
Vapor pressure and thermochemical properties of ZrCl4 for ZrC coating of coated fuel particles
LIU Chao(刘 超), LIU Bing(刘 兵), SHAO You-lin(邵友林),
LI Zi-qiang(李自强), TANG Chun-he(唐春和)
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
Abstract:
Vapor pressure of zirconium tetrachloride(ZrCl4) under vacuum and an argon pressure of 1×105 Pa was measured. The thermochemical changes of ZrCl4 during evaporation were studied by thermogravimetry-differential thermal analysis(TG-DTA), X-ray diffractometry(XRD), scanning electron microscopy(SEM) and energy-dispersive X-ray(EDX) analysis. At the same temperature, vapor pressures of ZrCl4 under vacuum and an argon pressure of 1×105 Pa are approximately the same. The vapor pressure exceeds 1×105 Pa at 340 ℃, which is high enough for ZrC coating of coated fuel particles. ZrCl4 sample is hydrolyzed to some extent to give ZrO2 and HCl, which however, has little influence on vapor pressure of ZrCl4 at high temperature. No ZrCl3 and Cl2 are produced by decomposition of ZrCl4 during evaporation, which is confirmed by thermodynamic calculation.
Key words:
vapor pressure; thermochemical change; ZrC coating; coated fuel particle;
1 Introduction
Zirconium tetrachloride is an important chemical raw material for many applications, such as pigment, waterproofing agent and tanning agent and catalyzer [1-4]. With the development of nuclear energy in the world[5-6], zirconium tetrachloride has also gained important applications in nuclear industry[7-8]. One of the most important applications is to prepare ZrC coating of coated fuel particles for high temperature gas cooled reactor. Several routes[9-15] have been developed to prepare ZrC coating, all of which utilized the reaction of a zirconium halide with a hydrocarbon gas. In these routes, accurate control of flow rate of zirconium source is crucial. ZrCl4 vapor route is an effective method to resolve this problem, in which flow rate of zirconium source can be adjusted by controlling heating temperature of ZrCl4 and flow rate of carrier gas. Thus, the vapor pressure and thermochemical changes of ZrCl4 during evaporation are vital for this application.
In the present study, vapor pressure of ZrCl4 under vacuum and an argon pressure of 1×105 Pa were measured and discussed. The thermodynamic change of ZrCl4 during evaporation was analyzed. The phase composition, particle size, morphology and element contents of ZrCl4 sample were investigated. Finally, reaction mechanism of ZrCl4 during evaporation was studied by thermodynamic calculation.
2 Experimental
2.1 Materials and set-up
Zirconium tetrachloride (ZrCl4, 98% purity) was obtained in Beijing Fine Chemical Co. Ltd., China. Set-up for measuring vapor pressure of ZrCl4 was especially designed due to its high sublimation point (331 ℃) and corrosivity. The set-up is mainly composed of controllable crucible resistance furnace, vacuum pump, pressure gauge, stainless steel vaporizer, etc, as shown in Fig.1.
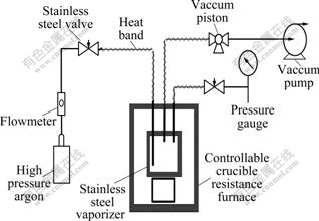
Fig.1 Set-up for measuring vapor pressure of ZrCl4
2.2 Vapor pressure of ZrCl4
Measurement of ZrCl4 vapor pressure under vacuum was as follows. At the first step, vaporizer was vacuumized for 30 min, and then heated at a rate of 5 ℃/min up to 340 ℃. It was isothermally treatment of 30 min every 20 ℃ interval and corresponding temperature and pressure were recorded. At the second step, a certain amount of ZrCl4 powder was loaded in the vaporizer, and the subsequent procedure was the same as that at the first step.
Measurement of ZrCl4 vapor pressure under an argon pressure of 1×105 Pa was as follows. At the first step, vaporizer was vacuumized for 30 min, then argon was introduced to 1×105 Pa, and subsequent procedure was the same as that under vacuum.
2.3 Thermochemical change of ZrCl4
The thermodynamic change of ZrCl4 during evaporation was studied by thermogravimetry- differential thermal analysis (TG-DTA, Netzsch STA 449c, Selb, Germany) in an argon atmosphere at a heating rate of 5 ℃/min. The composition analyses of ZrCl4 sample before and after evaporation were determined by X-ray diffraction(XRD) pattern from a Guinier-H?gg camera (D/MAX-ⅢB, Rigaku, Tokyo, Japan) with Cu Κα radiation and Si as an internal standard. The particle size, morphology and element contents of ZrCl4 sample before and after evaporation were characterized by a scanning electron microscope (S-3000N, Hitachi, Tokyo, Japan) coupled with an energy dispersive X-ray analysis system (PV7746121 ME, Sapphire, Tokyo, Japan). Finally, the reaction mechanism of ZrCl4 during evaporation was studied by thermodynamic calculation.
3 Results and discussion
Figs.2 and 3 show the vapor pressure of ZrCl4 under vacuum and an argon pressure of 1×105 Pa. By considering mixture of gas (or vapor) in vaporizer as ideal gas, the total pressure of mixture in vaporizer is equal to the sum pressure of each gas (or vapor) according to Dalton partial pressure law. So, vapor pressure of ZrCl4 is equal to the pressure difference of the first step and the second step, as shown in Figs.2 and 3.
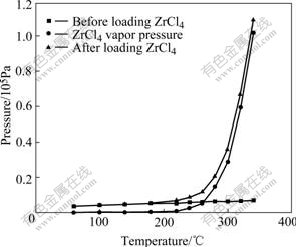
Fig.2 Measurement results of vapor pressure under vacuum
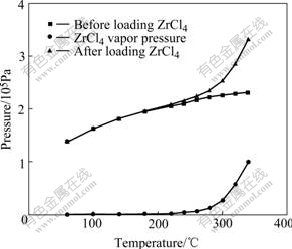
Fig.3 Measurement results of vapor pressure under argon pressure of 1×105 Pa
From Fig.2, between room temperature and 200 ℃, vapor pressure of ZrCl4 is very low and there is an obvious increase at about 210 ℃. This is because ZrCl4 sample is inevitably hydrolyzed to some extent due to absorption of water in air. Hydrolyzing product losts its bonded water and absorbed heat at about 210 ℃, which is confirmed by the obvious thermal mass loss in TG curve and the big endothermic peak in DTA curve at 210 ℃ (Fig.4). Above 210 ℃, vapor pressure increases quickly with the increase of temperature. The higher the temperature is, the quicker the vapor pressure increases. When the temperature reaches 340 ℃, vapor pressure pasts 1×105 Pa. From the DTA curve, there is a sharp endothermic peak at about 331 ℃ with a considerable mass loss in the TG curve. The endothermic peak at 331 ℃ is caused by the sublimation of ZrCl4. Pressure between 210 ℃ and 331 ℃ is mainly caused by ZrCl4 vapor. Besides, there are also some water vapor and chlorine hydride produced by dehydration of ZrOCl2·8H2O.
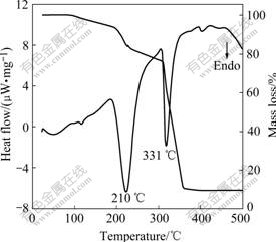
Fig.4 Thermogravimetric and differential thermal analysis curves of ZrCl4
By considering ZrCl4 vapor, HCl gas and water vapor as ideal gas, the total pressure(p) is linear with temperature(t) when the total molecules (HCl gas, ZrCl4 and water vapor) are constant according to Clapeyron equation:
pV=nRT (1)
where p is pressure; V is volume of gas (or vapor); n is molecules of the gas; R is a constant (8.314 J/(mol·K) for ideal gas) and T is thermodynamic temperature. During evaporation process, molecules of ZrCl4 vapor increase with increasing temperature and finally lead to a quick increase of vapor pressure.
From Fig.3, vapor pressure of ZrCl4 under an argon pressure of 1×105 Pa is similar to that under vacuum and passes 1×105 Pa at about 340 ℃. The similar changes can be found at about 210 ℃ and 340 ℃ as those under vacuum. Thus, it can be concluded that the same reactions happen at above temperatures in both cases.
Comparison of ZrCl4 vapor pressure under vacuum and an argon pressure of 1×105 Pa is shown in Fig.5. It can be seen that the increase rates of ZrCl4 vapor pressure with temperature in both cases are similar. The value under vacuum is a little larger than that under an argon pressure of 1×105 Pa at the same temperature. This is because external pressure makes it difficult for ZrCl4 molecule to escape from zirconium salt.
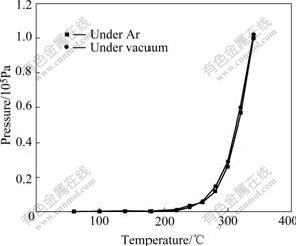
Fig.5 Vapor pressure of ZrCl4 at different temperatures
It is necessary to determine whether vapor pressure satisfies the requests of ZrCl4 vapor route to prepare ZrC coating of coated fuel particles. In terms of typical design parameters of coated fuel particles[16] (Table 1) and optimal process conditions of fluidized bed[17] (Table 2), flow rate of ZrCl4 is calculated to be 4.5 g/min. Whereas concentration of ZrCl4 vapor at 340 ℃ is calculated to be 4.64 g/L by Clapeyron equation. It is easy to satisfy the requests of ZrCl4 vapor route at such high vapor concentration. On the other hand, the concentration of ZrCl4 vapor varies with heating temperature. Thus, flow rate of ZrCl4 can be adjusted by controlling heating temperature of ZrCl4 and flow rate of carrier gas to obtain high performance ZrC coating of coated fuel particles.
Table 1 Typical design parameters of coated fuel particle

Table 2 Optimal process conditions of fluidized bed

SEM images of ZrCl4 sample before and after evaporation are shown in Fig.6. The particle sizes of ZrCl4 sample before evaporation distribute over 1-3 μm with spherical morphology and white color. After evaporation the particle appears to be in irregular shape with a size of 4-7 μm, grey color and several agglomerations. The observations in two images indicate that the composition of ZrCl4 sample before and after evaporation is changed. EDX semi-quantitative analysis results show that the ratio of Zr to Cl of ZrCl4 sample before evaporation is close to 1?4, and the ratio of Zr to O is close to 1?2 after evaporation. The result further indicates that the main composition of sample changes from ZrCl4 to ZrO2.

Fig.6 SEM images of ZrCl4 sample before(a) and after(b) evaporation
Fig.7 shows the XRD patterns of ZrCl4 sample before and after evaporation, respectively. Only three small diffraction peaks appear in Fig.7(a), which correspond to ZrCl4. There are several sharp diffraction peaks in Fig.7(b) corresponding to monoclinic ZrO2 (2θ= 28.180?, 31.468?, 34.147?) and tetragonal ZrO2 (2θ= 30.244?, 50.250?, 60.259?). ZrO2 is hydrolyzation product of ZrCl4 via following reactions:
ZrCl4+9H2O→ZrOCl2·8H2O+2HCl (2)
ZrOCl2·8H2O→ZrOCl2+8H2O (3)
ZrOCl2+H2O→ZrO2+2HCl (4)
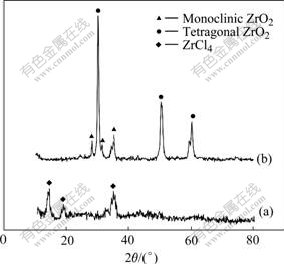
Fig.7 XRD patterns of ZrCl4 sample before(a) and after(b) evaporation
ZrCl4 sample is hydrolyzed to some extent. However, the amount is so small that it almost has no influence on vapor pressure of ZrCl4 at high temperature, which can also be seen from the remaining quantity of ZrCl4 sample after evaporation. It should be noted that a considerable mass loss appears at about 210 ℃ corresponding to the dehydration of ZrOCl2·8H2O in Fig.4. This is because ZrCl4 sample in TG measurement is very little and has large specific contact area with argon, which is different from actual evaporation process. So some measurements could be taken to obtain high performance ZrC coating during evaporation of ZrCl4, such as pumping for a few minutes at about 210 ℃ to remove HCl and H2O in ZrCl4 vapor.
No zirconium halide phases, such as ZrCl3 and ZrCl2, are found in Fig.7, indicating that no decomposition of ZrCl4 happens during evaporation by following reactions:
2ZrCl4→2ZrCl3+Cl2 (5)
ZrCl4→ZrCl4+Cl2 (6)
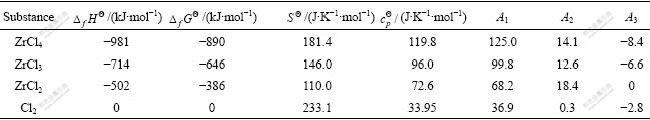
We can factually discuss the probability of reactions (5) and (6) in temperature range of evaporation by thermodynamic calculation. The main thermodynamic parameters are listed in Table 3. For reaction (5), if it happens in standard state (1×105 Pa, 298.15 K), the Gibbs energy change is
![]()
![]()
4.88 kJ/mol>0 (7)
Table 3 Thermodynamic parameters of some substance[18]
If it happens at 1×105 Pa and T, the Gibbs energy change is
![]() (8)
(8)
Enthalpy change ![]() and entropy change
and entropy change ![]() can be presented by
can be presented by
![]() (9)
(9)
![]() (10)
(10)
where ![]() and
and ![]() can be calculated:
can be calculated:
![]()
![]()
534 kJ/mol (11)
![]()
![]()
162 J/(K?mol) (12)
![]() is determined by A1, A2 and A3:
is determined by A1, A2 and A3:
![]() A1+A2×10-3 T+A3×10-5 T -2 (13)
A1+A2×10-3 T+A3×10-5 T -2 (13)
After integration of reactions (8)-(13), the Gibbs energy change for reaction (5) at 753.15 K is
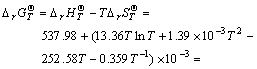
415.19 kJ/mol>0 (14)
From Eqns.(7) and (14), it can be seen that Gibbs energy change between 298.15 K and 753.15 K is above zero, which indicates that the reaction (5) will not happen spontaneously during evaporation.
The same as above calculations, the Gibbs energy changes of reaction (6) at 298.15 K and 753.15 K are shown as follows:
![]() =1 008 kJ/mol>0 (15)
=1 008 kJ/mol>0 (15)
![]() 537.48 kJ/mol>0 (16)
537.48 kJ/mol>0 (16)
Obviously reaction (6) will not happen spontaneously during vaporization, either.
4 Conclusions
1) Vapor pressure of ZrCl4 at 340 ℃ under vacuum and an argon pressure of 1×105 Pa exceeds 1×105 Pa, which can satisfy the requests of ZrCl4 vapor route for preparing ZrC coating of coated fuel particles.
2) Flow rate of ZrCl4 can be precisely adjusted by controlling heating temperature of ZrCl4 and flow rate of carrier gas.
3) ZrCl4 sample is hydrolyzed to some extent to give ZrO2 and HCl during evaporation, which however, has little influence on vapor pressure of ZrCl4 at high temperature. According to thermochemical properties of ZrCl4 during evaporation, some measurements could be taken to obtain high performance ZrC coating.
4) Both experimental and thermodynamic calculation results suggest that ZrCl4 doesn’t decompose to give ZrCl3 and Cl2 during evaporation.
References
[1] Editing Committee of Rare Metals. Handbook of rare metals [M]. Beijing: Metallurgical Industry Press, 1992: 274-280. (in Chinese)
[2] KUMAR V, KAUR S, KUMAR S. ZrCl4 catalyzed highly selective and efficient michael addition of heterocyclic enamines with α, β-unsaturated olefins [J]. Tetrahedron Letters, 2006, 47(39): 7001-7005.
[3] BABU K S, RAJU B C, SRINIVAS P V. Highly efficient and chemoselective cleavage of prenyl ethers using ZrCl4/NaBH4 [J]. Tetrahedron Letters,2003, 44(12): 2525-2528.
[4] SMITHA G, REDDY C S. ZrCl4-catalyzed aza-michael addition of carbamates to enones: Synthesis of Cbz-protected β-amino ketones [J]. Catalysis Communications,2007, 8(3): 434-436.
[5] WANG Ge-hua. Energy and sustainable development [M]. Beijing: Chemical Industry Press, 2005: 26-30. (in Chinese)
[6] MA Xu-quan. Development and application of nuclear energy [M]. Beijing: Chemical Industry Press, 2005: 6-23. (in Chinese)
[7] VENKATESH C, VADAKKAPATTU, LEE T W. Multi-dimensional simulations of the chemical vapor deposition for thermal barrier coatings using ZrCl4-H2-CO2-Ar gas mixtures [J]. Surface and Coatings Technology,2006, 201(3/4): 1065-1073.
[8] SAKAMURA Y, INOUE T, IWAI T. Chlorination of UO2, PuO2 and rare earth oxides using ZrCl4 in LiCl-KCl eutectic melt [J]. Journal of Nuclear Materials,2005, 340(1): 39-51.
[9] REYNOLDS G H, Chemical vapor deposition of ZrC on pyrocarbon-coated fuel particles [J]. J Nucl Mater, 1974, 50(2): 215-216.
[10] IKAWA K, IWAMOTO K. Coating microspheres with zirconium carbide [J]. J Nucl Mater, 1972, 45(1): 67-68.
[11] IKAWA K, IWAMOTO K. Coating microspheres with zirconium carbide-carbon alloy by iodide process [J]. J Nucl Sci and Tech, 1974, 11(6): 263-267.
[12] REYNOLDS G H. Chemical vapor deposition of isotropic carbon-zirconium carbide particle coatings [J]. J Nucl Mater, 1975, 56(2): 239-242.
[13] HOLLABAUGH C M. A new method for coating microspheres with zirconium carbide and zirconium carbide-carbon graded coats [J]. J Nucl Mater, 1975, 57(3): 325-326.
[14] WALLACE T C. Chemical vapor deposition of ZrC in small bore carbon-composite tubes [J]. Chemical Vapor Deposition, 1973, 4: 91-106.
[15] IKAWA K, IWAMOTO K. Coating microspheres with zirconium carbide-carbon alloy [J]. J Nucl Mater, 1974, 52(1): 128-130.
[16] TANG Chun-he, TANG Ya-ping. Design and manufacture of the fuel element for the 10MW high temperature gas-cooled reactor [J]. Nucl Eng Des, 2002, 218: 91-102.
[17] LI Qi-ming. Chemical vapor deposition of composite gradient coatings for coated fuel particles [D]. Beijing: Tsinghua University, 2000. (in Chinese)
[18] DEAN J A. Lange’s handbook of chemistry [M]. New York, USA: McGrqw-Hill Education Co., 1921: 6.142-6.148.
Foundation item: Project(863614202) supported by the National High-tech Research and Development Program of China
Corresponding author: LIU Chao; Tel: +86-10-89796095; E-mail: chao-liu04@mails.tsinghua.edu.cn


