B/Fe2O3共掺杂纳米TiO2可见光下的催化性能
李立清1, 刘宗耀2, 唐新村3, 唐 琳2, 郭三霞2, 李海龙2, 何益波2
(1. 中南大学 能源科学与工程学院, 长沙 410083;
2. 湖南大学 环境科学与工程学院, 长沙 410082;
3. 中南大学 化学与化工学院, 长沙 410083)
摘 要:
采用溶胶-凝胶法制备B/Fe2O3共掺杂TiO2复合光催化材料, 并用X射线衍射仪(XRD)、 扫描电镜(TEM)和紫外可见漫反射光谱(DRS)对粉体进行表征。 结果表明: 催化剂以锐钛矿存在的纳米颗粒, 直观地显示了物质的形貌, 掺杂B能极大提高催化剂的可见光响应。 以二氯苯酚(DCP)为降解物质, 在紫外和可见光下分别研究了复合催化剂的光催化活性。 掺杂B能使吸收光谱红移至可见光区, 而进一步掺杂Fe2O3大大提高了催化剂的活性。
关键词: 纳米TiO2; B/Fe2O3; 溶胶-凝胶; 共掺杂; 可见光; 光催化性能 中图分类号: O643.3
文献标识码: A
Photocatalytic activity of nano-TiO2 codoped with boron and Fe2O3 in visible region
LI Li-qing1, LIU Zong-yao2, TANG Xin-cun3, TANG Lin2,GUO San-xia2, LI Hai-long2, HE Yi-bo2
(1. College of Energy Science and Engineering, Central South University,Changsha 410083, China;
2. College of Environmental Science and Engineering,Hunan University, Changsha 410082, China;
3. School of Chemistry and Chemical Engineering, Central South University,Changsha 410083, China)
Abstract: Sol-gel method is used to prepare the photocatalysts. And it was characterized by X-ray diffractometry (XRD), TEM and UV-Vis diffuse reflectance spectra (DRS). The XRD results show that a structure composed only of anatase forms after calcination and the grain sizes are very small and separated uniformly, TEM shows analysis the characters of the figures, DRS results verify that TiO2 doped with B extends its absorption band significantly into the visible region. The 2, 4-dichlorophenol(DCP) was chosen as target pollutant, the degradation rate and the mineralization rate of the pollutants in the samples under both UV and visible light were investigated. The results show that the effect of B doping in TiO2 can extend the spectral response to the visible region and that the reaction activity is extraordinarily enhanced when it is further loaded with Fe2O3.
Key words: nano-TiO2; B/Fe2O3; sol-gel; codope; visible light; photocatalytic efficiency
自Fujishima发现受辐射TiO2 电极上光解水以来[1], TiO2以其优越的性能在污水处理和大气污染治理领域中引起了研究者广泛的兴趣[2-4]。 TiO2作为光催化剂, 产生的光生空穴和光生电子有很强的氧化还原能力, 可以有效地将污染物降解为CO2、 H2O和其他小分子[5]。 然而, 催化活性偏低和可见光利用率不高使光催化大规模应用受到限制, 所以如何拓宽吸收光谱至可见光区和提高催化剂活性成为研究热点。
为了提高催化剂活性, 人们对Cd-TiO2[6-7], Fe-TiO2[8-9], Ni-TiO2[10-11], V-TiO2[12-14], Mg-TiO2[15], Ce-TiO2[16]等金属离子掺杂进行了广泛的研究。 近来, 研究表明, TiO2掺杂非金属能有效地扩展吸收光谱至可见光区[17-23]。 其中非金属B掺杂成为最近的研究热点, Zhao等[21]用TiCl4和钛酸四正丁酯及硼酸合成了一系列不同掺杂比的Ni2O3/TiO2-xBx, 并研究了对氯酚的光催化降解效果; Chen等[22]通过Sol-gel方法制得了不同B掺杂比的催化剂, 并研究了B掺杂对TiO2纳米颗粒微观结构和光催化活性的影响; Jung等[23]通过Sol-gel方法合成了B2O3-SiO2/TiO2三元催化剂等。
本文作者通过简单Sol-gel方法制得非金属B和金属Fe2O3共掺杂复合催化剂粉体, 在结合XRD、 TEM和DRS等技术分析基础上, 考察了催化剂对DCP的光催化降解反应, 并研究了催化反应过程中TOC的变化。
1 实验
1.1 原料与制备工艺
Ti(OBu)4(化学纯), 冰醋酸、 硼酸、 无水乙醇、 FeCl3和2, 4-DCP均为分析纯, 使用前未经进一步处理, 用水均为去离子水。
以Ti(OBu)4为主要原料, HAC为抑制剂, 采用Sol-gel法合成催化剂。 首先将一定量H3BO3和FeCl3溶解到30mL无水乙醇中, 在磁力搅拌下加入10mL Ti(OBu)4, 搅拌2h, 然后在冰水浴磁力搅拌下, 缓慢滴加10mL无水乙醇、 1.3mL去离子水和1.0mL冰醋酸的混合溶液(滴加0.5h), 得到透明的溶胶。 然后在室温条件下搅拌2天得到半透明的凝胶, 60℃真空干燥后玛瑙研钵研磨, 最后在450℃煅烧4h(升温速率3℃/min)得到复合催化材料。 其中n(B): n(TiO2)取1.32%, n(Fe2O3)∶n(TiO2)取0.5%; 纯TiO2、 单一B掺杂TiO2和Fe2O3掺杂TiO2也以此方法制得。
1.2 催化剂粉体测试
XRD测试采用日本理学D/max2550VB+18kW转靶X射线衍射仪; 粉末紫外-可见吸收光谱(DRS)测试在装有积分球的UV3010(Hitachi, Japan)型紫外-可见分光光度计上进行, 以BaSO4 作参比; TEM测试采用JEM-3010高分辨透射电镜, 工作电压300kV ; DCP溶液的吸光度测试采用752型紫外光栅分光光度计; TOC采用美国OI公司生产的1010型TOC分析仪按湿法氧化法测定。
1.3 催化剂光催化活性测试
光催化反应以DCP溶液作为降解对象。 在自制的反应容器中, 将0.05g催化剂粉末放入盛有1.0×10-4 mol/L的100mL DCP溶液的烧杯中, 照射前, 超声波分散混合溶液2min, 然后黑暗中保持30min以达到吸附平衡。 紫外和可见光源分别用300W中压汞灯和300W卤素灯, 其中做可见光催化实验时用滤光片滤去420nm以下波长。 每隔30min取一次样, 离心分离后测其吸光度值, 由于其吸光度值与浓度呈很好的线性关系, 通过计算, 可以依此比较各个样品的光催化活性。
2 结果与讨论
2.1 XRD和形貌分析
图1所示为B和Fe2O3共掺杂、 B或Fe2O3单个掺杂及纯TiO2的X射线衍射谱。 由图可见, 所有样品经过煅烧后均为锐钛矿特征峰, 掺杂并没有引起TiO2晶型的变化, X射线衍射谱中没有出现B和Fe2O3相衍射峰, 可以推测这些晶体是以微晶
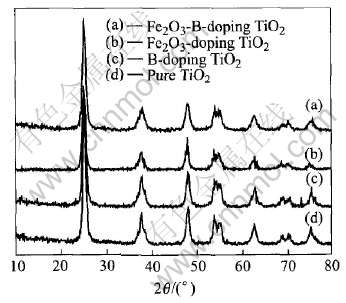
图1 不同掺杂TiO2样品的X射线衍射谱
Fig.1 XRD patterns of differently doped TiO2 samples
形式分散在TiO2的表面或进入TiO2晶格中。 其中B/TiO2晶格参数为a=b=3.7839nm和c=9.4552nm, 对比纯TiO2晶格参数a=b=3.7833nm和c=9.4996nm可知, B掺杂前后晶格参数a、 b基本一致, 参数c在掺杂B后有所下降, 说明掺杂的B进入TiO2晶格当中; 而进一步在B/TiO2材料中掺杂Fe2O3后, B-Fe2O3/TiO2共掺杂体系晶格参数a=b=3.7847nm和c=9.4536nm, 与B/TiO2材料晶格参数基本一样, 说明Fe3+并没有进入TiO2晶格中, 而是以Fe2O3微晶形式高度分散于TiO2相中。
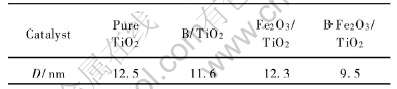
根据Scherrer公式算出晶粒的尺寸列于表1。 由表1数据可知, 所有样品粒径大小均为10nm左右。 图2所示为样品的TEM像。 由TEM像可知样品粒径比较接近, 均为10nm左右, 与Scherrer公式计算值非常吻合。
表1 不同掺杂TiO2样品粉末的粒径
Table 1 Particle size of differently doped TiO2 samples powders
2.2 UV-Vis DRS谱
图3所示为不同光催化剂的UV-Vis DRS谱, 由图3中曲线1和3可知, 微量掺杂Fe2O3前后的催化剂光响应性性能在大于420nm可见光部分略微增加, 而比较图3中曲线1和2可知, B的引入使复合半导体吸收限发生大幅红移, 使TiO2能带隙变窄, 在可见光下产生更多具有氧化和还原能力的光生载流子。
在UV-Vis DRS谱中, 采用直线外推法与长波方向的交点, 确定吸收曲线吸收边带边界的起始点λos, 由Eg=1240/λos计算出带宽, 结果列于表2。
由表2数据可知, B的掺杂使复合半导体吸收限发生大幅红移至可见光区, 能利用太阳光为能源进行催化反应。
表2 TiO2样品粉末的λos和带宽
Table 2 λos and band gaps of TiO2 samples powders
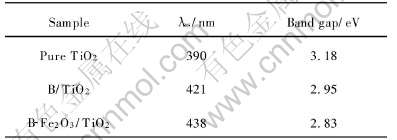
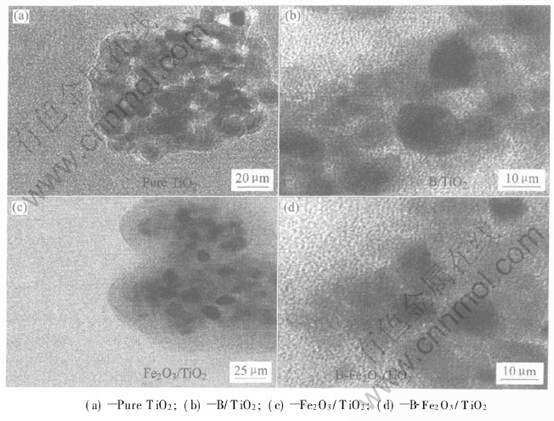
图2 不同掺杂TiO2样品的TEM像
Fig.2 TEM images of differently doped TiO2 samples
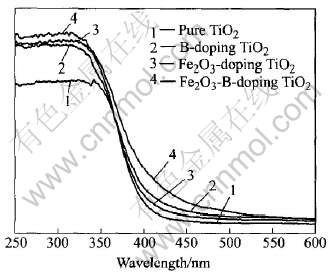
图3 不同掺杂TiO2样品的UV-Vis DRS谱
Fig.3 UV-Visible diffuse reflectance spectra of different doped TiO2 samples
2.3 Boron和Fe2O3掺杂对光催化活性的影响
绘制各个样品在紫外和可见光下催化DCP的浓度随时间的变化, 如图4所示。 由图4(a)可知, 在紫外光照射下, B/Fe2O3共掺杂和单一B或Fe2O3掺杂都取得较高的催化效率; 而在可见光下 (图4(b)), 只有B/Fe2O3共掺杂体系的具有高的
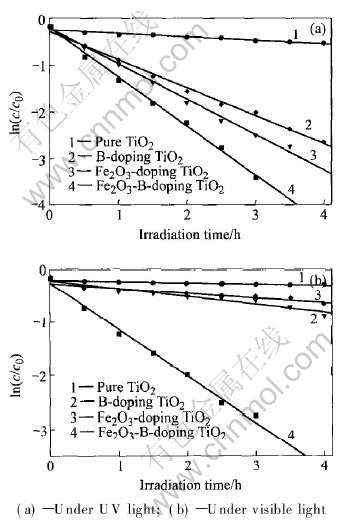
图4 不同催化剂光催化降解DCP溶液浓度随时间的变化
Fig.4 Photocatalytic degradation of DCP solutions
催化效率, 而单一B或Fe2O3掺杂催化剂活性提高不多。 结合XRD和DRS分析可知, B进入TiO2晶格中可能取代Ti-O键而形成Ti-B键, 而Fe2O3以微晶形式高度分散于TiO2材料中。 由于B对晶格的影响, B的p轨道和O的2p轨道杂化后使得禁带宽度减小而使可见光响应增强, 且B掺杂形成的内部带隙状态足够靠近TiO2的导带而产生电子耦合, 这样可以防止电子复合; 微晶Fe2O3则形成电子捕获陷阱有利于减少电子-空穴的简单复合, 并且促进表面光生电子-空穴的有效分离, 所以B/Fe2O3共掺杂体系极大地提高了可见光响应下的催化效率。
为研究DCP随时间的矿化情况, 测得不同时间下DCP溶液的TOC值, 如图5所示。 比较图5和4可知, 矿化曲线和降解曲线在紫外和可见光下变化趋势相同, 但降解曲线基本符合一级动力学反应模型, 而矿化则呈现零级动力学反应趋势。 有研究表明[24], Cl·在转化为Cl-前促进有机物的降
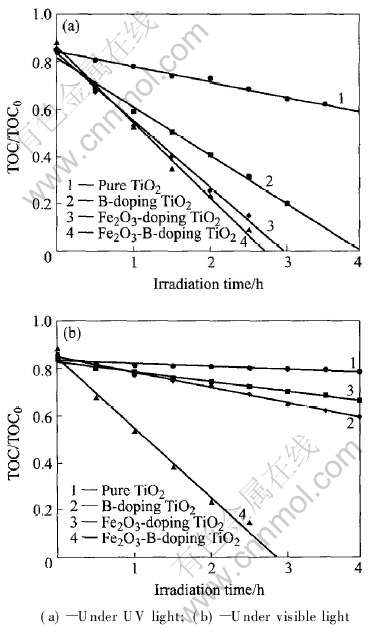
图5 不同催化剂光催化DCP溶液TOC时间的变化
Fig.5 Photocatalytic mineralization of DCP solutions
解, 而形成Cl-后将抑制有机物降解过程, 符合一级动力学反应趋势。 结合本实验分析可知, 总碳降解符合零级动力学反应, 而DCP降解符合一级动力学反应, 说明Cl-的作用可能仅是抑制DCP在催化剂表面的吸附来影响反应过程, 而对DCP的中间产物在催化剂表面的吸附基本没有抑制作用, 其作用机理有待进一步研究。
3 结论
1) 得出掺杂B能使吸收光谱移至可见光区, 在掺杂B基础上进一步掺杂Fe2O3极大地提高了催化剂的活性。
2) 得出DCP的降解反应符合一级动力学模型, 而DCP的TOC变化符合零级动力学模型。 简要地分析了中间机理, 可能是Cl-抑制了DCP在催化剂表面的吸附, 而Cl-对DCP的中间产物在催化剂表面的吸附基本没有抑制作用。
REFERENCES
[1]Fujishima A, Honda K. Electrochemical proteolysis of water at a semiconductor electorode[J]. Nature, 1972, 238: 37.
[2]Maldotti A, Molinari A, Amadelli R. Photocatalysis with organized systems for the oxofunctionalization of hydrocarbons by O2[J]. Chem Rev, 2002, 102: 3811-3836.
[3]Hoffmann M R, Martin S T, Choi W, et al. Environmental applications of semiconductor photocatalysis[J]. Chem Rev, 1995, 95: 69-96.
[4]Linsebigler A L, Lu G, Yates J T Jr. Photocatalysis on TiO2 surface[J]. Chem Rev, 1995, 95: 735-758.
[5]Nonami T, Hase H, Funakoshi K. Apatite-coated titanium dioxide photocatalyst for air purification[J]. Catal Today, 2004, 96: 113-118.
[6]Gopidas K R, Bohorquez M, Kamat P V. Photophysical and photochemical aspects of coupled semiconductors: charge-transfer processes in colloidal cadmium sulfide-titania and cadmium sulfide-silver (Ⅰ): Iodide systems[J]. J Phys Chem, 1990, 94: 6435-6640.
[7]Bessekhouad Y, Robert D, Weber J V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant[J]. J Phys Chem A, 2004, 163: 569-580.
[8]Dhananjeyan M R, Mielczarski E, Thampi K R, et al. Photodynamics and surface characterization of TiO2 and Fe2O3 photocatalysts immobilized on modified polyethylene films[J]. J Phys Chem B, 2001, 105: 12046-12055.
[9]Wang X H, Li J G, Kamiyama H, et al. Wavelength-sensitive photocatalytic degradation of methyl orange in aqueous suspension over iron(Ⅲ): Doped TiO2 nanopowders under UV and visible light irradiation[J]. J Phys Chem B, 2006, 110: 6804-6809.
[10]WANG Yan-qin, ZHANG Li, CHENG Hu-min, et al. The photocatalytic properties of transition metal ion-doped TiO2 nanoparticles[J]. Chemical Journal of Chinese Universities, 2000, 6: 958-960.
[11]Visinescu C M, Sanjines R, Lévy F, et al. Photocatalytic degradation of acetone by Ni-doped titania thin films prepared by dc reactive sputtering[J]. Applied Catalysis B: Environmental, 2005, 60: 155-162.
[12]Sarah K, Daniel R. Visible light driven V-doped TiO2 photocatalyst and its photooxidation of ethanol[J]. J Phys Chem B, 2001, 105: 2815-2819.
[13]Wu J C S, Chen C H. A visible-light response vanadium-doped titania nanocatalyst by sol-gel method[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163, 509-515.
[14]Iketani K, Sun R D, Toki M, et al. Sol-gel-derived VxTi1-xO2 films and their photocatalytic activities under visible light irradiation[J]. Mater Sci Eng B, 2004, 108: 187-193.
[15]Abothu I R, Rao A V P, Komarneni S. Nanocomposite and monophasic synthesis routes to magnesium titanate[J]. Mater Lett, 1999, 38(3): 186-189.
[16]Purans J, Azens A, Granqvist C G. X-ray absorption study of Ce-Ti oxide films[J]. Electrochimica Acta, 2001, 46: 2055-2058.
[17]Asahi R, Morikawa T, Ohwaki T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science, 2001, 293: 269-271.
[18]Sathish M, Viswanathan B, Viswanath R P, et al. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst[J]. Chem Mater, 2005, 17(25): 6349-6353.
[19]Tachikawa T, Takai Y, Tojo S, et al. Visible light-induced degradation of ethylene glycol on nitrogen-doped TiO2 powders[J]. J Phys Chem B, 2006, B110(26): 13158-13165.
[20]Khan S U M, Shahry M A, Ingler W B Jr. Efficient photochemical water splitting by a chemically modified n-TiO2[J]. Science, 2002, 297: 2243.
[21]ZHAO Wei, MA Wan-hong, CHEN Chun-cheng, et al. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2-xBx under visible irradiation[J]. J Am Chem Soc, 2004, 126: 4782-4783.
[22]CHEN Dai-mei, YANG Dong, WANG Qun, et al. Effects of boron doping on photocatalytic activity and microstructure of titanium dioxide nanoparticles[J]. Ind Eng Chem Res, 2006, 45: 4110-4116.
[23]Jung K Y, Park S B, Ihm S K. Local structure and photocatalytic activity of B2O3-SiO2/TiO2 ternary mixed oxides prepared by sol-gel method[J]. Applied Catalysis B: Environmental, 2004, B51: 239-245.
[24]CHEN Yi-chuan, Smirniotis P. Enhancement of photocatalytic degradation of phenol and chlorophenols by ultrasound[J]. Ind Eng Chem Res, 2002, 41: 5958-5965.
基金项目: 国家自然基金资助项目(20376019, 20576028); 湖南省建设厅科研资助项目
收稿日期: 2006-04-17; 修订日期: 2006-07-21
通讯作者: 李立清, 教授; 电话: 13807483619; E-mail: liqingli@hotmail.com


