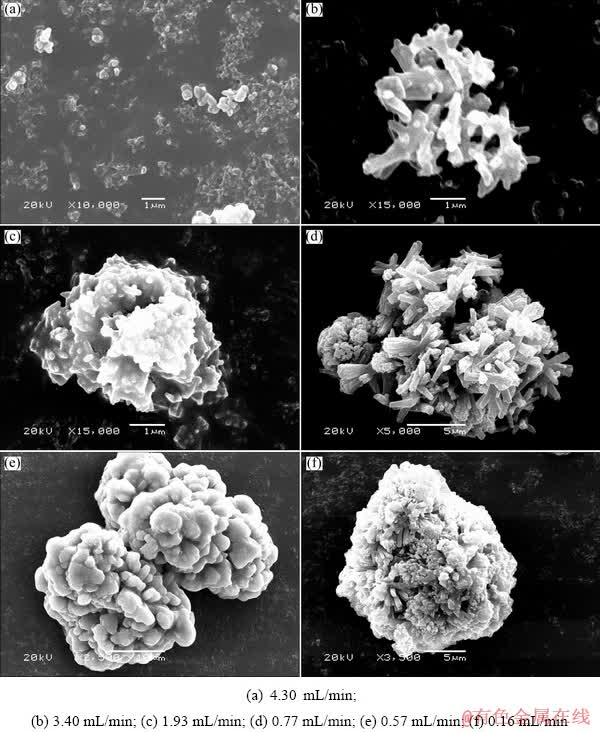
Fig. 4 Morphologies of cobaltous dihydroxycarbonate powders obtained at different feeding rates (60 °C, 60 min, pH 7.3, ammonium carbonate concentration of 100 g/L, cobalt sulfate concentration of 90 g/L)
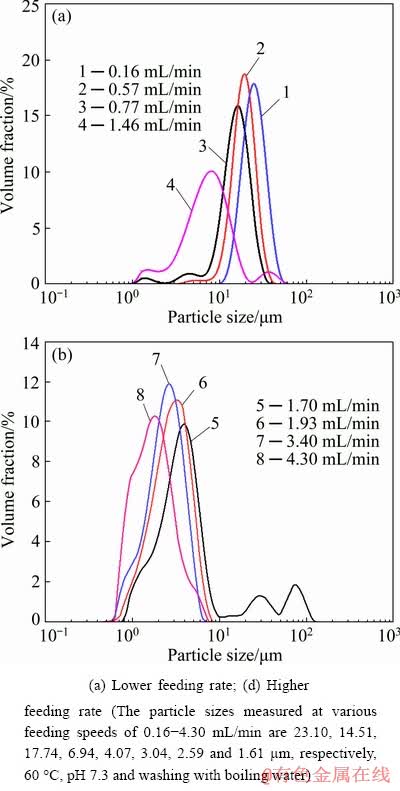
Fig. 5 Effect of feeding rate on particle size of cobaltous dihydroxycarbonate
The results in Fig. 4 and Fig. 5 show that a monodispersed micro-powder with average particle size of about 1.61 μm is obtained with a high cobalt sulfate solution feeding speed of 4.30 mL/min. Reducing the feeding rate of cobalt sulfate solution, the agglomerative precipitate particles will be formed and the apparent particle size of the precipitates increases slowly. And the morphology of the agglomerative particles changes with the cobalt sulfate solution feeding speed. A loose spherical agglomerative particle was formed by the agglomeration of rod-like nucleation when the cobalt sulfate solution feeding speed was controlled at 0.77-3.40 mL/min, while the dense spherical precipitate formed when the feeding speed was lowered to 0.57 and 0.16 mL/min. According to the results in Fig. 5, we can obviously see that the apparent particle sizes of the precipitates decrease with increasing the cobalt solution feeding speed, which are distributed in 0.5-10 μm with feeding speed of 1.70-4.30 mL/min and in 5-50 μm with feeding speed of 0.16-0.77 mL/min.
3.2 Hydrothermal transformation of cobaltous dihydroxycarbonate into cobalt carbonate
To obtain the low sulfur content spherical cobalt carbonate, a hydrothermal process was employed to transform the cobaltous dihydroxy- carbonate precursor into cobalt carbonate. Three cobaltous dihydroxycarbonate precursors prepared as previously mentioned synthesis procedures were adopted in the hydrothermal transformation experiments, and the variation of the sulfur content, phase transformation and morphology of the samples before and after the hydrothermal processing were investigated.
Table 2 shows that the sulfur contents of the hydrothermal-treated products are obviously lower than that of the cobaltous dihydroxycarbonate precursor and the sulfur content in the obtained product can be reduced to be less than 0.005 wt.% by the hydrothermal transformation reaction. Compared with the experiment results of Precursors 2 and 3 listed in Table 2, adding a certain amount of ammonium solution as additives in the hydrothermal process is beneficial to reducing the sulfur content in the hydrothermal treated products. It should be noted that the filtrate after the hydrothermal transformation process was in wine red color (the color of cobalt ion solution), which means that part of cobalt ion is dissolved in the liquors, indicating that a dissolution and reprecipitation process may happen in the hydrothermal transformation process which makes the sulfate ions get out of the precursor more efficiently.
Table 2 Effect of hydrothermal transformation on sulfur content of products

Consequently, the sulfur removal mechanism in the hydrothermal treatment process can be presumed as that the high sulfur content precursor with relatively high solubility dissolves and decomposes at elevated temperatures and the sulfate ion releases into the solutions simultaneously, and then cobalt carbonate precipitates due to its low solubility. The high solubility of cobalt sulfate and low sulfate concentration in the hydrothermal system make the low sulfur content in the final cobalt carbonate product.
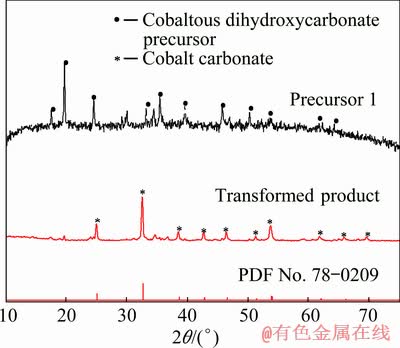
Fig. 6 XRD patterns of Precursor 1 before and after hydrothermal transformation
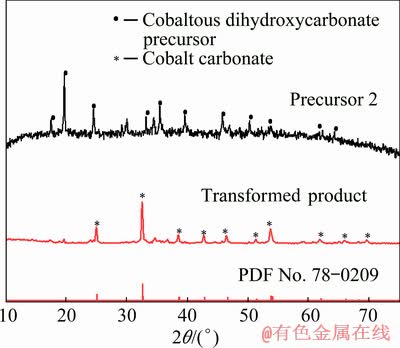
Fig. 7 XRD patterns of Precursor 2 before and after hydrothermal transformation
The results in Fig. 6 and Fig. 7 show that the precursors obtained by the reaction of cobalt sulfate solution with ammonium carbonate solution, no matter feeding methods, are of cobaltous dihydroxycarbonate, and the cobaltous dihydroxycarbonate precursors can be transformed into cobalt carbonate by the hydrothermal transformation process.
The morphologies of products obtained from the hydrothermal transformation reaction of the cobaltous dihydroxycarbonate precursors were examined by scan electron microscope, as shown in Fig. 8. Through SEM analysis, it can be found that the precursors of various morphologies were transformed into spherical cobalt carbonate after hydrothermal transformation, indicating that the morphologies of the precursors have little influence. Moreover, the spherical cobalt carbonate particles prepared after hydrothermal transformation are formed by the accumulation of flaky particles with developed pore structure and surface area, which can provide the basis for the preparation of high-performance battery materials.
4 Conclusions
(1) Spherical cobalt carbonate with sulfur content as low as 0.0030 wt.% can be prepared from cobalt sulfate solution by the two-stage process, in which sulfur-containing precursor of cobaltous dihydroxycarbonate precipitates by the reaction of cobalt sulfate solution and ammonium carbonate solution and then a hydrothermal process is conducted to transform the precursor into spherical cobalt carbonate with low sulfur content.
(2) Under the optimized conditions of temperature of 60 °C and final pH value of 7.3, cobaltous dihydroxycarbonate with the sulfur content less than 0.0115 wt.% is obtained by the reverse adding method with 90 g/L CoSO4 and 100 g/L (NH4)2CO3 solutions. Reducing the feeding rate is beneficial to the agglomeration and growth of cobaltous dihydroxycarbonate and obtaining spherical particles.
(3) The cobaltous dihydroxycarbonate precursor can be converted to spherical cobalt carbonate by a hydrothermal process in the temperature range of 125-150 °C, and the sulfur in the precursor can be removed efficiently.
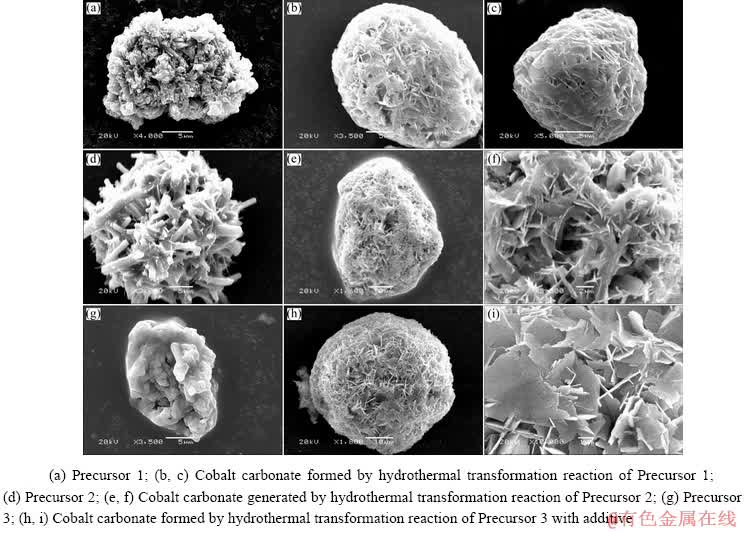
Fig. 8 SEM images of products before and after hydrothermal treatment with different cobaltous dihydroxycarbonate precursors
References
[1] XIAO J, WANG J F, LIU Y D, LI J, LIU Y X. Preparation of spherical cobalt carbonate powder with high tap density [J]. Journal of Central South University of Technology, 2006, 13: 642-646.
[2] ZHENG C, JIAN R H, PAN J L, ZOU C, WANG H, SHENG Y Y, ZHOU Q F, LIU W Q. Study on the preparation of battery grade cobalt carbonate from PTA oxidation residue [J]. Integrated Ferroelectrics, 2018, 189: 65-70.
[3] NASSAR M Y, AHMED I S. Hydrothermal synthesis of cobalt carbonates using different counter ions: An efficient precursor to nanosized cobalt oxide(Co3O4) [J]. Polyhedron, 2011, 30: 2431-2437.
[4] RAHIMI-NASRABADI M, NADERI H R, KARIMI M S, AHMADI F, POURMORTAZAVI S M. Cobalt carbonate and cobalt oxide nanoparticles synthesis, characterization and supercapacitive evaluation [J]. Journal of Materials Science: Materials in Electronics, 2017, 28: 1877-1888.
[5] KIM M J, HUH Y D. Morphology-controlled synthesis of octahedron and hexagonal plate of Co3O4 [J]. Materials Letters, 2011, 65: 650-652.
[6] HU Chang-wen, LIU Yuan-sheng. Review on production process of basic cobalt carbonate for petroleum refining catalysts [J]. Fine and Specialty Chemicals, 2015, 23: 37-38. (in Chinese)
[7] ZHENG Xing-fang, GUO Cheng-hua, ZHANG Jun. Preparation and characterization of cobaltosic oxide powder by precipitation method [J]. Chinese Journal of Spectroscopy Laboratory, 2012, 29: 1838-1840.
[8] WU Qing-sheng,. Production process improvement of basic cobalt carbonate [J]. Guangzhou Chemical Industry, 2017, 45(12): 171-182. (in Chinese)
[9] RAHIMI-NASRABADI M, AHMADI F, HAMDI S, ESLAMI N, DIDEHBAN K, GANJALI M R. Preparation of nanosized chromium carbonate and chromium oxide green pigment through direct carbonate and precursor thermal decomposition [J]. Journal of Molecular Liquids, 2016, 216: 814-820.
[10] EBRAHIMZADE H, KHAYATI G R, SCHAFFIE M. Thermal decomposition kinetics of basic carbonate cobalt nanosheets obtained from spent Li-ion batteries: Deconvolution of overlapping complex reactions [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1265-1274.
[11] CHAI Zi-fei, CHU Hong. Application of membrane technology in waste water with high content of ammonia nitrogen [J]. Technology of Water Treatment, 2015, 41: 103-106.
[12] SU Hua. Preparation of lithium-ion battery-grade cobalt sulfate by solvent extraction [J]. Rare Metals and Cemented Carbides, 2011, 39: 14-17. (in Chinese)
[13] SHEN L T, LI X B, ZHOU Q S, PENG Z H, LIU G H, QI T G, TASKINEN P. Sustainable and efficient leaching of tungsten in ammoniacal ammonium carbonate solution from the sulfuric acid converted product of scheelite [J]. Journal of Cleaner Production, 2018, 197: 690-698.
[14] WANG Xin-man, YUANG Jing, LI Qing-ge. A improvement of technological method for washing and purifying cobalt carbonate sediment [J]. Liaoning Chemical Industry, 2006, 35: 643-644. (in Chinese)
[15] SANKAR K V, SEO Y H, LEE S C, LIU S D, KUNDU A, RAY C T, JUN S C. Cobalt carbonate hydroxides as advanced battery-type materials for supercapatteries: Influence of morphology on performance [J]. Electroimica Acta, 2018, 259: 1037-1044.
[16] XING W, ZHUO S P, CUI H Y, ZHOU H C, SI W J, YUAN X, GAO X L, YAN Z F. Morphological control in synthesis of cobalt basic carbonate nanorods assembly [J]. Materials Letters, 2008, 62: 1396-1399.
[17] XIE Li-jing, JIN Xiao-qing, FU Guo-ru, ZHANG Zi-yu, YANG Yu-ying, HU Zhong-ai. Shape-controlled synthesis and structural characterization of cobalt basic carbonate by hydrothermal method [J]. Chemical Research and Application, 2010, 22: 985-988. (in Chinese)
[18] ZHANG Yu-qi, WU Jia-jing, CHEN Xing-gui, WU Ai-hua, YE Nan, GAO Zi-li, TANG Jian-cheng. Spherical cobalt carbonate synthesis by high pressure and its application in preparing ultra-fine spherical cobalt powders [J]. Jiangxi Science, 2018, 36: 834-837. (in Chinese)
[19] CHIANESE A, CONTALDI A, MAZZAROTTA B. Primary nucleation of sodium perborate in aqueous solutions [J]. Journal of Crystal Growth, 1986, 78: 279-290.
[20] NORE P, MERSMANN A B. Batch precipitation of barium carbonate [J]. Analytical Chemistry, 1993, 48: 3083-3088.
[21] GAO Jin, CHEN Yu-ling. Manufacturing of the cobalt hydroxide with higher density [J]. Cemented Carbide, 2001, 18: 77-80. (in Chinese)
[22] ZHANG Ke-cong. Modern crystal growth foundation (Book Ⅱ) [M]. Beijing: Science Press, 1987. (in Chinese)
[23] WANG Sheng, JI Hong-an. Novel technology of preparing cobalt oxide for lithium battery [J]. Nonferrous Metals, 2008, 60: 29-32. (in Chinese)
[24] LI Hong-gui. Principle of metallurgy [M]. Beijing: Science Press, 2005. (in Chinese).
从硫酸钴水溶液中制备球形碳酸钴
彭志宏,何 芳,贾 浩,李小斌,周秋生,齐天贵,刘桂华
中南大学 冶金与环境学院,长沙 410083
摘 要:采用硫酸钴溶液与碳酸铵溶液沉淀反应制备碱式碳酸钴前驱体。前驱体经水热转型处理获得低硫球形碳酸钴产品。实验结果表明:加料方式、沉淀反应的最终pH值和硫酸钴溶液浓度对碱式碳酸钴前驱体硫含量和形貌及前驱体颗粒尺寸分布均有明显的影响,在最佳条件下所得前驱体的硫含量为0.0115%(质量分数)。在125~150 °C下对前驱体进行水热处理,可使碱式碳酸钴转化为球形碳酸钴,其硫含量降低至0.0030%(质量分数)。
关键词:硫酸钴溶液;碱式碳酸钴;碳酸铵;球形碳酸钴;水热处理
(Edited by Wei-ping CHEN)
Foundation item: Project (51874372) supported by the National Natural Science Foundation of China
Corresponding author: Tian-gui QI; Tel/Fax: +86-731-88830453; E-mail: qitiangui@csu.edu.cn
DOI: 10.1016/S1003-6326(20)65231-1
Abstract: A precursor of cobaltous dihydroxycarbonate was firstly prepared by precipitation reaction of cobalt sulfate solution and ammonium carbonate solution, and then a hydrothermal process for the precursor was conducted to obtain the spherical cobalt carbonate with low sulfur content. The experimental results show that the feeding method, final pH value of the precipitation reaction slurry and the concentration of the cobalt sulfate solution have obvious effects on the sulfur content, morphology and particle size distribution of the precursor. The sulfur content of the precursor is 0.0115 wt.% under the optimized conditions. The hydrothermal treatment with temperatures of 125-150 °C can transform the precursor of cobaltous dihydroxycarbonate into spherical cobalt carbonate and decrease the sulfur content to 0.0030 wt.% in the obtained product.


