网络首发时间: 2019-07-29 19:29
基于锆铪溶剂萃取分离的萃取剂性能
王俊莲 付家帅 许文 王化军
北京科技大学土木与资源工程学院
摘 要:
溶剂萃取法是目前分离核级锆铪的主要方法。已工业化核级锆铪萃取分离技术MIBK(methyl isobutyl ketone,甲基异丁基酮)-硫氰化氢(HSCN)法、TBP(tributyl phosphate,磷酸三丁酯)-HNO3 法和TOA(trioctyl amine,三辛基胺)-H2 SO4 法均存在不足。学者们一直在努力研究和开发新的萃取剂,以实现核级锆铪的绿色高效分离。总结了自2000年来基于锆铪分离的萃取剂性能研究。按照萃取剂的结构特征将其分为中性萃取剂、酸性萃取剂和胺类萃取剂。中性萃取剂包括酮类萃取剂DIBK(diisobutyl ketone,二异丁基酮)和三烷基氧膦类萃取剂TOPO(trioctyl phosphine oxide,三辛基氧化膦)/Cyanex 921,Cyanex 923&Cyanex 925。酸性萃取剂包括有机磷/膦酸类萃取剂P204,P507,Cyanex 272,硫代膦酸类萃取剂Cyanex301,Cyanex 302,羟肟酸类萃取剂LIX 63,LIX 84-IC和羧酸类萃取剂Versatic acid 10。将含氨基基团的萃取剂均归于胺类萃取剂,包括季铵盐类萃取剂Aliquat 336,三烷基胺类萃取剂Alamine 300/TOA,Alamine 336,Alamine 308和TEHA(tri(2-ethylhexyl)amine,三(2-乙基己基)胺),含氨基的磷酸酯类萃取剂BEAP(bis(2-ethylhexyl)-1-(2-ethylhexylamino)propylphosphonate,二(2-乙基己基)-1-(2-乙基己基氨基)丙基磷酸酯),双酰胺荚蒾类萃取剂TODGA(N,N,N',N'-tetraoctyldiglycolamide,N,N,N',N'-四辛基-3-氧杂戊二酰胺)和异唑酮类萃取剂HPBI(3-phenyl-4-benzoyl-5-isoxazolone,3-苯基-4-苯甲酰基-5-异唑酮)、HTBI(3-phenyl-4-(4-toluoyl)-5-isoxazolone,3-苯基-4-(4-甲苯酰基)-5-异唑酮)、HFBTI(3-phenyl-4-(4-fluorobenzoyl)-5-isoxazolone,3-苯基-4-(4-氟代苯甲酰基)-5-异唑酮)。介绍了各类萃取剂萃取分离锆铪的主要性能及优缺点。
关键词:
锆 ;铪 ;萃取剂 ;溶剂萃取 ;分离 ;
中图分类号: TF841.4
作者简介: 王俊莲(1981-),女,河北廊坊人,博士,副教授,研究方向:有色金属湿法分离及二次资源回收,电话:18810496048,E-mail:wangjunlian306@163.com;
收稿日期: 2019-03-09
基金: 国家自然科学基金项目(51974026); 国家重点研发计划项目(2018YFC1900604)资助;
Performance of Various Extractants for Zirconium and Hafnium Separation by Solvent Extraction
Wang Junlian Fu Jiashuai Xu Wen Wang Huajun
Civil and Resource Engineering School,University of Science and Technology Beijing
Abstract:
Solvent extraction has always been the main Zr/Hf separation method. However,all the existing industrialized Zr/Hf solvent extraction separation methods,namely MIBK(methyl isobutyl ketone)-HSCN,TBP(tributylphosphate)-HNO3 and TOA(trioctylamine)-H2 SO4 have shortages. To realize Zr/Hf environment-friendly and efficient separation,novel extractants have always been studied. This paper summarized the studies of extractants on Zr/Hf separation since 2000. All the extractants were classified into three groups according to their structural characteristics. They were neutral extractants,acidic extractants and amine-based extractants. The neutral extractants included the ketone DIBK(diisobutyl ketone)and the trialkylphosphine oxides TOPO(trioctyl phosphine oxide)/Cyanex 921,Cyanex 923 and Cyanex 925. The acidic extractants included the organophosphoric/phosphonic/phosphinic acids P204,P507 and Cyanex 272,the thiosubstituted phosphinic acids Cyanex 301 and Cyanex 302,the hydroximic acids LIX 63,LIX 84-IC and the carboxylic acid Versatic acid 10. As to the amine-based extractant,it refered to all the extractans that contained amino group in this paper,concluding the quaternary ammonium salt Aliquat 336,the trialkylamines Alamine 300/TOA,Alamine 336,Alamine308 andtri(2-ethylhexyl)amine(TEHA),the neutral organophosphorous compound containing the amino groupbis(2-ethylhexyl)-1-(2-ethylhexylamino)propylphosphonate(BEAP),the amido podand N,N,N',N'-tetraoctyldiglycolamide(TODGA)and the isoxazolones 3-phenyl-4-benzoyl-5-isoxazolone(HPBI),3-phenyl-4-(4-toluoyl)-5-isoxazolone(HTBI)and 3-phenyl-4-(4-fluorobenzoyl)-5-isoxazolone(HFBTI). Their Zr/Hf separation performance and the main advantages and disadvantages were summarized.
Keyword:
zirconium; hafnium; extractants; solvent extraction; separation;
Received: 2019-03-09
锆铪是核军工、核电站等原子能工业不可或缺的高性能稀有金属材料。锆、铪对热中子吸收截面的巨大差异,导致锆铪在原子能领域的应用完全不同。高纯锆热中子吸收截面小(0.18 b),被用于反应堆的结构材料、铀燃料棒包壳材料等;高纯铪热中子吸收截面大(120 b),是小型热中子反应堆控制材料的首选。目前几乎所有的核潜艇、核动力航母等所用水冷反应堆均用高纯铪作控制棒。自然界中锆铪常常共存,铪含量为锆的1%~3%。锆铪必须深度分离,获得核级锆铪(要求锆中铪含量<100×10-6 )才能应用于原子能工业中。锆、铪同属IVB族金属元素,物理化学性质极为相近,离子半径近乎相同,分离极为困难。
随着我国军工、核电的快速发展及航母等军事舰艇、军事装备的升级换代,我国对核级锆、铪的总需求量快速增长。一艘3万马力的核潜艇所用的锆合金就达20~30 t。一个百万千瓦级压水堆核电站首次装料的核燃料所需锆材约为32 t,高燃耗反应堆每次换料大约需要8 t核级锆材。根据《2020~2030年中国电力供需及战略布局展望》,核电装机规划2020年达到5800万千瓦左右,2030年达到2.0亿千瓦,2050年4.0亿千瓦,届时核级锆材的年需求量将超过1万t。
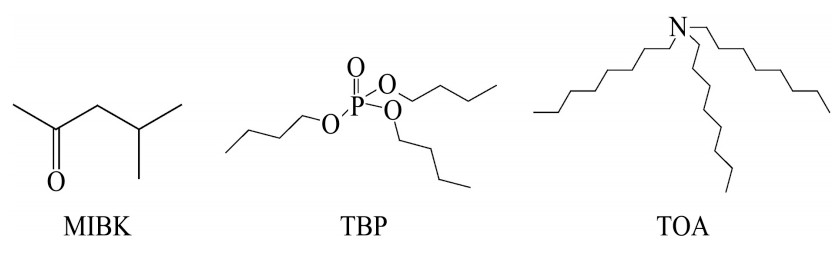
核级锆铪分离技术长期被国外垄断,我国依赖进口或花重金购买国外专利和生产工艺。美国甲基异丁基酮(methyl isobutyl ketone,MIBK)-硫氰化氢(HSCN)法生产的海绵锆约占全球2/3市场份额,法国塞佐斯公司利用ZrCl4 和HfCl4 在熔盐AlCl3 /KCl中饱和蒸气压的差异经精馏获得高纯ZrCl4 (亦称CEZUS法),该法每年生产的核级海绵锆约为2000 t。此外,法国磷酸三丁酯(tributyl phosphate,TBP)-HNO3 法和日本三辛基胺(trioctyl amine,TOA)-H2 SO4 法也是已工业化的核级锆铪分离技术,但所生产的核级海绵锆占全球市场份额很小。这些核级锆铪分离技术均存在不足,如MIBK-HSCN法MIBK水溶性大易损失,闪点低易燃,气味大操作环境差,且会产生高浓度含氨氮、SCN- 、CN- 和有机质的废水;CEZUS法属于火法分离,对设备耐腐蚀性要求高,连续抽送、控制蒸汽流和防止空气湿度污染等技术复杂,投资大,分离系数小(βZr/Hf ≈2),至少需要90级塔板才能获得核级锆、铪产品;TBP-HNO3 -HCl法容易乳化,体系酸度大,导致试剂消耗量大和设备腐蚀性强,生产成本是MIBK-HSCN法2倍;TOA-H2 SO4 法萃取容量小,工艺流程长,分离系数小(βZr/Hf ≈8~10)
[1 ,2 ,3 ]
。萃取剂MIBK,TBP和TOA结构式如图1所示。
开发具有我国自主知识产权的核级锆铪绿色高效分离技术,是打破国外核级锆铪分离技术垄断、保障我国国防安全、满足日益增长的电力需求和成为世界核电强国的关键。学者们在这方面做了很多研究工作。锆铪分离有诸多方法,如熔盐萃取法
[4 ,5 ]
、熔盐精馏法
[6 ]
、分步结晶法、离子交换法
[7 ]
、溶剂萃取法等。其中溶剂萃取法处理量大、设备简单、成本低、易于实现生产连续化,是核级锆铪分离的主要方法。萃取剂是开发新型核级锆铪萃取分离体系、实现核级锆铪绿色高效分离的关键。将对用于锆铪分离的萃取剂进行分类阐述。
1 中性萃取剂
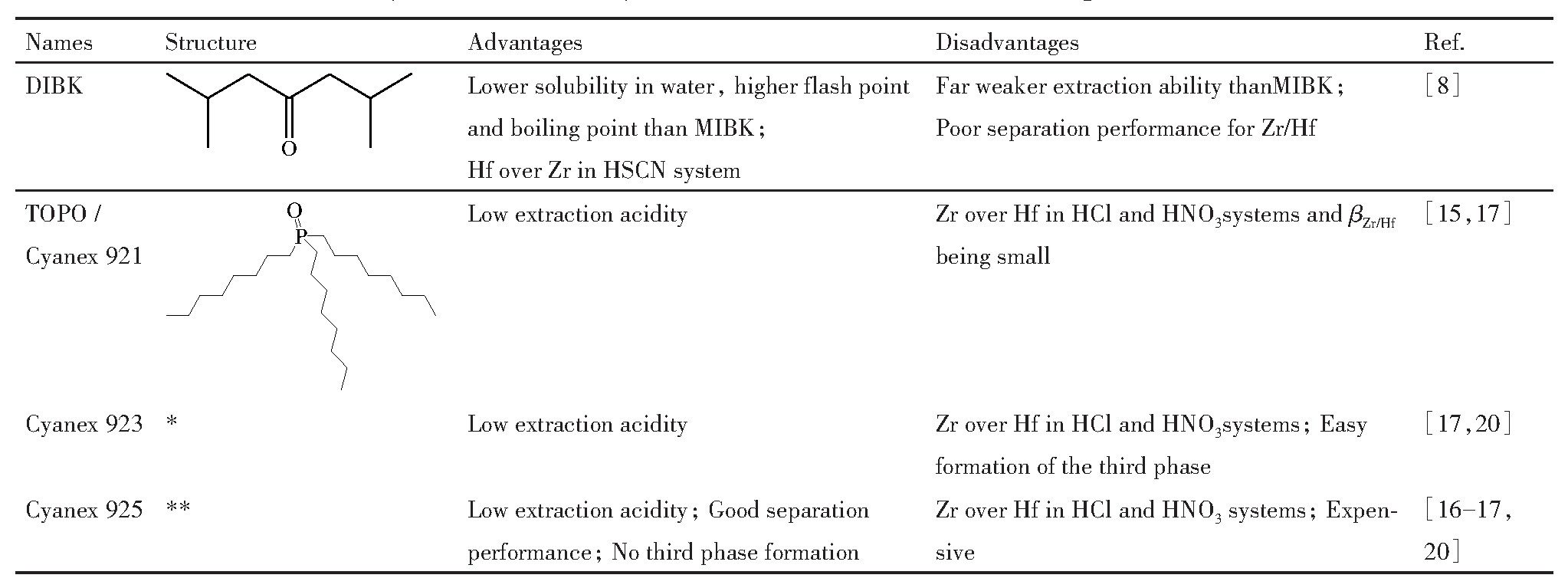
1.1 酮类萃取剂
针对MIBK水溶性大、闪点低、易燃的缺点,开发二异丁基酮(diisobutyl ketone,DIBK)-HSCN萃取体系
[8 ]
。DIBK比MIBK水溶性小,闪点高、沸点高,可减少萃取过程中萃取剂损失,改善操作环境。HSCN体系下,DIBK优先萃取Hf,但萃取能力远小于MIBK,锆铪分离系数很小(βHf/Zr =1.23)。HCl,HNO3 和H2 SO4 体系下,DIBK优先萃取Zr,萃取能力远小于HSCN体系下对Zr,Hf的萃取能力,且锆铪分离系数亦很小(βZr/Hf <1.4)。为解决DIBK-HSCN法对锆铪萃取能力弱、分离系数小的不足,徐志高课题组
[9 ,10 ,11 ]
进一步研究了DIBK-P204和DIBK-TBP混合萃取体系分离Zr、Hf的性能。HSCN体系下,DIBK-P204和DIBK-TBP均优先萃取Hf,锆铪分离系数βHf/Zr 分别可达到10.5和9.3。
HSCN体系下,HfO2+ 与SCN- 生成的络合物Hf(OH)2 (SCN)2 稳定性优于ZrO2+ 与SCN- 生成的络合物Zr(OH)2 (SCN)2 ,故HfO2+ 优先被萃入有机相。该体系下,酮类萃取剂萃取锆铪为溶剂化萃取机制。MIBK萃取HfO2+ 的萃合物结构为Hf(OH)2 (SCN)2 ·2MIBK(低酸度下)或Hf(SCN)4 ·2MIBK(高酸度下)
[12 ]
;DIBK-P204混合萃取剂体系萃取ZrO2+ 和HfO2+ 的萃合物结构分别为Zr(SCN)3 ·HA2 ·DIBK和Hf(SCN)3 ·HA2 ·DIBK
[13 ]
;DIBK-TBP混合萃取剂体系萃取ZrO2+ 和HfO2+ 的萃合物结构分别为Zr(SCN)4 ·TBP·DIBK和Hf(SCN)4 ·TBP·DIBK
[14 ]
。
图1 萃取剂MIBK,TBP和TOA结构式
Fig.1 Extractant structures of MIBK,TBP and TOA
酮类萃取剂于HSCN体系下优先萃取Hf,萃取分离锆铪过程中传质少,但会产生高浓度含氨氮、SCN- 和CN- 的废水,造成环境污染。
1.2 三烷基氧膦类萃取剂
针对中性磷类萃取剂TBP-HNO3 -HCl法分离锆铪易乳化、体系酸度高的不足,研究了三烷基氧膦类萃取剂三辛基氧化膦(trioctyl phosphine oxide,TOPO)/Cyanex 921,Cyanex 923,Cyanex 925分离锆铪新体系。TOPO是氰特公司产品Cyanex 921的主要成分。HCl体系下,TOPO优先萃取Zr,较佳的水相酸度为2.5~4.0 mol L-1 HCl,锆铪分离系数βZr/Hf 可达到6.1
[15 ]
;Cyanex 925对Zr,Hf的最佳分离酸度为3.5 mol·L-1 HCl,亦是优先萃取Zr,βZr/Hf 可达37
[16 ]
。HNO3 体系下,Cyanex 921,Cyanex 923和Cyanex 925对Zr,Hf的萃取率均随HNO3 浓度的增大先增大后减小,在2 mol·L-1 HNO3 处达到最大值。3种萃取剂均优先萃取Zr,萃取能力顺序为Cyanex925>Cyanex 921>Cyanex 923。最优条件下,Cyanex921,Cyanex 923和Cyanex 925对锆铪分离系数βZr/Hf 分别为17.0,21.4和40.7。萃取容量为0.4 mol·L-1 Cyanex 925可负载0.69 g·L-1 Zr和0.22 g·L-1 Hf
[17 ]
。
三烷基氧膦类萃取剂萃取锆铪亦为溶剂化萃取机制。HNO3 体系下,萃合物的结构通式为ZrO(NO3 )2 ·x L和HfO(NO3 )2 ·x L
[17 ]
;HCl体系下,萃合物的结构通式为ZrCl4 ·x L和HfCl4 ·x L
[16 ,18 ]
。其中x表示萃合物中萃取剂的分子数,L表示三烷基氧膦类萃取剂分子,如Cyanex 921,Cyanex 923,Cyanex 925等。
基于三烷基氧膦的混合萃取剂体系,P204萃取能力太强,导致3~4 mol·L-1 HCl酸度下TOPO-P204体系下锆铪分离性能非常差;Aliquat 336,Alamine 336,Alamine 308是胺类萃取剂,低酸度(<5mol·L-1 HCl)下对Zr,Hf无萃取能力,导致TOPO-Aliquat 336,TOPO-Alamine 336,TOPO-Alamine308混合萃取体系对Zr,Hf萃取效率变差
[15 ]
。TOPO-P507混合萃取体系在HNO3 体系中亦优先萃取Zr。当水相酸度为2 mol·L-1 HNO3 时,βZr/Hf 随TOPO/P507比值增大而增大,可达17.8
[19 ]
。伊朗核科学技术研究所Taghizadeh课题组
[20 ]
研究了Cyanex923-TBP混合萃取体系HNO3 溶液中萃取分离Zr,Hf性能。相比TBP-HNO3 法,水相酸度由7 mol·L-1 降至3.5 mol·L-1 ,萃取容量增大30%以上,锆铪分离系数βZr/Hf 可高达186。
TOPO/Cyanex 921,Cyanex 923和Cyanex 925在HCl和HNO3 体系下萃取酸度均低于TBP-HNO3 -HCl体系,3种萃取剂均优先萃取Zr,萃取过程中传质大。此外,TOPO/Cyanex 921分离系数小(TBP-HNO3 -HCl法βZr/Hf 为30~40
[3 ]
),Cyanex 923萃取过程中易产生第三相
[20 ]
,Cyanex 925是国外公司产品,萃取剂成本较高。Cyanex 923-TBP混合萃取体系水相酸度比TBP-HNO3 法低,不易乳化,且锆铪分离系数高。然而混合萃取体系在长期的萃取分离工业生产中很难维持混合萃取体系中各萃取剂之间的固定比例。中性萃取剂的结构式及萃取分离Zr,Hf优缺点总结于表1中。
2 酸性萃取剂
这类萃取剂包括有机磷/膦酸类萃取剂(P204,P507,Cyanex 272)、硫代膦酸类萃取剂(Cyanex301,Cyanex 302)、羟肟酸类萃取剂(LIX 63,LIX84-IC)、羧酸类萃取剂(Versatic acid 10)等。
2.1 有机磷/膦酸类萃取剂
有机磷/膦酸类萃取剂是研究和应用最多、最广的一类萃取剂。HCl体系下,P204,P507和Cyanex272均优先萃取Zr。在HCl浓度介于1~4 mol·L-1 时,属于溶剂化作用机制。低酸度下(1 mol·L-1 HCl),Zr,Hf萃取率P204>P507>Cyanex 272;随着HCl浓度增大(2~4 mol·L-1 HCl),Zr,Hf萃取率均增大,Zr,Hf萃取率P507>P204>Cyanex 272。相同条件下,Cyanex 272对Zr,Hf分离性能最好,βZr/Hf 可达8.7,而P204仅为1.9,P507为4.6。这类萃取剂与LIX 63,Versatic acid 10,Alamine 336和Ali-quat 336的混合萃取体系均呈现反协同效应,但LIX 63的加入可提高Zr,Hf分离系数。P204-LIX63,P507-LIX 63和Cyanex 272-LIX 63混合体系对Zr,Hf分离系数分别可提高至8.0,8.2和9.7
[21 ]
。
表1 中性萃取剂的结构式及萃取分离Zr,Hf优缺点 下载原图
Table 1 Structures,advantages and disadvantages of neutral extractants for Zr/Hf separation
*Cyanex 923:A mixture of trialkylphosphine oxides R3 PO(14%),R2 R'PO(42%),RR'2 PO(31%)and R’3 PO(8%),where R standing for n-octyl,R'denotes n-hexyl.Its average molecular weight being 348 g·mol-1 ;**Cyanex 925:A mixture of trialkylphosphine oxides R3 PO and R2 R'PO,where R standing for 2,4,4’-trimethylpentyl,R’denotes n-octyl.Its average molecular weight being 386 g·mol-1
HNO3 体系下,P204,P507和Cyanex 272均优先萃取Zr,萃取能力P204>P507>Cyanex 272。对Zr,Hf分离性能P204<P507<Cyanex 272,分离系数βZr/Hf 分别可达12.9,13.8和14.2。P507与TBP,Versatic acid 10,TOA的混合萃取体系对Zr,Hf萃取分离性能影响不大;P507-LIX 63混合萃取体系在HNO3 浓度>2 mol·L-1 时,对Zr呈现正协同效应,对Hf呈现反协同效应,当水相酸度为2 mol·L-1 HNO3 时,βZr/Hf 最大,可达46
[19 ,22 ]
。伊朗核科学与技术研究院Torab-Mostaedi课题组
[23 ]
的研究表明,0.5%Cyanex 272-20%TBP(v/v)混合萃取体系对Zr呈现正协同效应,对Hf呈现反协同效应,水相酸度为2.5 mol·L-1 HNO3 时,βZr/Hf 可达到99.7。HNO3 体系下为溶剂化萃取机制,萃合物结构为ZrO(NO3 )2 ·2HA
[19 ]
。
H2 SO4 体系下,P204,P507和Cyanex 272均优先萃取Hf
[24 ,25 ,26 ]
。水相酸度在0.5~6 mol?L-1 H2 SO4 下,Hf,Zr萃取率随着H2 SO4 浓度增加而减小,萃取能力P204>>Cyanex 272。3 mol?L-1 H2 SO4 下,Cyanex272对Hf,Zr分离系数βHf/Zr 可达9.6,此时Hf,Zr萃取率分别仅为22.5%和2.9%;相同条件下P204对Hf,Zr分离系数βHf/Zr 为12.9,此时Hf,Zr萃取率分别为84.7%和30.1%
[21 ]
。然而生产实践中P204-H2 SO4 锆铪分离分离系数仅为2~4
[6 ]
。H2 SO4 体系下,萃取机制为阳离子交换机制
[27 ,28 ]
。P204-LIX63和Cyanex272-LIX63混合萃取体系对Hf,Zr呈现反协同效应
[21 ]
。
有机磷/膦酸类萃取剂在HCl和HNO3 体系下,优先萃取Zr,萃取过程中传质大,且锆铪分离系数小;H2 SO4 体系下,优先萃取Hf,但分离系数小。P204萃取能力强,反萃酸度高;Cyanex 272对Hf,Zr萃取能力弱。
2.2 硫代膦酸类萃取剂
印度斯温卡泰斯瓦拉大学Reddy课题组
[29 ,30 ,31 ]
和伊朗核科学技术研究院Kamal课题组
[32 ]
研究了Cyanex 301和Cyanex 302萃取Zr、Hf性能和机制。HCl体系下,当水相酸度<0.1 mol·L-1 时,Cyanex301和Cyanex 302均优先萃取Hf,且随着HCl浓度增大,Zr,Hf萃取率降低。萃取机制属于阳离子交换机制,萃合物组成分别为ZrOA2 和HfOA2 。当水相酸度为0.01 mol·L-1 HCl时,对Zr萃取能力Cyanex 301>Cyanex 302,萃取容量Cyanex 301<Cyanex302。负载Hf的Cyanex 301有机相,用8 mol·L-1 H2 SO4 才能将Hf反萃完全,用8 mol·L-1 HCl反萃时,反萃率仅为60%。负载Hf的Cyanex 302有机相,也需用8 mol·L-1 H2 SO4 才能反萃完全。Cyanex301对Hf,Zr最大分离系数βHf/Zr 为7.0。
硫代膦酸类萃取剂Cyanex 301,Cyanex 302的配位原子S电负性小,属于软配体,而Zr(IV),Hf(IV)属于硬离子,理论上这类萃取剂不适于锆铪分离,分离锆铪性能不及有机磷/膦酸类萃取剂。
2.3 羟肟酸类萃取剂
羟肟酸类萃取剂LIX 63在HNO3 体系下优先萃取Zr,萃取能力远小于P507。HNO3 体系下,Zr,Hf萃取率随HNO3 浓度的增大先增大后减小。低酸度下(<1.5 mol·L-1 ),属于溶剂化萃取机制,萃合物结构分别为Zr(NO3 )2 ·2LIX 63和Hf(NO3 )2 ·2LIX 63。水相酸度为2 mol·L-1 HNO3 时,Zr,Hf分离效果最好,βZr/Hf 可达186.3。反萃困难,1~7 mol·L-1 的HCl和H2 SO4 均不能实现Zr,Hf完全反萃
[22 ]
。HCl体系下,LIX 63亦是优先萃取Zr,萃取能力远远小于Cyanex 272
[21 ]
。
LIX 84-IC在HCl体系中,优先萃取Hf,Hf,Zr萃取率随HCl浓度增加先迅速减小而后缓慢增大,在1 mol·L-1 HCl处达到最小值。低酸度下(<1 mol·L-1 ),属阳离子交换机制,萃合物结构分别为ZrOA2 和HfOA2 ;高酸度下为溶剂化萃取机制。0.1 mol·L-1 LIX 84-IC有机相在水相酸度为0.01 mol?L-1 HCl对Zr的萃取容量仅为75.2 mg·L-1 ,1~8 mol·L-1 HCl和H2 SO4 对有机相中负载的Zr反萃率仅为28%左右
[33 ,34 ]
。
羟肟酸类萃取剂LIX 63和LIX 84-IC对锆铪萃取能力很弱,萃取容量小,反萃困难。
2.4 羧酸类萃取剂
羧酸类萃取剂Versatic acid 10对Zr,Hf萃取能力很弱,单独萃取分离锆铪的研究很少。在HCl体系下,Versatic acid 10优先萃取Zr。随HCl浓度的增大,Zr,Hf萃取率均减小,属于阳离子交换机制,萃合物组成分别为ZrOA2 ·2HA和HfA4
[21 ,35 ]
。
酸性萃取剂的结构式及萃取分离Zr,Hf优缺点总结于表2中。
3 胺类萃取剂
3.1 季铵盐类萃取剂
Aliquat 336在HCl体系中,低酸度下(<5 mol·L-1 HCl),Zr,Hf萃取率为零;水相酸度>5 mol·L-1 HCl时,Zr,Hf萃取率随HCl浓度增加而增大,Zr萃取率大于Hf
[36 ]
。Aliquat 336在HCl体系下对Zr,Hf萃取能力弱于三烷基胺类萃取剂Alamine 308,Alamine 336,Alamine 300和三(2-乙基己基)胺(tri(2-ethylhexyl)amine,TEHA)。水相酸度9 mol·L-1 HCl下,Zr,Hf分离系数βZr/Hf 可达42.3。HCl体系下,负载Zr,Hf的有机相用去离子水即可实现反萃率>99%
[36 ,37 ]
。HCl体系下,萃合物的结构为(R3 R'N)n-4 ZrCln
[1 ]
。。H2 SO4 体系中,现象正好相反,低酸度下(<1 mol·L-1 H2 SO4 )Zr,Hf萃取率随水相酸度增大而迅速减小,酸度继续增大(1~3 mol·L-1 H2 SO4 )Zr,Hf萃取率均为零。H2 SO4 体系下亦是优先萃取Zr,Zr,Hf萃取率相差不大,分离性能不佳
[38 ]
。
3.2 三烷基胺类萃取剂
HCl体系中,三烷基胺类萃取剂对Zr,Hf的萃取规律与季铵盐类萃取剂Aliquat 336类似。低酸度下(<5 mol·L-1 HCl),Zr,Hf萃取率为零;水相酸度>5 mol?L-1 HCl时,Zr,Hf萃取率随HCl浓度增加而增大,Zr萃取率大于Hf。萃取能力大小顺序为:TEHA>Alamine 308>Alamine 336>Alamine 300。TEHA在水相酸度为8 mol·L-1 HCl时对Zr,Hf分离性能最好,βZr/Hf 可高达316.5;相同酸度下,Alamine 308,Alamine 336和Alamine 300对Zr,Hf的最大分离系数分别为80.1,91.7和87.5。用低浓度盐酸(<1.0 mol·L-1 HCl)即可实现Zr,Hf近乎完全反萃;高浓度HCl(>7 mol·L-1 HCl)可实现Hf选择性反萃。用H2 SO4 作为反萃剂时,低浓度下(<1.0 mol·L-1 H2 SO4 )即可实现Hf选择性反萃
[37 ,39 ]
。
H2 SO4 体系中,萃取现象与HCl体系下完全不同,TEHA,Alamine 308,Alamine 336和Alamine300对Zr,Hf的萃取率也呈现不同的规律。水相酸度介于0~3 mol?L-1 H2 SO4 时,TEHA对Zr,Hf无萃取能力,萃取率均近乎为零;Alamine 308对Zr,Hf萃取率随H2 SO4 浓度增大呈现先迅速增大后缓慢减小的规律,0.5 mol·L-1 H2 SO4 时,Zr,Hf分离性能最好,βZr/Hf 最大为12.4;Alamine 300为萃取剂时,随H2 SO4 浓度增大Zr萃取率先增大后缓慢减小,Hf萃取率一直缓慢减小,βZr/Hf 最大为10.4;Alamine 336对Zr,Hf萃取率均随H2 SO4 浓度增大而降低,在H2 SO4 >0.3 mol·L-1 时Hf萃取率就近乎为零,在H2 SO4 =3 mol·L-1 时,Zr的萃取率也降为零
[38 ]
。在高浓度HCl体系(8 mol·L-1 )和低浓度H2 SO4 体系(0.1mol·L-1 )下,Alamine 336萃取锆铪出现乳化现象
[40 ]
。
表2 酸性萃取剂结构式及萃取分离Zr,Hf优缺点 下载原图
Table 2 Structures,advantages and disadvantages of acidic extractants for Zr/Hf separation
HCl体系下,属于阴离子交换机制,萃合物的结构为(R3 NH)n-4 ZrCln
[1 ]
。H2 SO4 体系下,低酸度时为加合物形成机制,萃合物结构为(R3 NH2 SO4 )2 Zr(SO4 )2 ;高酸度下为阴离子交换机制,萃合物结构为(R3 NH)2 Zr(SO4 )3
[38 ]
。
季铵盐和胺类萃取剂优先萃取Zr,萃取过程中传质大;在HCl体系下萃取酸度高(7~9 mol·L-1 HCl),试剂消耗量大,对设备腐蚀严重;H2 SO4 体系下分离系数小。
3.3 其他含氨基类萃取剂
长春应化所廖伍平课题组
[41 ]
开发了含磷酰基的新型胺类萃取剂二(2-乙基己基)-1-(2-乙基己基氨基)丙基磷酸酯(bis(2-ethylhexyl)-1-(2-ethylhexylamino)propylphosphonate,BEAP)。BEAP在HCl、HNO3 和H2 SO4 体系下均优先萃取Zr。不同体系下,Zr,Hf萃取率大小顺序为HCl<HNO3 <H2 SO4 。H2 SO4 体系下,Zr,Hf分离系数最大,0.6mol·L-1 H2 SO4 时βZr/Hf 为6.8;属于溶剂化萃取机制,萃合物结构分别为ZrO(HSO4 )2 ·3BEAP和HfO(HSO4 )2 ·2BEAP。HNO3 体系下βZr/Hf <3.2,HCl体系下βZr/Hf <2。30%BEAP正辛烷溶液在水相酸度为0.75 mol·L-1 H2 SO4 时对Zr,Hf的萃取容量分别为26.80和25.15 g·L-1 。1~5 mol·L-1 HCl和HNO3 难以将Zr,Hf反萃完全,反萃效率HCl<HNO3 。
埃及原子能管理局热实验室中心Saleh
[42 ]
研究了双酰胺荚醚类萃取剂N,N,N',N'-四辛基-3-氧杂戊二酰胺(N,N,N',N'-tetraoctyldiglycolamide,TODGA)于HNO3 体系下萃取分离Zr,Hf性能。随HNO3 浓度增大,Zr,Hf萃取率均增大,Zr萃取率大于Hf,锆铪分离系数βZr/Hf <2.8。
印度斯温卡泰斯瓦拉大学Reddy课题组
[43 ]
合成并研究了新型异唑酮类萃取剂3-苯基-4-苯甲酰基-5-异唑酮(3-phenyl-4-benzoyl-5-isoxazolone,HP-BI)、3-苯基-4-(4-甲苯酰基)-5-异唑酮(3-phenyl-4-(4-toluoyl)-5-isoxazolone,HTPI)和3-苯基-4-(4-氟代苯甲酰基)-5-异唑酮(3-phenyl-4-(4-fluorobenzoyl)-5-isoxazolone,HFBPI)萃取分离Zr,Hf性能和机制。HCl体系下,Zr,Hf萃取率均随HCl浓度增大而减小。对Zr萃取能力HPBI>HTPI>HFBPI;对Hf萃取能力HFBPI>HPBI>HTPI。3种萃取剂均优先萃取Zr,对Zr,Hf分离性能均较差(βZr/Hf <2.5)。萃取机制属于阳离子交换机制,萃合物结构分别为ZrOA2 和HfOA2 。
BEAP,TODGA和异唑酮类萃取剂HPBI,HT-PI和HFBPI均优先萃取Zr,萃取过程中传质大,锆铪分离系数小。
胺类萃取剂的结构式及萃取分离Zr,Hf优缺点总结于表3中。
表3 胺类萃取剂的结构式及萃取分离Zr,Hf优缺点 下载原图
Table 3 Structures,advantages and disadvantages of amine-based extractants for Zr/Hf separation
4 结语
现有分离Zr,Hf萃取体系或萃取剂水溶性大,产生高浓度含氨氮、SCN- ,CN- 和有机质的废水,环境污染大;或萃取酸度高,导致试剂消耗量大和对设备腐蚀性强;或萃取过程容易乳化,造成分相困难;或萃取容量小,效率低;或锆铪分离系数小,难以实现核级锆铪分离;或反萃困难;或萃取剂价格昂贵。虽然协同萃取体系一定程度上能够弥补单一萃取剂体系存在的不足,但在长期的萃取分离工业生产中很难维持混合萃取体系中各萃取剂之间的固定比例。研究和开发对锆铪萃取酸度低、分离系数大、萃取容量高、不易乳化、容易反萃、环境友好、结构简单易合成的新型绿色高效萃取剂一直是不断努力追求的目标,是锆铪分离领域的研究热点。
自然界中铪含量为锆的1%~3%,优先萃含量少的铪可减少萃取级数,降低试剂消耗量,节约时间和电力成本,且设备占地面积小。优先萃铪的萃取剂很少,包括酮类萃取剂、有机磷/膦类萃取剂、硫代膦酸类萃取剂和部分羟肟酸类萃取剂(如LIX84-IC)。酮类萃取剂在HSCN体系下优先萃取铪,但该萃取体系会产生高浓度含氨氮、SCN- 和CN- 的废水,造成环境污染;硫代膦酸类萃取剂软配体S原子理论上不适用于Zr(IV),Hf(IV)硬离子萃取分离;羟肟酸类萃取剂优先萃铪与否与萃取剂分子结构密切相关。有机磷/膦类萃取剂在H2 SO4 体系下优先萃铪,是除酮类萃取剂-HSCN体系外最具开发潜力的优先萃铪萃取体系。这类萃取剂中P204萃取能力太强,锆铪分离系数小。对锆铪分离性能Cyanex 272>P507>P204,但Cyanex 272对锆铪分离系数依然小,且萃取能力弱。故新型有机磷/膦类萃取剂是开发优先萃铪新体系的重要研究方向,值得学者们关注。
参考文献
[1] Banda R,Lee M S.Solvent extraction for the separation of Zr and Hf from aqueous solutions[J].Separation and Purification Reviews,2015,44(3):199.
[2] Li P H,Xu Z G,Chi R A,Zhao J,Wang L J,Xu Y L.Research progress in hydrometallurgical separation of zirconium and hafnium[J].Chinese Journal of Rare Metals,2016,40(5):499.(李攀红,徐志高,池汝安,赵骏,王力军,徐源来.湿法分离锆铪的研究进展[J].稀有金属,2016,40(5):499.)
[3] Huang D F,Zhou M,Li C X,Kong D C,Yang L F,Xiao S H.Current technological states in the preparation of atomic-grade ZrO2 and HfO2 and its research fornew process[A].Progress Report on China Nuclear Science&Technology[C].Beijing:Chinese Nuclear Society,2009.188.(黄代富,周密,李春湘,孔冬成,杨立峰,肖少华.制备原子能级二氧化锆(铪)工艺技术现状与新工艺的研究[A].中国核科学技术进展报告[C].北京:中国核学会.2009.188.)
[4] Zheng S H.Experimental Study on Extraction of Zirconium and Hafnium by Fluoride Chloride Mixed Salt System[D].Ma'anshan:Anhui University of Technology,2017.35.(郑仕鸿.氟氯混合盐体系熔盐萃取分离锆铪的实验研究[D].马鞍山:安徽工业大学,2017.35.)
[5] Chai Y Q.Study on the Separation of Zirconium and Hafnium by Molten Salt Extraction[D].Ma’anshan:Anhui University of Technology,2017.39.(柴延全.熔盐萃取法分离锆铪的研究[D].马鞍山:安徽工业大学,2017.39.)
[6] Zhu X F.Pyrometallurgical separation method for zirconium oxide/hafnium oxide mixtures[P].Chinese Patent:CN201310682029.8,2014.(朱兴峰.氧化锆/氧化铪混合物的火法分离方法[P].中国专利,CN201310682029.8,2014.)
[7] Felipe E C B,Ladeira A C Q.Separation of zirconium from hafnium by ion exchange[J].Separation Science and Technology,2018,53(2):330.
[8] Xu Z G,Wang L J,Chi R A,Zhang L.Solvent extraction and separation of hafnium from zirconium with DIBK[J].Nonferrous Metals(Extractive Metallurgy),2012,(3):35.(徐志高,王力军,池汝安,张力.DIBK溶剂萃取法分离锆铪[J].有色金属(冶炼部分),2012,(3):35.)
[9] Xu Z G,Wang L J,Wu M,Xu Y L,Chi R,Li P H,Zhao J.Separation of zirconium and hafnium by solvent extraction using mixture of DIBK and P204[J].Hydrometallurgy,2016,165:275.
[10] Xu Z G,Wang L J,Wu Y K,Chi R A,Wu M,Yang HF.Thermodynamics of solvent extraction of zirconium and hafnium with DIBK-P204 system[J].Chinese Journal of Rare Metals,2013,37(2):266.(徐志高,王力军,吴延科,池汝安,吴明,阳慧芳.DIBK-P204体系萃取锆铪的热力学研究[J].稀有金属,2013,37(2):266.
[11] Xu Z G,Wang L J,Wu Y K,Chi R A,Zhang L,Wu M.Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system[J].Transactions of Nonferrous Metals Society of China,2012,22(7):1760.
[12] Lin Z H.Extraction separation of zirconium and hafnium with methyl isobutyl ketone(MIBK)[J].Rare Metals Letters,2007,26(1):93.(林振汉.用甲基异丁基酮萃取分离锆铪的工艺评价[J].稀有金属快报,2007,26(1):93.)
[13] Xu Z G,Wang L J,Wu Y K,Chi R A,Li P H,Yang HF.Mechanisms of extraction and separation of zirconium and hafnium by DIBK-P204 system[J].Chinese Journal of Nonferrous Metals,2013,23(7):2061.(徐志高,王力军,吴延科,池汝安,李攀红,阳慧芳.DIBK-P204体系萃取分离锆和铪的机制[J].中国有色金属学报,2013,23(7):2061.)
[14] Xu Z G,Wang L J,Wu Y K,Chi R A,Zhang L.Mechanisms of extraction and separation of zirconium and hafnium using mixtures of DIBK and TBP[J].Chinese Journal of Nonferrous Metals,2012,22(8):2374.(徐志高,王力军,吴延科,池汝安,张力.DIBK-TBP体系萃取分离锆铪的机制[J].中国有色金属学报,2012,22(8):2374.)
[15] Banda R,Lee H Y,Lee M S.Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants[J].Journal of Radioanalytical and Nuclear Chemistry,2013,298(1):259.
[16] Da Silva A,El-Ammouri E,Distin P A.Hafnium/zirconium separation using Cyanex 925[J].Canadian Metallurgical Quarterly,2000,39(1):37.
[17] Nayl A A,El-Nadi Y A,Daoud J A.Extraction and separation of Zr(IV)and Hf(IV)from nitrate medium by some CYANEX extractants[J].Separation Science and Technology,2009,44(12):2956.
[18] El-Ammouri E,Distin P A.Hafnium extraction from acidic chloride solutions by Cyanex 923[J].Solvent Extraction and Ion Exchange,1996.14:871.
[19] Wang L Y,Lee H Y,Lee M S.Solvent extraction separation of Zr and Hf from nitric acid solutions by PC 88Aand its mixture with other extractants[J].Metals and Materials International,2015,21(1):166.
[20] Taghizadeh M,Ghanadi M,Zolfonoun E.Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923[J].Journal of Nuclear Materials,2011,412(3):334.
[21] Wang L Y,Lee H Y,Lee M S.Solvent extractive separation of zirconium and hafnium from hydrochloric acid solutions by organophosphorous extractants and their mixtures with other types of extractants[J].Chemical Engineering Communications,2015,202(10):1289.
[22] Wang L Y,Lee M S.Separation of zirconium and hafnium from nitric acid solutions with LIX 63,PC 88A and their mixture by solvent extraction[J].Hydrometallurgy,2014,150:153.
[23] Yadollahi A,Saberyan K,Torab-Mostaedi M,Charkhi A,Pourjavid M R.Solvent extraction separation of zirconium and hafnium from nitric acid solutions using mixture of Cyanex-272 and TBP[J].Radiochimica Acta,2018,106(8):675.
[24] Min S H,Lee M S.Selective separation of hafnium from sulfuric acid solution containing Zr by solvent extraction with PC88A[J].Korean Journal of Metals and Materials,2015,53(1):51.
[25] Lee M S,Banda R,Min S H.Separation of Hf(IV)-Zr(IV)in H2 SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations[J].Hydrometallurgy,2015,152:84.
[26] Banda R,Min S H,Lee M S.Selective extraction of Hf(IV)over Zr(IV)from aqueous H2 SO4 solutions by solvent extraction with acidic organophosphorous based extractants[J].Journal of Chemical Technology and Biotechnology,2014,89(11):1712.
[27] Wang L Y,Lee M S.Development of a separation process for the selective extraction of hafnium(IV)over zirconium(IV)from sulfuric acid solutions by using D2EH-PA[J].Hydrometallurgy,2016,160:12.
[28] Wang L Y,Lee M S.A review on the aqueous chemistry of Zr(IV)and Hf(IV)and their separation by solvent extraction[J].Journal of Industrial and Engineering Chemistry,2016,39:1.
[29] Reddy B R,Kumar J R,Raja K P,Reddy A V.Solvent extraction of Hf(IV)from acidic chloride solutions using Cyanex 302[J].Minerals Engineering,2004,17(7-8):939.
[30] Reddy B R,Kumar J R,Reddy A V.Solvent extraction oil zirconium(IV)from acidic chloride solutions using the thiosubstituted organophosphorus acids Cyanex 301and 302[J].Journal of Chemical Technology and Biotechnology,2004,79(11):1301.
[31] Kumar J R,Reddy B R,Reddy K J,Reddy A V.Liquid-liquid extraction of tetravalent hafnium from acidic chloride solutions using bis(2,4,4-trimethylpentyl)dithiophosphinic acid(Cyanex 301)[J].Separation Science and Technology,2007,42(4):865.
[32] Saberyan K,Meysami A H,Rashchi F,Zolfonoun E.Proposal of a new Hf(IV)/Zr(IV)separation system by the solvent extraction method[J].Chinese Journal of Chemistry,2008,26(11):2067.
[33] Reddy B R,Kumar J R,Reddy A V.Solvent extraction of zirconium(IV)from acid chloride solutions using LIX84-IC[J].Hydrometallurgy,2004,74(1-2):173.
[34] Reddy B R,Kumar J R.Studies on liquid-liquid extraction of tetravalent hafnium from weakly hydrochloric acid solutions by LIX 84-IC[J].Separation and Purification Technology,2005,42(2):169.
[35] Lee H Y,Kim S G,Oh J K.Stoichiometric relation for extraction of zirconium and hafnium from acidic chloride solutions with Versatic Acid 10[J].Hydrometallurgy,2004,73(1-2):91.
[36] Poriel L,Favre-Reguillon A,Pellet-Rostaing S,Lemaire M.Zirconium and hafnium separation,Part 1.Liquid/liquid extraction in hydrochloric acid aqueous solution with aliquat 336[J].Separation Science and Technology,2006,41(9):1927.
[37] Banda R,Lee H Y,Lee M S.Separation of Zr from Hf in hydrochloric acid solution using amine-based extractants[J].Industrial&Engineering Chemistry Research,2012,51(28):9652.
[38] Wang L Y,Lee M S.Separation of Zr and Hf from sulfuric acid solutions with amine-based extractants by solvent extraction[J].Separation and Purification Technology,2015,142:83.
[39] Banda R,Lee H Y,Lee M S.Separation of Zr and Hf from strong hydrochloric acid solution by solvent extraction with TEHA[J].Journal of Radioanalytical and Nuclear Chemistry,2013,295(2):1537.
[40] Conradie E W,van der Westhuizen D J,Nel J T,Krieg H M.The separation of zirconium and hafnium from(NH4 )3 Zr(Hf)F7 using amine-based extractants[J].Journal of the Southern African Institute of Mining and Metallurgy,2016,116(10):915.
[41] Chen S,Zhang Z F,Kuang S T,Li Y L,Huang X W,Liao W P.Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP[J].Hydrometallurgy,2017,169:607.
[42] Saleh A S.Solvent extraction of Zr(IV)and Hf(IV)with N,N,N',N'-tetraoctyldiglycolamide[J].Journal of Radioanalytical and Nuclear Chemistry,2012,292(3):1109.
[43] Reddy B R,Kumar J R,Reddy A V.3-phenyl-4-acyl-5-isoxazolones as reagents for liquid-liquid extraction of tetravalent zirconium and hafnium from acidic chloride solutions[J].Journal of the Brazilian Chemical Society,2006,17(4):780.