Trans. Nonferrous Met. Soc. China 23(2013) 2743-2750
Mineralogical characterization and leaching behavior of Nigerian ilmenite ore
A. A. BABA1,2, S. SWAROOPA2,3, M. K. GHOSH2, F. A. ADEKOLA1
1. Department of Chemistry, University of Ilorin, P. M. B. 1515, Ilorin 240003, Nigeria;
2. Hydro & Electrometallurgy Department, CSIR-Institute of Minerals and Materials Technology, Bhubaneswa 751013, India;
3. CSIR-Central Road Research Institute, New Delhi 110025, India
Received 31 October 2012; accepted 6 April 2013
Abstract:
The characterization and H2SO4 leaching behavior of a Nigerian ilmenite ore following mechanical activation and alkali roasting were investigated. The effects of NaOH/ore ratio, H2SO4 concentration, leaching and roasting temperature on the Ti recovery from the milled ore were examined. The results show that mechanical activation significantly enhances the dissolution of ilmenite ore. Under the leaching conditions of 90 °C, 60% (v/v) H2SO4 and 4 h, about 72% Ti extraction was obtained from a milled ore roasted at 850 °C with 60% NaOH. X-ray diffraction (XRD) phase analysis of the roasted mass, water treated residue and leach residue supports the reaction mechanism and experimental results.
Key words:
ilmenite; leaching; mechanical activation; roasting; titanium;
1 Introduction
Processing of the ilmenite ore for the production of pigment grade TiO2 often becomes difficult due to high iron content. Because of the limitation in the availability of natural rutile resources, many studies have focused on the beneficiation of ilmenite into synthetic rutile [1,2]. The known reserves of titanium oxides are decreasing, so other titanium minerals must be used, ilmenite being the immediate choice due to its abundance [3,4]. Hydrometallurgical processing of ilmenite requires drastic leaching conditions basically with hot concentrated mineral acids. The main industrial route is the sulfate process. In the sulfate process, the ilmenite ore is dissolved in concentrated sulfuric acid solution (~ 85%) at a temperature of nearly 200 °C to prepare a titanyl sulfate solution. This solution is further purified and hydrolyzed to produce pure TiO2 [5].
In order to meet the demand for high-grade titanium ores, commercial practices including roasting and smelting are followed to upgrade the ilmenite resources by removing iron oxides and other impurities. Smelting produces a titania-rich slag and a molten iron by-product (South Africa, Canada and Norway). Ilmenite roasting to synthetic rutile involves two process routes, namely Becher process (Australia) using ilmenite grade of 57%-63% TiO2 and Benelite process-roasting followed by acid leaching step (USA, India and Malaysia) and can treat a wide range of 50%-63% TiO2 [6].
Although sulfate process constitutes about 50% of global TiO2 production, but high acid concentration, temperature and exothermic nature of reaction may lead to disastrous operating problem unless well controlled, besides severe environmental problems arising from generation of H2S and SO2 gases and release of waste acid during hydrolysis. In order to achieve enhanced Ti recovery, mechanical activation through high energy ball milling is often employed prior to leaching and has been found to significantly accelerate the dissolution rate by increasing their chemical reactivity [7-9]. Increased reactivity of mechanically activated minerals can be attributed to various reasons such as decrease of particle size and increase of specific surface area, changes in the crystalline structure and disorder, and occurrence of chemical reaction [10,11].
Besides increasing the recovery of valuable components, mechanical activation of mineral leads to the decreased decomposition temperature and reduction of reagent consumption [12,13]. Many practicable routes without mechanical activation also have been proposed by several authors aiming at developing a new economic and environmentally acceptable extraction process for high titanium recovery [14-16]. MANHIQUE et al [14] demonstrated a process based on NaOH roasting of titanium ore, hydrolysing fused cake and dissolution in acid. About 80% of titanium was recovered using 2:1 of NaOH to FeTiO3 (in mole ratio), for 1 h fusion at 850 °C. The presence of ternary phases (NaFeTiO4/Na2Fe2Ti3O10) was dominant at 850 °C contrary to binary phases (Na2TiO3/NaFeO4) at 550 °C. Under the optimum leaching conditions of S/L 0.26, 75 °C and 15 min, 85% of NaOH was recovered. Leaching obeyed the mechanism model of shrinking core.
ITOH et al [15] used microwave irradiation technique for the fast oxidation of ilmenite to pseudobrokite (Fe2TiO5) and TiO2 followed by magnetic separation; dilute H2SO4 leaching of pseudobrokite and hydrolysis led to high recovery of TiO2.
It is evident that the dissolution behavior of ilmenite depends largely on its phase constitution and textural characteristics determined by the degree of weathering and is likely to vary from one deposit to the other [17,18].
The objective of the present study was to examine the low temperature acid leaching performance of a Nigerian ilmenite ore following mechanical activation and alkali pretreatment. The mineralogical and morphological characteristics of the ore were also examined. Establishment of optimum consumption of reagents like acid and alkali as well as low operating leaching conditions were emphasized in the present study. Solid intermediate products and final leach residue were characterized by XRD and SEM to corroborate the observation in roasting and leaching.
2 Experimental
2.1 Materials
The ilmenite ore used for this study was collected from Sagbe, Ifelodun Local Government Area of Kwara State, Nigeria. The ore was first crushed and ground and sieved into size of 90-125 μm. All reagents used were analytical grade and distilled water was used in the preparation of all solutions.
2.2 Characterization of ore/residue
For the elemental analysis, 0.5 g ground ore sample was first fused with 10 g KHSO4 at 800 °C and then the fused mass was dissolved in 1:1 HCl solution. Titanium analysis was carried out by UV/Visible spectrophotometer and other metal ions using Perkin Elmer AAnalyst 200 atomic absorption spectro- photometer.
The mineralogical phases of the ilmenite ore, intermediate solid sample and the leach residue were examined by X-ray diffractometry (XRD) using Philips XPERT-PRO PW 3050/60 X-ray diffractometer with Cu Kα1 radiation generated at 30 mA and 40 kV. The morphology was observed by scanning electron microscope (JEOL JSM-6510). Particle size distribution of the ore and activated ore was measured with CILAS 1064 liquid particle size analyzer.
2.3 Mechanical activation of ilmenite
For mechanical activation work, a twin-bowl INSMART planetary ball mill (Model PBM-07) of overall capacity 500 mL and useful capacity 250 mL was used. Milling was carried out at 300 r/min using 10 mm diameter stainless steel balls keeping the ball to powder mass ratio at 10:1. Milling was carried out for 1 h.
2.4 Roasting
Approximately 5 g of the activated ore together with the varied amounts of NaOH (50%, 60%, 70% and 80% in mass fraction) or Na2CO3 were mixed along with a small amount of water in a nickel crucible and placed inside a muffle furnace. The roasting temperature was varied from 550 °C to 850 °C keeping the roasting time constant at 2 h. After required roasting time, the crucible was cooled inside before removing the roasted product.
2.5 Treatment of roasted products
The roasted products were leached with water to remove eventual unreacted NaOH and to hydrolyze the products. Some impurities were also removed in this process. The slurry was filtered and washed thoroughly with warm distilled water until pH of about 9.5 of the wash solution was attained. The hydrolyzed solids were first air-dried followed by oven drying at about 80 °C for 2 h. This treated residue was further leached with H2SO4 solution.
2.6 Leaching studies
Leaching of the unmilled and milled-roasted-treated ilmenite ore was carried out in a 250 mL double-walled cylindrical glass reactor. The reactor lid had a provision for reflux condenser and sampling port. Hot water from a constant temperature water bath was circulated between the walls of the reactor to achieve the required temperature of the leaching medium. The agitation was accomplished using a magnetic paddle by keeping the glass reactor over a magnetic stirrer. The reactor was first filled with required amount of H2SO4 solution and then heated to the prefixed temperature followed by addition of sample. The mixture was agitated to a constant stirring speed of 480 r/min. At regular intervals, samples were taken and immediately filtered. The titanium content in the solution was determined using a UV-visible spectrophotometer (Perkin Elmer Lamda 35). The leach residues were washed thoroughly and oven-dried at about 80 °C for 24 h prior to the phase analysis by X-ray diffraction.
2.7 Spectrophotometric analysis of titanium
The spectrophotometric analysis of Ti in the leach liquor is based on the reaction of H2O2 with H3PO4 under acidic conditions, forming yellow-red TiO(SO4) complex [19]. For each determination, 5 mL of the aliquot was accurately pipetted to a 100 mL volumetric flask to which 10 mL H2SO4 (1:4), 2 mL H3PO4 and 3-4 drops of H2O2 solutions were added. The volume was made up to the mark with distilled water. The solution was left at room temperature for 20 min for color development. The absorbance of yellow-red complex was measured at 420 nm. Titanium concentration in the sample was appropriately determined from the calibration curve obtained with standard solutions.
2.8 Calculation of Ti leaching efficiency
The Ti extraction (η) in each test was calculated from the amount of the Ti in the leach liquor at a particular time to that of total Ti in the feed sample. Here the feed sample refers to the amount of ore sample used in roasting. After roasting although there is a mass loss there will not be any change in total Ti amount. Since the total roasted mass is taken for water leaching and the total solid from this step is used for acid leaching. The following relationship for Ti leaching efficiency calculation in each test is nearly accurate assuming that there is no loss of Ti during the water leaching.

where ρ is the Ti concentration in the leach liquor; V is the volume of the leach solution; m is the mass of Ti in the ore sample.
3 Results and discussion
3.1 Chemical analysis and characterization
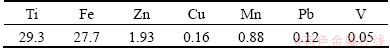
The chemical composition of the ilmenite ore studied is presented in Table 1.
Table 1 Elemental composition of ilmenite ore (mass fraction, %)
The X-ray patterns of the unmilled and milled ore samples are illustrated in Fig. 1. The XRD pattern of the unmilled sample (Fig. 1(a)) indicates that the ore is primarily comprised of ilmenite (JCPDS No. 89—2811) and rutile (JCPDS No. 89—8304) phases. However, after high energy milling for 60 min (Fig. 1(b)), major diffraction peaks of the corresponding phases slightly broadened and their intensity decreased due to the refinement of crystalline grains and the accumulation of lattice distortion linked with ball impact and collision [8,10].
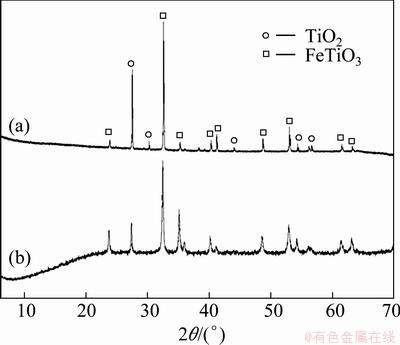
Fig. 1 XRD patterns of unmilled (a) and milled for 60 min (b) ilmenite ore
As there is no other discernible iron containing phases like Fe2O3 or Fe3O4, the total Fe can be safely assumed to be only associated with the ilmenite phase. Under this assumption, TiO2 (in ilmenite) content may be taken as 39.5%. And 9.3% TiO2 will be as rutile phase amounting to total TiO2 content in the ore as 48.8%.
The crystal lattice strain (ε) and the crystallite size (D) can be calculated from the line broadening of the peaks using Scherrer’s formula:
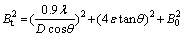 (1)
(1)
where Bt is the full width at half maximum (FWHM) intensity of the peak, λ is the wavelength of the radiation used, D is the average crystallite size, ε is the strain, θ is the diffraction angle and B0 is the instrumental line broadening.
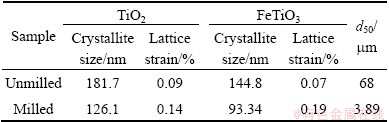
Table 2 summarizes the milling effect on crystallite size (D) and lattice strain (ε). Crystallite size and lattice strain were calculated from the XRD data for both FeTiO3 and TiO2 phases using X’pert HighScore Plus  (Ver. 3.0d) software. It is observed from Table 2 that the crystallite size decreases and lattice strain increases significantly in comparison with the unmilled samples. After high energy ball milling for 60 min, d50 is reduced from 68 mm to 3.89 mm.
(Ver. 3.0d) software. It is observed from Table 2 that the crystallite size decreases and lattice strain increases significantly in comparison with the unmilled samples. After high energy ball milling for 60 min, d50 is reduced from 68 mm to 3.89 mm.
Table 2 Effect of mechanical activation on crystallite size and lattice strain
The SEM images in the backscattered electron- imaging pattern of unmilled and mechanically activated ilmenite samples are shown in Fig. 2. As seen in Fig. 2, the particle size of activated ore was smaller than that of the unmilled ore due to the release of surface energy resulting from the formation of aggregations of tiny particles. The unmilled ore appears mostly as sub-round to sub-angular particles. Conversely, the activated powder consists of particles in the submicron to micron range and is predominantly angular in shape caused by the fracture of the original particles while retaining original shape in some. Particles are often round, which may be as a result of combination of abrasion of the edges and physical deformation during milling [17,20].

Fig. 2 SEM images of unmilled (a) and activated (b) ilmenite ore
3.2 Direct H2SO4 leaching of milled titanium ore
After mechanical activation, the particle size of ilmenite ore is significantly smaller than that of non- activated ore coupled with decrease in crystallite size and increase in lattice strains of the constituent phases. The decrease of particle size and the lattice distortion can both enhance the decomposition of minerals [13,21]. Initially, direct H2SO4 leaching without any pretreatment was carried out with the milled ore. The leaching curves under different H2SO4 concentrations at 80 °C are shown in Fig. 3.
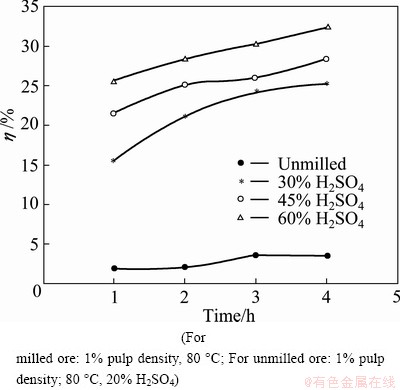
Fig. 3 Effect of H2SO4 concentration on Ti extraction
The results shown in Fig. 3 indicate that mechanical activation greatly improves the leaching recovery. Whereas the unmilled ore sample results in only about 3.5% extraction, but the milled sample yields about 32% maximum recovery in 4 h. However, the extraction rates increase moderately with increasing H2SO4 concentration.
3.3 Roasting-leaching studies for Ti extraction
During the alkali roasting of ilmenite ore, the following reactions will occur. High temperature decomposition of alkali can be represented by the following reactions:
2NaOH→Na2O+H2O (2)
Na2CO3→Na2O+CO2 (3)
Sodium oxide formed from the reaction (2) or (3) will in turn react with the rutile or ilmenite phase.
4FeTiO3+2Na2O+O2→4NaFeTiO4 (4)
TiO2+Na2O→Na2TiO3 (5)
The possibility of formation of single titanate and ferrate through the breakdown of ilmenite structure is also not ruled out [14].
4FeTiO3+6Na2O+O2→4Na2TiO3+4NaFeO2 (6)
Ilmenite can also form a ferrate and a ternary phase simultaneously.
12FeTiO3+6Na2O+3O2→4NaFeTi3O8+8NaFeO2 (7)
During the initial treatment step with water, alkali fused product especially single titanate and ferrate will be hydrolyzed.
NaFeO2+H2O→NaOH+FeOOH (8)
Na2TiO3+2H2O→2NaOH+TiO(OH)2 (9)
However, ternary phase NaFeTiO4 or NaFeTi3O8 will not be hydrolyzed under this condition and will react only in the presence of acid.
During the acid leaching step of the water treated residue, the following reactions will occur:
TiO(OH)2+H2SO4→TiO(SO4)+2H2O (10)
2NaFeTiO4+6H2SO4→Na2SO4+2TiO(SO4)+Fe2(SO4)3+ 6H2O (11)
2NaFeTi3O8+10H2SO4→Na2SO4+6TiO(SO4)+Fe2(SO4)3+10H2O (12)
2FeOOH+3H2SO4→Fe2(SO4)3+4H2O (13)
3.3.1 Comparison of different roasting agent on Ti extraction
Two different roasting agents, Na2CO3 and NaOH, were used during roasting of milled ilmenite ore at 750 °C and treated with water prior to leaching with 30% (v/v) H2SO4 solution at 90 °C. The results are depicted in Fig. 4.
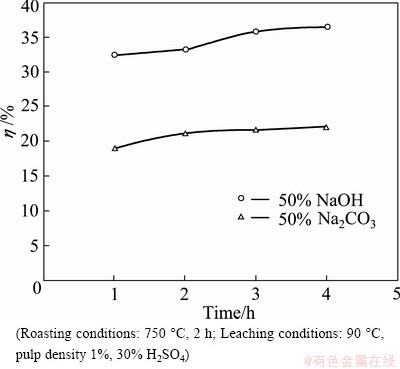
Fig. 4 Effects of different roasting agents on Ti extraction
It is evident that the use of NaOH as a roasting agent for the milled ore is more effective in extracting Ti as compared with Na2CO3. Using 50% NaOH as roasting agent, about 36% Ti extraction was achieved contrary to 22% Ti extraction using 50% Na2CO3. Hence for further studies, NaOH was adopted as roasting agent. One of the reasons may be Na2O generation capacity of the two alkalis. NaOH produces 1.5 times more Na2O (0.77 g/g) than Na2CO3 (0.49 g/g) and similar ratio is observed in the extraction results.
3.3.2 Influence of NaOH amount
The variation of NaOH/ore ratio (by mass) on roasting of milled ilmenite at 750 °C was examined. The NaOH/ore ratio was varied from 0.5 to 0.8 (i.e 50%-80%) keeping the time and temperature constant (2h, 750 °C) during roasting. The roasted product was water treated and then leached in 30% H2SO4 at 90 °C and 1% pulp density. Figure 5 presents the Ti recovery results at different NaOH/ore ratios.
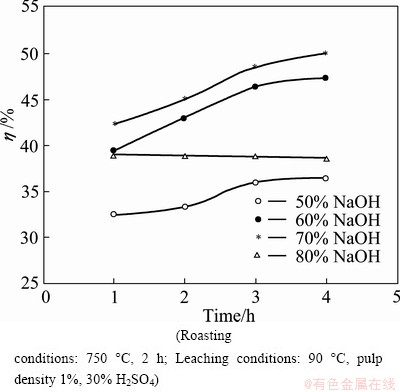
Fig. 5 Effect of NaOH amount on Ti extraction
The results illustrated in Fig. 5 indicate that increased addition of NaOH enhances the Ti extraction up to NaOH/ore ratio of 70%. Beyond 70% NaOH, there is a sharp decrease in titanium recovery. This may be due to excess liquid volume resulting from the use of excess alkali. If the melt volume increases beyond a certain point, it is likely to limit the diffusion of oxygen molecule to the solid-liquid interface, which in turn may affect the reactions (4) and (6) stated above [22]. Therefore, 60% NaOH was chosen as an optimum amount for further studies.
3.3.3 Influence of H2SO4 concentration
The effect of H2SO4 concentration on the Ti extraction from a roast ore was examined after being roasted at 750 °C with 60% NaOH. Leaching was carried out at 90 °C for a period of 4 h. As shown in Fig. 6, Ti extraction increases consistently with the increase of H2SO4 concentration as well as with time. By increasing the acid concentration from 30% to 60 % (v/v), the titanium recovery is increased from 47% to 65%.
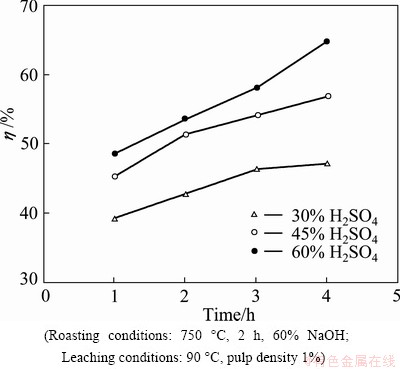
Fig. 6 Effect of H2SO4 concentration on Ti extraction from roast ore

Fig. 7 Effect of leaching temperature on Ti extraction from roasted product
3.3.4 Influence of leaching temperature
The effects of leaching temperature variation on the Ti extraction from the milled-roasted-treated ilmenite in 60% (v/v) H2SO4 solution are illustrated in Fig. 7.
Figure 7 indicates that the dissolution of Ti from the roasted ore is strongly dependent on the leaching temperature. At the leaching temperature of 90 °C, Ti recovery reached 65% in contrast to 54% at a moderately low temperature of 70 °C. It is also observed that the maximum extraction occurs in the initial 1 h period after which the extraction increases steadily.
3.3.5 Influence of roasting temperatures
In order to examine the effect of roasting temperature on the extraction efficiency of titanium, the roasting temperature was varied from 550 to 850 °C, keeping time and alkali amount constant at 1 h and 60%. The roasted sample was leached (after water treating) under the standard conditions of 90 °C, 1% pulp density and 60% H2SO4 solution. The results of the influence of roasting temperatures are presented in Fig. 8.
The results in Fig. 8 point out that the roasting temperature has strong influence on the Ti extraction efficiency. For instance, roasting at 550 °C yields about 53.5% Ti extraction, whereas increasing the roasting temperature to 850 °C increases recovery to 72.2%. The Ti recovery of 72.2% seems to be commercially attractive [7].
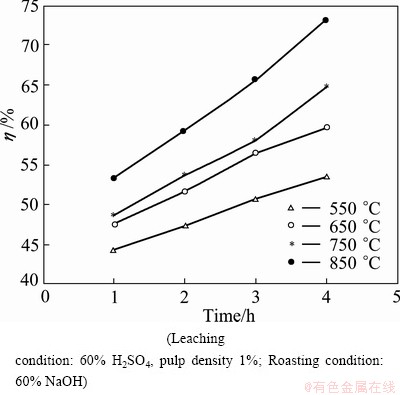
Fig. 8 Effect of roasting temperature on Ti extraction
3.4 Characterization of roasted products and leach residues
The SEM images of the roasted ore, hydrolyzed residue and acid leaching residue along with the original milled ore are shown Fig. 9. It is clearly observed that smooth and nearly cuboidal grains (Fig. 9(a)) of milled ilmenite are completely transformed to porous and nodular clusters of particles after alkali fusion (Fig. 9(b)). During the water treatment of the roasted mass, excess alkali is removed along with water soluble titanates/ ferrates causing the agglomerated form to collapse and particles become mostly free (Fig. 9(c)). Final acid leaching residue mostly contains unreacted rutile and ilmenite which were not converted during alkali fusion.
In order to elucidate the reaction mechanism, three solid samples, i.e. roasted ore, water treated roasted ore and acid leaching residue were characterized by XRD and the results are shown in Fig. 10. In the diffractogram of roasted mass (Fig. 10(a)), the presence of phases like NaFeO2, Na2TiO3, NaFeTiO4 and NaFeTi3O8 are clearly indicated which are as per the reactions shown in Eqs. (4)-(7). The presence of unreacted FeTiO3 or TiO2 could not be identified in the diffractogram, which may be due to the less amount compared with the other phases or their peak intensity might be reduced due to the formation of new phases with somehow bigger grain sizes. Figure 10(b) indicates a new phase FeOOH which was solely formed due to Reaction (8). However, other hydrolyzed product titanyl hydroxide [TiO(OH)2] could not be detected because this phase is generally XRD insensitive due to its amorphous nature. As pointed out earlier, ternary phases like NaFeTiO4 and NaFeTi3O8 remain inert during water treatment and are observed in the XRD pattern. Figure 10(c) corresponds to the XRD pattern of final acid leaching residue and comprises of TiO2 and FeTiO3 phases which did not react during roasting step and reappeared due to the disappearance of all other phases through water treatment and acid leaching.
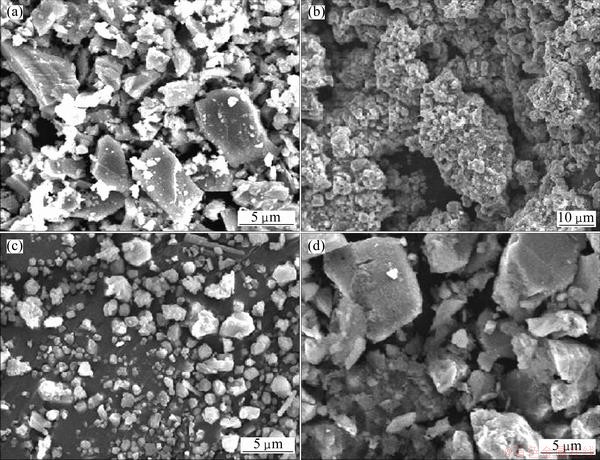
Fig. 9 SEM images of milled ilmenite (a), roasted sample (b), water treated sample (c) and acid leaching residue (d)
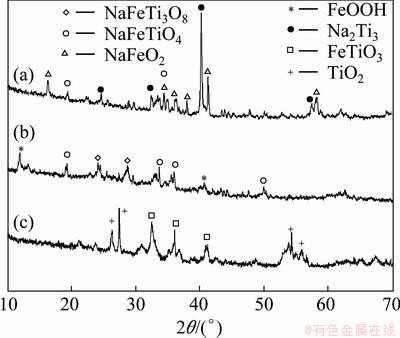
Fig. 10 XRD patterns of roasted ilmenite (a), water treated roasted ore (b) and acid leaching residue (c)
4 Conclusions
The XRD characterization of the ore reveals that it consists of ilmenite (FeTiO3) and rutile (TiO2) phases. High energy ball milling is very effective in mechanical activation of both the phases as evidenced by the decrease in crystallize size and the increase in lattice strains. Low temperature (80 °C) direct sulfuric acid leaching is not feasible even for the activated sample (the maximum 32% Ti recovery with 60% H2SO4). NaOH is more effective as roasting agent in contrast to Na2CO3. Under the optimized roasting conditions of 850 °C, NaOH/ore ratio of 0.6 for 1 h, about 72% Ti recovery was achieved by leaching with 60% H2SO4 at 90 °C in 4 h. Phase identification through XRD patterns of roasted mass, water treated residue and acid leach residue corroborated the reaction mechanism and experimental results.
Acknowledgements
One of the authors, Dr. BABA wishes to thank University of Ilorin, Nigeria for permission to honour CSIR-TWAS Fellowship award; Academy of Sciences for the Developing World, Trieste, Italy and Council for Scientific and Industrial Research, New Delhi, India for the award of 2010 CSIR-TWAS Fellowship for Postdoctoral Research. The authors are grateful to Dr. BALE of Geology and Mineral Sciences Department, University of Ilorin, Nigeria for supplying the ore used for this work; and the Institute of Minerals and Materials Technology, Bhubaneswar-751013, India, for providing facilities used for this research.
References
[1] WANG Y, QI T, CHU J, ZHAO W. Removal of iron from ilmenite by KOH leaching–oxalate leaching method [J]. Rare Metals, 2010, 29(1): 9-16.
[2] ITOH S, SATO S, ONO J, OKADA H, NAGASAKA T. Feasibility study of the new rutile extraction process from natural ilmenite ore based on the oxidation reaction [J]. Metallurgical and Materials Transactions B, 2006, 37: 979-982.
[3] da SILVA G C, da GUNHA J W S, DWECK J, ALFONSO J C. Liquid–liquid extraction (LLE) of iron and titanium by bis-(2-ethyl- hexyl) phosphoric acid (D2EHPA) [J]. Minerals Engineering, 2008, 21: 416-419.
[4] SARKER M K, RASHID A K M B, KURNY A S W. Kinetics of leaching of oxidized and reduced ilmenite in dilute hydrochloric acid solutions [J]. International Journal of Mineral Processing, 2006, 80(2): 223-228.
[5] LIANG B, LI C, ZHANG Y. Leaching kinetics of Panzhihua ilmenite in sulfuric acid [J]. Hydrometallurgy, 2005, 76: 173-179.
[6] LASHEEN T A I. Chemical beneficiation of Rosetta ilmenite by direct reduction leaching [J]. Hydrometallurgy, 2005, 76: 123-129.
[7] LI C, LIANG B, GUO L, WU Z. Effect of mechanical activation on the dissolution of Panzhihua ilmenite [J]. Minerals Engineering, 2006, 19: 1430-1438.
[8] CHEN Y, WILLIAMS J S, CAMPBELL S J, WANG G M. Increased dissolution of ilmenite induced by high-energy ball milling [J]. Materials Science and Engineering A, 1999, 271: 485-490.
[9] ZHANG L, HU H, WEI L, CHEN Q, TAN J. Effects of mechanical activation on the HCl leaching behavior of titanaugite, ilmenite, and their mixtures [J]. Metallurgical and Materials Transactions B, 2010, 41: 1158-1165.
[10] WEI L, HU H, CHEN Q, TAN J. Effects of mechanical activation on the HCl leaching behavior of plagioclase, ilmenite and their mixtures [J]. Hydrometallurgy, 2009, 99: 39-44.
[11] SASIKUMAR C, SRIKANTH S, MUKHOPADHYAY N K, MEHROTRA S P. Energetic of mechanical activation-application to ilmenite [J]. Minerals Engineering, 2009, 22(6): 572-574.
[12] KALINKIN A M, KALINKINA E V, MAKAROV V N. Mechanical activation of natural titanite and its influence on the mineral decomposition [J]. International Journal of Mineral Processing, 2003, 69: 143-155.
[13] ZHANG Yang, ZHENG Shi-li, DU Hao, XU Hong-bin, ZHANG Yi. Effect of mechanical activation on alkali leaching of chromite ore [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 888-891.
[14] MANHIQUE A J, FOCKE W W, MADIVATE C. Titania recovery from low grade titano ferrous minerals [J]. Hydrometallurgy, 2011, 109: 230-236.
[15] ITOH S, SUGA T, TAKIZAWA H, NAGASAKA T, 2007. Application of 28 GHz microwave irradiation to oxidation of ilmenite ore for new rutile extraction process [J]. The Iron and Steel Institute of Japan International, 2007, 47(10): 1416-1421.
[16] NAGASAKA T, ITOH S. Preparation of rutile: Japanese. 331992 [P]. 2003.
[17] SASIKUMAR C, RAO D S, SRIKANTH S, RAVIKUMAR B, MUKHOPADHYAY N K, MEHROTRA S P. Effect of mechanical activation on the kinetics of sulfuric acid leaching of beach sand ilmenite from Orissa, India [J]. Hydrometallurgy, 2004, 75: 189-204.
[18] CHERNET T. Mineralogical and textural constraints on mineral processing of the Koivusaarenneva ilmenite ore, Kalvia, Western Finland [J]. International Journal of Mineral Processing, 1999, 57: 153-165.
[19] BROWN F W, SMITH H. Analysis of coal ash by atomic absorption spectrometric and spectrophotometric methods [R]. USGS Bulletin, 1971.
[20] WELHAM N J, LLEWELLYN D J. Enhanced dissolution of tantalite/columbite following milling [J]. International Journal of Mineral Processing, 1998, 61: 145-154.
[21] FERNANDEZ-BERTRAN J F. Mechanochemistry: An overview [J]. Pure and Applied Chemistry, 1999, 71(4): 581-586.
[22] TATHAVADKAR V D, ANTONY M P, JHA A. The soda-ash roasting of chromite minerals: Kinetics considerations [J]. Metallurgical and Materials Transactions B, 2001, 32: 593-602.
尼日利亚钛铁矿的矿物学特征和浸出行为
A. A. BABA1,2, S. SWAROOPA2, M. K. GHOSH2, F. A. ADEKOLA1
1. Department of Chemistry, University of Ilorin, P. M. B. 1515, Ilorin 240003, Nigeria;
2. Hydro & Electrometallurgy Department, CSIR-Institute of Minerals and Materials Technology, Bhubaneswar 751013, India;
3. CSIR-Central Road Research Institute, New Delhi 110025, India
摘 要:研究尼日利亚钛铁矿矿物学特征和经机械活化和碱性焙烧处理后的浸出行为。研究了NaOH/矿石比、H2SO4浓度、浸出和焙烧温度对钛回收率的影响。结果表明,机械活化对钛铁矿石的浸出有明显的增强作用。钛铁矿经机械活化后,加入60%NaOH在850 °C下焙烧,在温度为90 °C, 经60%H2SO4浸出4 h下的浸出率为72%。对焙烧矿、水处理后残渣和酸浸滤渣的XRD物相分析证实了反应机理和实验结果。
关键词:钛铁矿;浸出;机械活化;焙烧;钛
(Edited by Xiang-qun LI)
Corresponding author: A. A. BABA; Tel: +23-48035010302; E-mail: alafara@unilorin.edu.ng
DOI: 10.1016/S1003-6326(13)62792-2
Abstract: The characterization and H2SO4 leaching behavior of a Nigerian ilmenite ore following mechanical activation and alkali roasting were investigated. The effects of NaOH/ore ratio, H2SO4 concentration, leaching and roasting temperature on the Ti recovery from the milled ore were examined. The results show that mechanical activation significantly enhances the dissolution of ilmenite ore. Under the leaching conditions of 90 °C, 60% (v/v) H2SO4 and 4 h, about 72% Ti extraction was obtained from a milled ore roasted at 850 °C with 60% NaOH. X-ray diffraction (XRD) phase analysis of the roasted mass, water treated residue and leach residue supports the reaction mechanism and experimental results.


