Trans. Nonferrous Met. Soc. China 23(2013) 1783-1788
Interaction forces between muscovite and silica surfaces in electrolyte solutions measured with AFM
Hao JIANG, Zhen XIE, Guo-rong LIU, Ya-wen YU, Ding ZHANG
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 8 May 2012; accepted 14 September 2012
Abstract:
Interaction forces between a silica colloidal sphere and a muscovite flat surface in electrolyte solutions were directly measured with an atomic force microscope (AFM). The results showed a significant impact of time, electrolyte concentration and solution pH on both long-range (non-contact) and adhesion (pull-off) force. A strong long-range repulsive force was observed under conditions of lower electrolyte concentration and higher solution pH, while a weak long-range attractive force was observed in the higher electrolyte concentration and lower pH solutions. With the electrolyte concentration increasing, the interaction forces decreased from strong repulsive force to strong attractive force. The measured long-range forces were monotonically repulsive at pH 5.8-10.2 and changed in a small scale. However, when the solution pH decreased to 3.4, a weak attractive force was observed at a separation distance of 22-32 nm. In low electrolyte concentration and pH solutions, the adhesion force between the muscovite and silica is large. With increasing the electrolyte concentration and solution pH, the adhesion force decreased and became relatively stable at last. The measured interaction forces were fitted well with the classical DLVO theory.
Key words:
interaction forces; atomic force microscope; muscovite; colloidal probe; DLVO;
1 Introduction
The mica is one kind of clay mineral widely distributed in ore. Due to its distinctive properties including electric insulation, heat resistance, chemical resistance and so on, the mica is extensively used in a wide rang of applications, such as paper making, oil frilling, water pollutant removal, oil sands industry and consumer products [1]. It is very meaningful to study the surface properties of mica.
Generally, the surface of mineral becomes charged in solution. As the point of zero charge (PZC) of muscovite is very low, it is always charged negatively. The electrostatic force dominates the interaction force between muscovite particles. The free ions of electrolyte would adsorb to the charged mineral surface in solution, which makes the surface electrical properties different [2]. Both the electrolyte concentration and solution pH can affect the surface properties of mineral, and the interaction force between particles would be different [3].
Colloid force measurements between two solid surfaces have been reported extensively in Refs. [4-6]. DUCKER et al [7,8] used the AFM to study the interaction forces between silica surfaces in solution environment. They found that the force profiles fitted well with the classical DLVO theory when the distance was greater than 3 nm. The experiment force profiles were between the constant charge curve and the constant potential curve. ALCANTAR et al [9] used a surface force apparatus to study the interaction between mica surfaces in pure and mixed NaCl and CaCl2 solution. Their results showed that the short-range colloidal forces depended on the type and concentration of the cations present in the solution. YOON and RAVISHANKAR [10,11] studied the hydrophobic force systematically with AFM. They found that there were only short-range hydrophobic forces when the cationic surfactants were present alone. LIU et al [12,13] studied the interaction forces between bitumen surfaces with AFM. They found that the double layer electrostatic force was dominant in non-contact force when the bitumen was good. When the bitumen was tailings, both the double layer electrostatic force and hydrophobic force contribute to the non-contact force. RUTLAND et al [14,15] found that a hydrophobic monolayer formed and purely attractive hydrophobic interactions were measured. The bilayer formation occurred at the critical micelle concentration (CMC) for low pH and below the CMC at higher pH.
The interaction forces can be measured with either the surface force apparatus (SFA) or the atomic force microscope (AFM). The measured forces can be analyzed by the classical DLVO theory [16]:
FT=FV+FE (1)
where FT is the total force between two surfaces, FE is the double layer electrostatic force between the over lapping electrostatic double layers, and FV is the London-van der Waals force.
However, when the situation is more complicated, the extended DLVO theory can be used to analyze the experimental curves [12]:
FT=FV+FE+FHA+FHR+FS (2)
where FHA is the hydrophobic force, FHR is the hydration force and FS is the steric force.
The interaction colloidal force of silica-silica and muscovite-silica measured with AFM technique is summarized in the present work. We study the impacts of electrolyte concentration and solution pH on the interaction force. The classical DLVO theory was used to fit the experiment force profiles. The results of the present study would be useful to predict the interaction forces between mineral surfaces in solution environment.
2 Experimental
2.1 Materials
The purchased silica microspheres with 5 μm in diameter were used for preparation of silica probe for AFM measurement. Ultra-flat silica substrate and muscovite substrate all were purchased. Reagent grade HCl and NaOH (AR) were used as pH modifiers. Ultrahigh pure KCl (>99.999%) was used as the supporting electrolyte. Reagent acetic acid and absolute ethanol were utilized as the cleaning solvents. Ultra water with a resistivity of 18.2 MΩ·cm was prepared with an ultra-pure water machine.
2.2 Silica probe and substrate preparation
There were many methods to prepare colloidal probe [17,18]. The most common way was to use epoxy to glue the colloidal particle on the cantilever. In our research, a 5 μm-radius silica sphere was glued with a two-component epoxy onto the tip of a short, wide beam AFM cantilever. The spring constant of the cantilever was calculated automatically by the AFM through the “Thermal tune” button. The method of calculating the spring constant can be seen from the manual of AFM. The glued probe particle was allowed to expose to the air for more than 1 h to let the epoxy dry. Prior to each set of experiments, the probe particles were thoroughly rinsed with ultra water, ethanol and ultra water again and then dried in the air. Figure 1 shows the prepared silica probe in our lab.
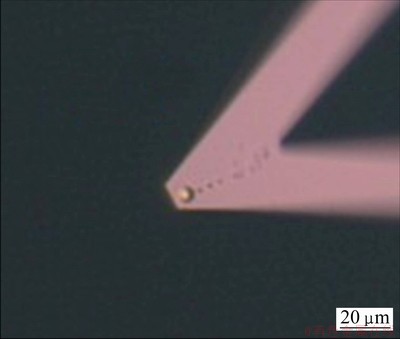
Fig. 1 A 5 μm radius silica probe on triangle cantilever
Before the experiment, the muscovite substrate was cleaned successively with ultra water, ethanol, acetic acid and ultra water orderly. The prepared substrate then was dried with ultra-pure grade nitrogen.
2.3 Surface forces measurement
Direct force measurements were carried out by using a Picoforce atomic force microscope. In each experiment, the surface of muscovite was fresh. The force—distance curve obtained in ultra-pure water at pH 5.7 was compared with that in Ref. [19] to ensure that the muscovite surfaces were clean and the AFM was well adjusted. A range of concentration solutions of KCl were prepared in ultra-pure water. Requisite amounts of these stock solutions were injected into the chamber. The measurements were initiated after 1 h of equilibration. After completion of the force measurements with one KCl concentration, the muscovite and silica surfaces were separated by about 23 μm from each other and then the solution in the chamber was changed to another concentration. In each force measurement, the adhesion force and the jump distance were also measured. At least two sets of measurements were conducted at a given reagent condition. All the experiments completed in the contact mode.
In the force measurements, two surfaces jump into contact when the slope of the force curve becomes equal to or greater than the spring constant (k). By successively increasing k, we can measure stronger force at closer distance.
3 Results and discussion
3.1 DLVO theory calculation
In continuum theory, the potential distribution is determined from the Poisson-Boltzmann equation which is a second-order differential equation. To solve this equation, certain boundary conditions have to be assumed previously. Two boundary conditions are often used: it is assumed either that the surface charges remain constant (constant charge) or that the surface potentials remain constant (constant potential). The force between an AFM tip with radius of curvature R and a flat surface is given by assuming constant potentials of the sample ψS and the tip ψT [20,21]:
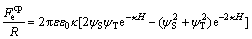 (3)
(3)
where ε is the permittivity of the medium, ε0 is the vacuum permittivity and κ-1 is the Debye length. Under constant charge conditions, the electrostatic double layer force is
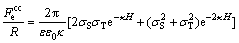 (4)
(4)
where σS and σT are the surface charge densities of sample and tip, respectively.
To different geometries, there are different formulas to be used to calculate the London-van der Waals interaction force. For sphere-flat surface, the van der Waals forces are fitted by
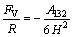 (5)
(5)
where A132 is the Hamaker constant, R is the radius of the colloidal sphere and H is the separation distance.
Figure 2 shows the force vs distance curve obtained in 1 mmol/L KCl solution at pH 6.8. The upper curve is based on constant charge model, while the lower one represents the constant potential model. Both have been carried out in the conditions of Hamaker constant A132 of 4.6×10-21J, Debye length κ-1 of 9.4 nm, the surface potential ψ0 of -52 mV and the surface charge σ0 of -0.0036 C/m2. It can be seen that the force curves of two calculating methods are overlapped when the distance is greater than 25 nm, and they well fit the experiment curve at this distance. When the separation distance is smaller than 20 nm, the two theoretical curves separate from each other, meanwhile the constant potential curve can fit the experiment curve better than the constant charge curve. This phenomenon is consistent with that in Refs. [8-15]. Thus, the subsequent experiment force profiles are fitted by the constant potential calculation.
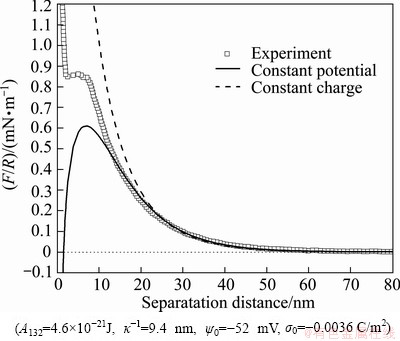
Fig. 2 Interaction forces (F/R) between silica surfaces as function of separation distance in solution containing 1 mmol/L KCl at pH 6.8
3.2 Effect of time
To ensure a representative force profile, force measurements were carried out at a number of different locations on the muscovite substrate. In general, the force profiles were found to be fairly reproducible. For the convenience of illustration, only one typical (most repeated) force profile was reported under a given condition. Whenever appropriate, all the scattered data were reported to show variation and to emphasize a trend. An average value or a distribution of adhesion force was reported.
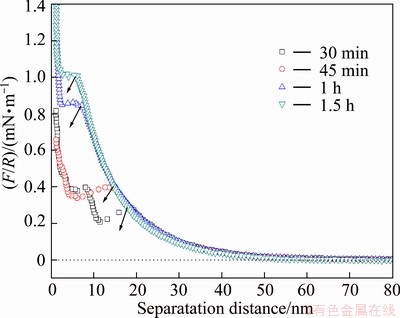
Fig. 3 Normalized interaction force (F/R) profile as function of time of silica surfaces in 1 mmol/L KCl solution at pH 6.8 (arrow shows jump distance)
As shown in Fig. 3, the repulsive long-range forces rise with increasing time. However, the jump distance is shortened as the time increases. About 1 h is required to obtain reproducible force profiles. This type of time-dependent behavior can be attributed to the adsorption of polar molecule (K+) on the silica surfaces. To ensure that the surface is fully equilibrated prior to collecting force data, 1.5 h of incubation time is allowed prior to each force measurement. The results have demonstrated that the interaction forces between silica surfaces are certainly time-dependent.
3.3 Effect of electrolyte concentration
The interaction forces between muscovite and silica surfaces were measured as a function of KCl concentration in the solution of pH 6.8. As shown in Fig. 4, the repulsive long-range forces are depressed by increasing potassium chloride (KCl) concentration.
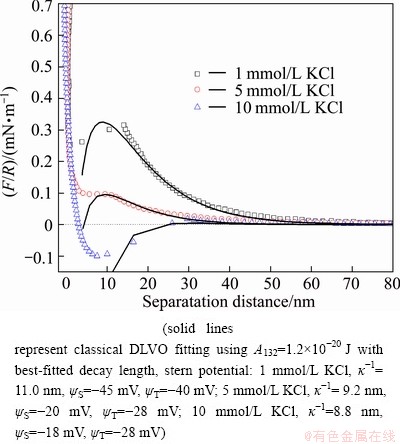
Fig. 4 Normalized interaction forces (F/R) between muscovite and silica surfaces as function of separation distance at pH 6.8 in solutions of different KCl concentrations
In the solution containing 1-5 mmol/L KCl, the long-range forces show repulsive at a separation distance smaller than 30 nm and jump-in at a separation distance of about 12 nm, with a strong repulsion at a separation distance of less than 5 nm. However, in a solution containing 10 mmol/L KCl, a strong attractive force at a separation distance of 9-28 nm emerges, a strong repulsive force at a separation distance of 9 nm adds to the total force and the force profile changes to repulsive at 4 nm. This additional strong repulsive force at a small distance (4-5 nm) is attributed to the hydration force as the muscovite surface is highly hydrophilic. The magnitude and range of long-range force decrease with increasing salt concentration, demonstrating a compression of the electrostatic double layer by KCl addition. The long-range force profiles can be well fitted with the classical DLVO theory. The results have further confirmed that the long-range interaction forces between muscovite and silica surfaces are controlled by the electrostatic double layer force at a lower salt concentration, while the van der Waals force becomes dominant at a higher salt concentration.
It is well known that the adhesion force corresponds to how strong the two surfaces are attached to each other, while non-contact forces (or so-called long-range forces in the literature) indicate how difficult two surfaces approach each other. Table 1 shows the change of the adhesion force between muscovite and silica surfaces in KCl of different concentrations. It is observed that the adhesion force is much greater than the long-range repulsive (approach force) at all salt concentrations. The magnitude of adhesion forces decreases with increasing the salt concentration. When the concentration is higher than 5 mmol/L, the adhesion forces remain at about 0.5 mN/m. Adhesion forces are attributed to the molecular or atomic interaction between the two surfaces after their contact. This type of molecular or atomic interaction depends on the nature of molecules on the two surfaces, which are impacted by conditions such as pH, electrolyte, and divalent ions. In addition, the adhesion forces are also affected by the roughness of the surfaces.
Table 1 Adhesion force (F/R) between muscovite and silica surfaces as function of electrolyte (KCl) concentration at pH 6.8
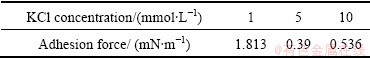
In summary, a higher salinity reduces the repulsive force, and diminishes the adhesion force. The results suggest that the interaction forces and adhesion forces between muscovite and silica are significantly impacted by the salt concentration.
3.4 Effect of solution pH
Solution pH is recognized as an important operating parameter in mineral flotation. In general, the flotation is completed in the acid or alkaline environment. The effect of solution pH on the interaction forces between muscovite and silica surfaces is shown in Fig. 5.
The forces measured between the muscovite and silica surfaces in 1 mmol/L KCl solutions of varying pH values of 3.4, 5.8, 8.3, and 10.2 are shown in Fig. 5. For the pH values of 5.8, 8.3 and 10.2, the interaction forces are monotonically repulsive. The value of the repulsive force increases with increasing the solution pH in a small scale. The change is not as great as that with different electrolyte concentrations. It can be seen that the measured repulsive force profiles are nearly coincided in a weak acid and alkaline solution. A similar observation was reported elsewhere [22]. However, when the solution pH decreases to 3.4, a weak attractive force is observed at a separation distance of 22-32 nm. This phenomenon suggests that the long-range interaction forces between muscovite and silica surfaces are pH-dependent in solution environments. There is a weak attractive force at lower pH, while there are long-range repulsive forces at higher pH. It is indicated that the interaction forces are dominated by the repulsive electrostatic double layer force at pH values of 5.8, 8.3, and 10.2. The point of zero charge (PZC) of muscovite is 1, and the point of zero charge of silica is 2-3.7. It is expected that for solutions of pH values above the point of zero charge of muscovite and silica, an electrostatic repulsion between the unequally charged negative surfaces is expected. For solutions of pH values between the PZCs of the muscovite and silica, an electrostatic attraction between the oppositely charged surfaces is expected. The force profiles obtained with muscovite and silica surfaces in simple electrolyte solutions at pH 3.4 agree very well with such expectations. Anyway, there is an jump-in phenomenon at all the pH values. The force profiles at pH values of 5.8, 8.3 and 10.2 can be fitted well by the classical DLVO theory.
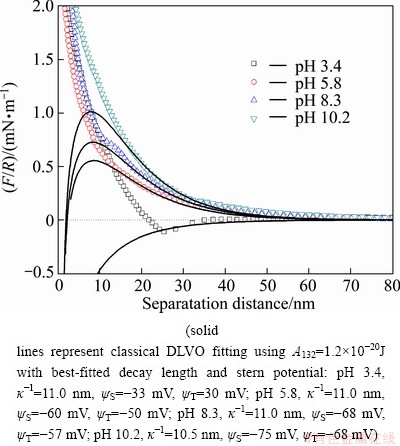
Fig. 5 Normalized interaction forces (F/R) between muscovite and silica surfaces as function of separation distance in 1 mmol/L KCl solution at different solution pH values
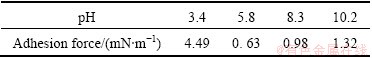
As shown in Table 2, with increasing the solution pH, the normalized adhesion forces drop abruptly from 4.5 mN/m at pH 3.4 to 0.6 mN/m at pH 5.8. The adhesion forces at pH values of 5.8, 8.3, and 10.2 are very small compared to those at pH 3.4. This phenomenon is consistent with the measured interaction forces between muscovite and silica at pH values of 5.8, 8.3, and 10.2. The results suggest that the surface properties of muscovite in weak acid and alkaline environment are hardly unchanged. However, the interfacial tension of muscovite at very low pH is much stronger than that at higher pH. This phenomenon should be attributed to the crystal structure of muscovite which make it become stable in weak acid and alkaline environment.
Table 2 Adhesion force (F/R) between muscovite and silica surfaces as function of pH in 1 mmol/L KCl solutions
4 Conclusions
1) The results clearly show that the solution pH and electrolyte concentration have a significant impact on both the long-range force and adhesion forces. The equilibration time has impact on the jump-in distance.
2) At lower concentration of electrolyte, there is a strong repulsive force and adhesion force, while higher concentration of electrolyte (KCl) can substantially reduce both the repulsive and the adhesion forces. A lower solution pH corresponds to a weak attractive force at a separation distance of 22-32 nm. The interaction forces which are all long-range repulsive forces are hardly changed at higher pH.
3) The adhesion force decreases with increasing the solution concentration and pH. There is a large adhesion force of 4.49 mN/m at pH 3.4, while there are very small adhesion forces in alkaline solution.
4) The non-contact interaction force profiles can be reasonably described by the classical DLVO theory. The electrostatic double layer force dominates the non-contact force for the system of muscovite-silica at lower electrolyte concentration as well as higher solution pH. While at higher electrolyte concentration or lower pH, the van der Waals force dominates the non-contact force at close distance.
References
[1] ZHU Li-ping. The experimental study of recovering mica from crushed mica tailings [D]. Hebei: Hebei University of Technology, 2005: 4-6. (in Chinese)
[2] MILLER J D, YALAMANCHILI M R. Surface charge of alkali halide particles as determined by laser-Doppler electrophoresis [J]. Langmuir, 1992, 8: 1464-1469.
[3] LIU Xin-xing, HU Yue-hua. Atomic force microscopy and its application in mineral processing [J]. Mining and Metallurgical Engineering, 2000(20): 32-35. (in Chinese)
[4] GUPTA V, MILLER J D. Surface force measurements at the basal planes of ordered kaolinite particles [J]. Journal of Colloid and Interface science, 2010, 344: 362-371.
[5] TYRRELL J W G, ATTARD P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force-separation data [J]. Langmuir, 2002, 18: 160-167.
[6] HARTLEY P G., LARSON I, SCALE P J. Electrokinetic and direct force measurements between silica and mica surfaces in dilute electrolyte solutions [J]. Langmuir, 1997, 13: 2207-2214.
[7] DUCKER W A, SENDEN T J, PASHLEY R M. Measurement of forces in liquids using a force microscope [J]. Langmuir, 1992, 8: 1831-1836.
[8] BUTT H J, JASCHKE M, DUCKER W. Measuring surface forces in aqueous electrolyte solution with the atomic force microscope [J]. Bioelectrochemistry and Bioenergetics, 1995, 38: 191-201.
[9] ALCANTAR N, ISRAELACHVILI J, BOLES J. Forces and ionic transport between mica surfaces: Implication for pressure solution [J]. Geochimica et Cosmochimica Acta, 2003, 67(7): 1289-1304.
[10] YOON R H, RAVISHANKAR S A.Long-rang hydrophobic forces between mica surfaces in dodecylammonium chloride solution in the presence of dodecanol [J]. Journal of Colloid and Interface Science, 1996, 179: 391-402.
[11] YOON R H, RAVISHANKAR S A.Long-rang hydrophobic forces between mica surfaces in alkaline dodecylammonium chloride solution [J]. Journal of Colloid and Interface Science, 1996, 179: 403-411.
[12] LIU Jiang-jun, XU Zheng-he, MASLIYAH J. Interaction forces in bitumen extraction from oil sands [J]. Journal of Colloid and Interface Science, 2005, 287: 507-520.
[13] LIU Jiang-jun, XU Zheng-he, MASLIYAH J. Colloidal forces between bitumen surfaces in aqueous solutions measured with atomic force microscope [J]. Journal of Colloid and Interface Science, 2005, 260: 217-228.
[14] RUTLAND M W, PARKER J L. Surface forces between silica surfaces in cationic surfactant solution: adsorption and bilayer formation at normal and high pH [J]. Langmuir, 1994, 10: 1110-1121.
[15] RUTLAND M, WALTERNO A, CLAESSON P. pH-dependent Interaction of mica surfaces in aqueous dodecylamonium/ dodecylamine solutions [J]. Langmuir, 1992, 8: 176-183.
[16] QIU Guan-zhou. Interactions between particles and fine particles flotation [M]. Changsha: Central South University Press, 1993: 26-119. (in Chinese)
[17] XU Hui. Study of the hydrophobic force using the atomic force microscopy [D]. Hangzhou: Zhejiang University, 2006: 32-34. (in Chinese)
[18] ZANG Li-jie. Preparation of silica colloid probe and its applications on simulation of interaction force measurements between particles [D]. Shijiazhuang: Hebei University, 2009: 29-47. (in Chinese)
[19] HERDER P C. Interactions between mica surfaces in dodecyl- and octyammonium chloride solutions [J]. Journal of Colloid and Interface Science, 1990, 134: 346-356.
[20] BUTT H J, CAPPELLA B, KAPPL M. Force measurements with the atomic force microscope: Technique, interpretation and applications [J]. Surface Science Reports, 2005, 59: 47-55.
[21] LIANG Yun-cheng, HILAL N, LANGSTON P. Interaction forces between colloidal particles in liquid: Theory and experiment [J]. Advances in Colloid and Interface Science, 2007, 134-135: 151-166.
[22] ZHAO Hong-ying, BHATTACHARJEE S, CHOW R. Probing surface charge potentials of clay basal planes and edges by direct force measurements [J]. Langmuir, 2008, 24: 12899-12910.
运用原子力显微镜研究电解质溶液中白云母与二氧化硅间的相互作用力
蒋 昊,谢 珍,刘国蓉,俞亚文,张 丁
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:运用原子力显微镜研究了二氧化硅胶体探针(直径,5 μm)与白云母表面之间在电解质溶液环境下的相互作用力。试验考察了平衡时间、电解质浓度和溶液pH值对相互作用力的影响。实验结果表明,电解质浓度和溶液pH值对白云母/二氧化硅之间相互作用力及粘附力都产生显著的影响。在低电解质浓度和高的溶液pH值条件下,观察到一个很强的长程排斥力;而在高电解质浓度和低pH值条件下,两表面之间产生一个弱的长程吸引力。随着电解质浓度的增加,相互作用力从强的排斥力变化到强的吸引力。当溶液pH值在5.8~10.2时,相互作用是单调的排斥力;当溶液pH值降低至3.4时,在距离22~32 nm处出现一个弱的吸引力。在低浓度或低pH值下,白云母与二氧化硅之间的粘附力比较大,随着电解质浓度和溶液pH值的增加,粘附力不断减小,最后相对稳定。运用经典DLVO理论对相互作用力进行理论计算,与实验曲线基本一致。
关键词:相互作用力;原子力显微镜;白云母;胶体探针;DLVO
(Edited by Hua YANG)
Foundation item: Project (50974134) supported by the National Natural Science Foundation of China; Project (2005CB623701) supported by the National Basic Research Program of China
Corresponding author: Hao JIANG; Tel: +86-731-88830204; E-mail: jianghao-1@126.com
DOI: 10.1016/S1003-6326(13)62661-8
Abstract: Interaction forces between a silica colloidal sphere and a muscovite flat surface in electrolyte solutions were directly measured with an atomic force microscope (AFM). The results showed a significant impact of time, electrolyte concentration and solution pH on both long-range (non-contact) and adhesion (pull-off) force. A strong long-range repulsive force was observed under conditions of lower electrolyte concentration and higher solution pH, while a weak long-range attractive force was observed in the higher electrolyte concentration and lower pH solutions. With the electrolyte concentration increasing, the interaction forces decreased from strong repulsive force to strong attractive force. The measured long-range forces were monotonically repulsive at pH 5.8-10.2 and changed in a small scale. However, when the solution pH decreased to 3.4, a weak attractive force was observed at a separation distance of 22-32 nm. In low electrolyte concentration and pH solutions, the adhesion force between the muscovite and silica is large. With increasing the electrolyte concentration and solution pH, the adhesion force decreased and became relatively stable at last. The measured interaction forces were fitted well with the classical DLVO theory.


