Trans. Nonferrous Met. Soc. China 23(2013) 1789-1796
Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite
Pei-pei WANG1, Wen-qing QIN1, Liu-yi REN1, Qian WEI1, Rui-zeng LIU1, Cong-ren YANG1, Shui-ping ZHONG2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Comprehensive Utilization of Low-Grade Ores, Shanghang 364200, China
Received 23 May 2012; accepted 23 April 2013
Abstract:
Solution chemistry and flotation of cassiterite in the presence of alkyl hydroxamic acid collector were investigated. Results show that the alkyl hydroxamic acid is able to float fine cassiterite very well at relatively alkaline conditions, and the pH range of better flotability broadens with the increase of chain length of the collector. Moreover, the metal cations influence the flotation mostly depending on the pH of the medium and to some extent on the concentration of metal cations. Theoretical calculation on the group electronegativity indicates that the alkyl chain length of about seven is of great superiority in the synthetization of hydroxamic acid collector for cassiterite flotation. A co-adsorption between molecules and ions onto cassiterite surface is considered according to the lg c—pH diagram analysis. In terms of zeta potential and infrared spectra, the main interaction involves electric charge interaction, hydrogen bonding interaction and complexation, with the proposed formation of an O, O-five membered rings chelate compound.
Key words:
fine cassiterite; flotation; alkyl hydroxamic acid; chelate;
1 Introduction
As the most commonly used metal all over the world, tin is usually found as oxide minerals in nature and is extracted from tin-containing ores [1,2], among which the most widespread is cassiterite (SnO2). In the view of its ultrafine dissemination, great density and crisp property [3], cassiterite is more likely to be crushed into fine particles in the process of exploitation [4]. Coarse cassiterite particles are usually recovered by gravity separation, but for fine particles, flotation is inefficient [5,6]. Cassiterite is virtually insoluble in water while its surface properties are strongly influenced by acids and alkalis [7]. Flotation is a physic-chemical separation process that utilizes the difference in surface properties of the valuable minerals and the unwanted gangue minerals [8]. With the development of mineral processing, exploitation of chemical products and the increasing innovation of measuring and testing techniques for reagents, extensive studies have been conducted to improve the flotation performance of cassiterite. Nevertheless, the property of the fine ore has not been systematically understood [9].
Currently, the research orientations to enhance the recovery of fine cassiterite can be classified into two basic groups: the first is to investigate the pretreatment of fine minerals before flotation, using methods such as ultraviolet and photoelectron spectroscopy to investigate the collecting mechanisms [10-12] of cassiterite; the second is to exploit new equipments and synthesize new flotation reagents. Ideally, collectors with good selectivity or flotability are of great interest. Hydroxamic acids (RCONHOH) have been known for a long time. However, their importance has not been fully recognized until the last 20 years, after a large amount of information had been accumulated on their chemical role as efficient binding ligands for metal cations [13,14]. Cassiterite particles give a good flotation response to hydroxamate collectors because of the strong complexation of the reagents [15,16]. Currently, much of attention has been paid to the salicylhydroxamic acid or benzohydroxamic acid which has a pronounced selectivity, but for the particles in the size <38 μm, the recovery is insufficient [3,11]. So, the alkyl hydroxamic acid with better collecting ability is worth being investigated. In this work, the mechanisms of fine cassiterite with alkyl hydroxamic acids were reported through flotation tests, group electronegativity calculations, solution chemistry analyses, electrophoretic mobility and infrared spectra measurements. In addition, the effects of metal cations on the flotation were further discussed.
2 Experimental
2.1 Materials
A rough high-grade cassiterite concentrate was generated by gravity separation of a tin ore from Gaofeng Mining Co., Ltd. in Guangxi, China. The concentrate was further purified to remove trace of ferreous mineral using magnetic separation, followed by adding dilute sulfuric acid and stirring for about one week to remove CaCO3, and shaking table to remove SiO2. Chemical analyses exhibited that the resultant powders contained 95.49% SnO2. X-ray diffraction (XRD) results are shown in Fig. 1. No peaks of any impurities were detected, which manifested that SnO2 was obtained with high purity and deemed sufficiently pure for research purposes. Finally, the cassiterite concentrate was ground in a porcelain ball mill and then through hydraulic classification to yield two different particle sizes as 20-38 μm and <20 μm, respectively. Subsequently, the cassiterite concentrate was oven-dried after washing with distilled water several times to adjust the pH at about 7. The 20-38 μm size fraction was taken for the flotation test and the <20 μm size fraction was further ground to ultra-fine size in an agate mortar for the electrokinetic and infrared spectra measurements.

Fig. 1 XRD pattern of cassiterite concentrate
Alkyl hydroxamic acids with carbon chain length of 7-9 were synthesized in the laboratory according to the procedure mentioned by previous studies [10,17]. With grease, hydroxylamine hydrochloride and formic acid as raw materials, three hydroxamic acids were synthesized. The final resultants were considerably high with purity of approximately 90%. As white flaky crystals have sparse water-solubility, the alkyl hydroxamic acid is usually used as an aqueous solution which is less than 0.5% in mass fraction. All other reagents, except the collectors, were of analytical reagent grade, including H2SO4 and NaOH used for pH adjustment, and all experiments were carried out with distilled water only.
2.2 Flotation
Experiments were carried out in a microflotation cell with a volume of approximately 40 mL. The contents of the cell were stirred at 1600 r/min by a four-blade impeller. Each test was carried out by equilibrating 2.0 g of cassiterite with alkyl hydroxamic acid of known concentration. An aqueous solution of sodium hydroxide or sulfuric acid was used to adjust the pH of the pulp. The procedures involved in the micro-flotation tests are revealed in Fig. 2. At last, concentrate and bottom product were washed with distilled water, filtered, dried, weighed and analyzed.
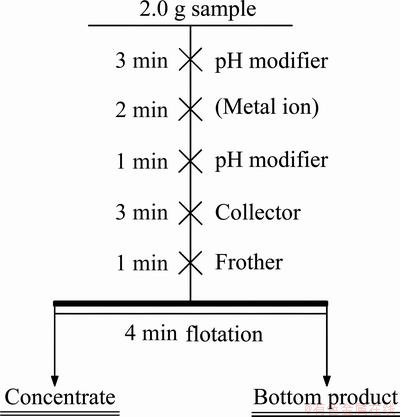
Fig. 2 Flotation flowchart of cassiterite
2.3 Measurement of electrophoretic mobility
The electrophoretic mobility determinations were carried out using a JS94H micro-electrophoresis apparatus, which was produced by the digital technology equipment of Zhongchen Co., Ltd. in Shanghai, China. In each test, 0.1 g of finely ground cassiterite (<5 μm) was dispersed in 50 mL aqueous solution and agitated for 3 min to form the suspensions containing 0.2% solids. The suspension was conditioned by the addition of the appropriate reagents to adjust the pH in the range of 3-10 for the zeta potential measurements. An average of four independent measurements was taken for each electrophoretic mobility point, and the reproducibility of the data was checked.
2.4 Infrared spectroscopy
Infrared spectra of the nonyl hydroxamic acid, cassiterite mineral, and their interaction product were obtained via the KBr pellet method in a Fourier transform infrared (FTIR) spectrometer (Nicolet model Nexus 670, USA). The interaction product was obtained by contacting fine cassiterite minerals (<2 μm) with a nonyl hydroxamic acid solution (1.5 g/L) at a pH about 6.5. In the preparation of the interaction product, the excess reagent was removed by filtering the suspensions and washing the residues with distilled water three times. Successively, the products were dried at the room temperature.
3 Results and discussion
3.1 Flotation of cassiterite with alkyl hydroxamic acids
At alkyl hydroxamic acid concentration of 30 mg/L, the effect of the pH on the flotation of cassiterite is given in Fig. 3. The maxima of flotation recovery of roughly around 90% occurs at about pH 10 with all the three collectors, and the pH region of better flotability broadens with the increase of the chain length of the collector. When the pH surpasses 10, a sharp decline is noticed, interestingly, which coincides with both the lg c—pH (concentration logarithm vs pH) diagrams and the zeta potentials analyses in the following test.
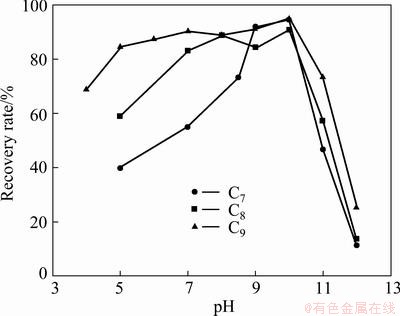
Fig. 3 Effect of pH on flotation recovery of cassiterite at collector concentration of 30 mg/L
Effect of concentration of hydroxamate collectors on the flotation of cassiterite under selected pH conditions are presented in Fig. 4. The curves delineate that there is subtle difference between the C8 (octyl hydroximic acid) and C9 (nonyl hydroximic acid) collectors, and all the recovery maxima occur at the same concentration of about 30 mg/L. As the collector concentration shifts to higher degree, a slight decrement is noticed, attesting the reported data [10,18].
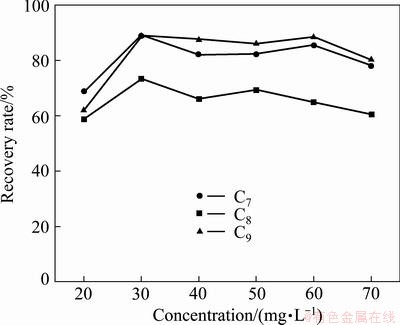
Fig. 4 Effect of collector concentration on flotation recovery of cassiterite at pH 8.0
Study and calculation showed that the collecting ability of collectors depended on the structure of polar group in molecule. ZHU [19] and his co-workers advanced one of the most important criteria that group electronegativity calculation was used as the basis to synthetize a number of collectors for a variety of minerals. Also, the equation of the requirement for the hydrophile-lipophile equilibrium of collector in flotation was presented to the flotation chemistry, which can be described as
 (1)
(1)
where N1 denotes the carbon chain length; χg stands for the eletronegativity of the alkyl hydroxamic acid group; χL and χM correspond to the electronegativities of the elements O and Sn, respectively. Based on Ref. [19], the relevant data are χg =3.8, χL =3.5 and χM =1.8. After calculation, the value of N1 was obtained as 6.5, which indicates that the alkyl chain length of about seven (octyl hydroxamic acid) is of great superiority in the synthetization of hydroxamic acid collector for cassiterite flotation, basically according with the flotation results.
3.2 Effect of metal cations on flotation of cassiterite
The unavailable metal cations such as ferric, aluminum, ferrous, copper ions and their hydrolytic products are one of the major factors that influence the flotation process [14]. Many of the commonly occurring gangue minerals associated in the lode type of tin ore are more soluble than cassiterite in the aqueous phase. Besides, some of the metal cations are artificially joined in the prior flotation flowsheet [10]. As a result, it is of great value to investigate the effects of some metal cations on the cassiterite flotation to get a better insight into the whole process.
Nonyl hydroxamic acid gives better flotability in a broad pH range according to Fig. 3, and it is adopted in the following research and measurements.
Figure 5 delineates the flotation response of cassiterite at different pH with metal cations concentration of 10-5 mol/L. In the absence of metal cations, a 30 mg/L nonyl hydroxamic acid solution gives the highest recovery rate of about 94.87%. With the action of Cu2+ and Fe3+, the flotation of cassiterite is slightly increased within a wide pH range, and the peak recovery rate can reach 96.68% with Fe3+ at pH about 10. For Fe2+, it has almost the same action as Fe3+ in acidic pulp conditions, but with the pH surpassing the neutral conditions, high oxidation rate impacts the Fe2+, thereby influencing the chemical environment and inversely depressing the recovery. Former documents [20] have mentioned that Fe2+ directly influences the stableness of hydroxamic acids due to three reasons: reduction impact in acidic pulp condition; complexation effect with hydroxamic acid; decomposition function to the hydroxylamine in alkaline solution. Nevertheless, as the cassiterite treated with Al3+ further reacts with collector, a distinct lower recovery rate of about 46.8% is noticed at pH about 6. When the pH shifts to more acidic or more alkaline, inhibition phenomenon is weakened.

Fig. 5 Effect of pH on flotation of cassiterite with metal cations
Figure 6 gives the flotation response of cassiterite at different concentrations of metal cations carried out at a fixed pH about 6.5. No remarkable variation is noticed with the Fe3+ or Fe2+ concentration of less than 10-3 mol/L. Moreover, cassiterite flotation is apparently restrained when the concentrations of Al3+ and Cu2+ exceed 10-5 and 10-4 mol/L, respectively, particularly with the later one. This may attribute to the consumption of surfactant in bulk chemical reaction, and high concentration of metal cations causes an unfavorable adsorption thereafter.
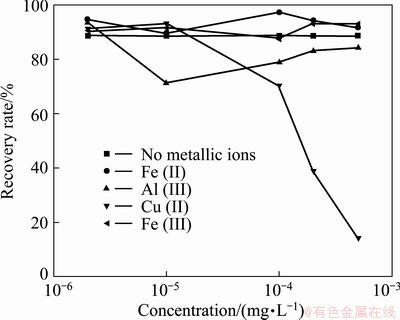
Fig. 6 Effect of concentration on flotation of cassiterite with metal cations
An interesting result is obtained compared with the results of previous paper [10] that with the collector concentration of 10-5 mol/L, the presence of Fe2+ or Al3+ enhanced the recovery but the presence of Fe3+ inversely affected the recovery in the pH range of 4-7. Drop in the flotation recovery was noticed when the concentrations of Fe3+, Fe2+ and Al3+ exceeded 4×10-6, 1×10-3 and 7×10-5 mol/L, respectively. Two reasons may be responsible for this incoherence. First, this particular size fraction was obtained using both screening and hydraulic classification, so the size is entirely different. Second, the different collectors were used. Anyway, the activation or depression of cassiterite flotability of these ions with different pH values or concentrations of metal cations was demonstrated in the experiments.
3.3 Electrokinetics
A significant step forward in delineating the physical chemistry of flotation system is bringing the concepts of the electrical double layer and electrokinetic potentials into widespread use in the interpretation of flotation phenomena [21]. Previous published data on the electro-surface properties of cassiterite such as point of zero charge (PZC), isoelectric point (IEP), and electrokinetic potential are limited. For oxide minerals, hydrogen and hydroxyl ions have long been considered to be potential-determining. The IEP is also the point of zero charge [22]. The floatation capability of fine cassiterite is enhanced notably with nonyl hydroxamic acid, as mentioned in Fig. 3. To better understand the interaction mechanism between them, electrokinetic phenomena of mineral suspensions were investigated. At room temperature about 25 °C, zeta potentials of cassiterite in the absence and in the presence of nonyl hydroxamic acid with the concentrations of 30, 50 mg/L were measured, and the results are demonstrated in Fig. 7.
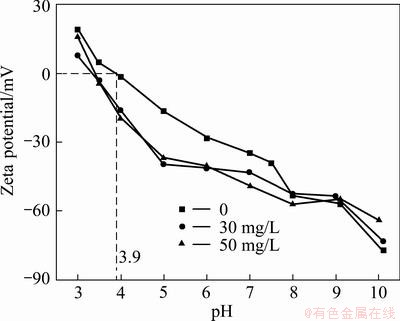
Fig. 7 Electrophoretic mobility of cassiterite in the presence of nonyl hydroxamic acid
The pure mineral exhibits PZC at pH around 3.9, namely, the surface of cassiterite particles is positively charged at pH<3.9, but negatively charged in adverseness. According to Ref. [23], the pHpzc value lies in the region of 3.8-5.5 depending on the sample origin, type of electrolyte and the determination procedure. Our measured data are in excellent agreement with those reported in Ref. [23]. The presence of nonyl hydroxamic acid shifts the curve of zeta potential as a function of pH to the left and makes the zeta potential of casslterite more negative. This might be attributed to the H3O+ withdrawl from the surface as a consequence of collector adsorption [24]. But with increasing the pH to strong alkaline conditions (pH>8), the gap between the zeta potentials of cassiterite in the presence and absence of nonyl hydroxamic acid gradually diminish. No remarkable difference is found between the zeta potentials at the collector concentrations of 30 mg/L and 50 mg/L. The trends were observed that the flotation recovery decreases sharply at pH>10 and none enhancement emerges with collector concentration exceeding 30 mg/L, which is consistent with above interpretation.
The interaction between the medium ions and the oxide surface plays an important role in regulating the surface charge. From the perspective of molecular structure, nonyl hydroxamic acid is an anionic surfactant, so the adsorption of the ionized collector on the surface of cassiterite that causes the zeta potential more negative is understandable. Early similar investigation [13] proposed that the adsorption of anionic hydroxamate collector on the negatively charged surface of cassiterite was not sufficiently caused by an electrostatic attraction alone. Moreover, specific adsorption or/and some chemically based compounds [25] may be involved.
3.4 Solution chemistry method in flotation of cassiterite
As noted in Refs. [4,26] the lgc—pH diagram of the collector was usually used to analyze the solution chemistry of collectors, and the pKa was referred to the constant of dissociation equilibrium [27]. Thereby in the aqueous solution, there is:
HB H++B-,
H++B-,
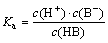 (2)
(2)
According to the quality balance, there is
cB=c(HB)+c(B-) (3)
Substituting Eq. (3) into Eq. (2) obtains
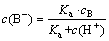 (4)
(4)
 (5)
(5)
Taking the logarithm of both Eqs. (4) and (5) yields
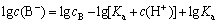 (6)
(6)
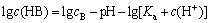 (7)
(7)
where cB =2.0×10-4, pKa =10.98, namely,
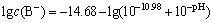 (8)
(8)
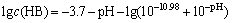 (9)
(9)
From the above equation results, the hydrolytic products of nonyl hydroxamic acid alter under varying pH. The lg c—pH diagram is illustrated in Fig. 8. When pH<10.98, HB plays a dominant role in the hydrolytic components. However, B- preponderates over the former with pH>10.98.

Fig. 8 Relation between lg c and pH of hydrolytic components in solution of nonyl hydroxamic acid
As known from solution chemistry analyses, the solubility of nonyl hydroxamic acid in strong acidic condition (pH<4) is relatively low and this seems to suggest that collector may adsorb on the cassiterite in the form of molecule. The non-ionic carbon chains within collector molecules may associate easily, forming sub-layer adsorption in the anti-direction [28], thus causing decrease in hydrophobicity and flotability of cassiterite. Other standpoints [29] have performed that the protonation of the hydroxamic acid occured in solution, which reduced the quantity of collector ions reacting with metal cations. Besides, the surface of the mineral could not form stable compound with collector in the strong acidic environment [25], which rendered the fall of the recovery.
Within the pH range of 4-11, the coadsorption between molecules (HB) and ions (B-) in the pulp solution is considered. This seems to suggest that the interaction involves both electric charge interaction contributions and also effects from hydrogen bonding and complexation [26]. On the other hand, electrokinetics and microflotation results at this pH range show that the nonyl hydroxamic acid/mineral interaction is strong enough to surpass the electrostatic repulsion between the negatively charged surface and the collector anion [24]. This ion-molecule coadsorption pattern increases the activity of the collector and accordingly enhances the flotability of cassiterite. The optimum pH range for the flotation of cassiterite is 4-11, within which the coadsorption takes place.
When pH>10.98, though the dissociation degree of collector is amplified, the competition adsorption from OH- brings about reduction of the complexation chance for nonyl hydroxamic acid [25]. This is one of the reasons. The other may be attributed to the enhanced electrostatic repulsion between negatively charged cassiterite [30] and homogeneously charged collector anions that render the decline of recovery.
3.5 Infrared spectroscopy
The selectivity of mineral flotation with hydroxamate depends on a balance between at least two characteristics: the mineral solubility and the stability constant of the complex hydroxamate/lattice cation. The most direct collecting form of the reagent is the existence of valid adsorption on the surface of minerals. Usually, the special functional groups within the collectors can chemically bond up with metal cations on the surface of minerals [31]. To further investigate the interaction mechanisms, infrared spectra of the nonyl hydroxamic acid, cassiterite mineral and precipitates (cassiterite- nonyl hydroxamic acid) are presented and compared with each other in Fig. 9.

Fig. 9 FTIR spectra of nonyl hydroxamic acid (a), cassiterite (b), and cassiterite (c) after connection with nonyl hydroxamic acid solution
Organo-metallic compound shows adsorption peak
of 4000-600 cm-1 that mainly caused by the ligand vibration [32]. Both oxygen atoms from carbonyl and hydroxyl in group of the hydroxamic acid contain unpaired electrons, which can easily bond with the metal cations [33]. The nonyl hydroxamic acid exhibits two adsorption bands at 3254.1 and 2923.6 cm-1, which are associated with N—H and O—H stretching vibration adsorption band, respectively [34]. The infrared spectra of cassiterite gives a prominent peak in the spectral range of 700-500 cm-1, especially the one at 695.1 cm-1, which corresponds to the characteristic peak of SnO2 [13]. In the system of cassiterite/nonyl hydroxamate, flotation occurs in a pH range over the PZC, thereby collector adsorption takes place under electrostatic repulsion conditions. The cassiterite infrared spectra after collector adsorption at pH of about 6.5 present remarkable absorption bands: disappearance of O—H stretching vibration adsorption band at 3274.3 cm-1 due to the possible formation of O-metal bond [13,35]; the adsorption of stretching vibration band of N—H and its shift from 2923.6 to 2925.4 cm-1; both of the two bands in the range of 2856.2-2925.4 cm-1 and 1453.6-1521.2 cm-1 precisely assigned to the stretching vibration and bending vibration absorption of C—H in —CH3 and —CH2 groups; at 1521.2 cm-1 corresponding to original hydroxamate bands at 1569.8 cm-1, which is the new band, i.e., formed after adsorption, proving that chemisorption has occurred. Tentatively, the new bands could be assigned to the stretching vibration of the C=O bonds due to the conjugation effect from N atoms or formation of hydrogen bond within molecules [23], and also the stretching vibration and bending vibration absorption of N—H in —N—OH groups may be involved. Therefore, there is evidence of chemisorption by the reaction between nonyl hydroxamate and cassiterite, which is in the form of Sn-nonyl hydroxamic acid compounds. From the aspect of chemical adsorption, researchers [25] have studied the cassiterite mineral after the treatment of nonyl hydroxamate potassium with the symbol of C14. The infrared spectra results showed that the chemical adsorption exists, and the resultant product may be proposed as a chelate of O, O-five membered rings with Sn2+ other than O,N-four membered rings with Sn2+ [34,36]. Besides, specific adsorption like hydrogen bonding between collector-mineral or collector-collector may be involved, which coincides with the proof from the above discussion.
4 Conclusions
1) The maxima of flotation recoveries occur at about pH 10 with all three alkyl hydroxamic acid collectors, and the pH region of better flotability broadens with the increase of the chain length of the nonpolar group of collector. The alkyl chain length of about seven is of great superiority in the synthetization of hydroxamic acid collector for cassiterite flotation.
2) Fe3+ and Cu2+ have somewhat activation on the flotation system whereas other ions such as Al3+ strongly depress cassiterite flotation which mostly depends on the pH of the medium and to some extent on the concentration of metal cations. The cassiterite-nonyl hydroxamic acid system tolerates higher concentrations of Fe3+ or Fe2+ than Al3+ or Cu2+ species.
3) Through the lg c—pH diagram analyses, a coadsorption between molecules (HB) and ions (B-) in the pulp solution is considered, which increases the activity of the collector and accordingly promotes the flotability of cassiterite.
4) The pure mineral exhibits a PZC at pH around 3.9, while the presence of nonyl hydroxamic acid shifts the curve of zeta potential to the left and makes the zeta potential of cassiterite more negative. Infrared spectroscopic studies reveal that the main interaction can be attributed to chemical adsorption of nonyl hydroxamic acid onto cassiterite surface. In general, electric charge interaction, hydrogen bonding interaction and complexation are involved, with the resultant product proposed as a chelate of O, O-five membered rings with Sn2+.
References
[1] POTAPOVA E, GRAHN M, HOLMGREN A, HEDLUND J. The effect of calcium ions and sodium silicate on the adsorption of a model anionic flotation collector on magnetite studied by ATR-FTIR spectroscopy [J]. Journal of Colloid and Interface Science, 2010, 345(1): 96-102.
[2] BROCCHI E A, MOURA F J. Chlorination methods applied to recovery refractory metals from tin slag [J]. Minerals Engineering, 2008, 21(2): 150-156.
[3] SREENIVAS T, SRINIVAS K, NATARAJAN R, PADMANABHAN N P H. An integrated process for the recovery of tungsten and tin from a combined wolframite-scheelite-cassiterite concentrate [J]. Mineral Processing and Extractive Metallurgy Review, 2004, 25(3): 193-203.
[4] QIN Wen-qing, WANG Pei-pei, REN Liu-yi, WEI Qian, PENG Zhi-bing, GU Yan-ling. Effect of matching relationship between particles and bubbles on the flotation of fine cassiterite [J]. Journal of China University of Mining and Technology, 2012, 41(3): 420-424. (in Chinese)
[5] YE Xue-jun, LU Bing-jun, FENG Zhang-fa, CHEN Jin-quan. Test and application of sawtooth wave jigger for recovering fine cassiterite [J]. Metal Mine, 2009(2): 134-136. (in Chinese)
[6] LIU Ya-ying, BI Xian-wu, WU Li-yan, YIN Bing. Occurrence of tin in a thousand tons tailing dam in Shizhuyuan [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2009, 28(14): 344-348. (in Chinese)
[7] QIN Wen-qing, REN Liu-yi, WANG Pei-pei, YANG Cong-ren, ZHANG Yan-sheng. Electro-flotation and collision-attachment mechanism of fine cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 917-924.
[8] WILLS B A, NAPIER-MUNN T J. Mineral processing technology [M]. Netherlands: Elsevier Ltd, 2006: 267.
[9] XU Yang-bao, QIN Wen-qing, LIU Hui. Mineralogical characterization of tin-polymetallic ore occurred in Mengzi, Yunnan Province, China [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 725-730.
[10] SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces, 2001, 205(1-2): 47-59.
[11] QIN Wen-qing XU Yang-bao, LIU Hui, REN Liu-yi, YANG Cong-ren. Flotation and surface behavior of cassiterite with salicylhydroxamic acid [J]. Industrial and Engineering Chemistry Research, 2011, 50(18): 10778-10783.
[12] LI Hai-pu, ZHANG Sha-sha, JIANG Hao, LI Bin, LI Xing. Effect of degree of substitution of carboxymethyl starch on diaspore depression in reverse flotation [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1868-1873.
[13] WU X Q, ZHU J G. Selective flotation of cassiterite with benzohydroxamic acid [J]. Mineral Engineering, 2006, 19(14): 1410-1417.
[14] ZENG Qing-hua, ZHAO Hong, WANG Dian-zuo. Influence of metal cations on cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(1): 98-101.
[15] GHOSH K K, SINHA D, SATNAMI M L, DUBEY D K, SHRIVASTAVA A, PALEPU R M, DAFONTE P R. Enhanced nucleophilic reactivity of hydroxamate ions in some novel micellar systems for the cleavage of parathion [J]. Journal of Colloid and Interface Science, 2006, 301(2): 564-568.
[16] GLORIUS M, MOLL H, GEIPEL G, BERNHARD G. Complexation of uranium(VI) with aromatic acids such as hydroxamic and benzoic acid investigated by TRLFS [J]. Journal of Radioanalytical and Nuclear Chemistry, 2008, 277(2): 371-377.
[17] LIN Qiang. The synthesis of new flotation agents and research on the relationship between structure and performance [D]. Changsha: Central South University of Technology, 1989: 28-54. (in Chinese)
[18] ZHU Yu-shuang, ZHU Dan. Adsorption thermodynamics of octyl hydroxamic acid on cassiterite [J]. Nonferrous Metals, 1994, 46(1): 24-28. (in Chinese)
[19] ZHU Jian-guang. Flotation reagent [M]. Changsha: Central South University of Technology Press, 1993: 202-205. (in Chinese)
[20] ZHOU Yu-lin, YUAN Hao. Improvement of synthesis process of salicylhydroxamic acid [J]. Modern Mining, 2009(1): 47-50. (in Chinese)
[21] FUERSTENAU D W, PRADIP. Zeta potential in the flotation of oxide and silicate minerals [J]. Advances in Colloid and Interface Science, 2005, 114-115(30): 9-26.
[22] ALVAREZ-SILVA M, URIBE-SALAS A, MIRNEZAMI, M, FINCH J A. The point of zero charge of phyllosilicate minerals using the mular-roberts titration technique [J]. Mineral Engineering, 2010, 23(5): 383-389.
[23] BOGDANOVA N F, KLEBANOV A V, ERMAKOVA L E, SIDOROVA M P, ALEKSANDROW D A. Adsorption of ions on the surface of tin dioxide and its electrokinetic characters in 1:1 electrolyte solutions [J]. Colloid Journal, 2004, 66(4): 409-417.
[24] PAVEA O, BRANDAO P R G, PERES A E C. Adsorption of oleate and octyl-hydroxamate on to rare-earths minerals [J]. Mineral Engineering, 1996, 9(3): 357-366.
[25] ZHU Yu-shuang, ZHU Jian-guang. The chemical principle of flotation reagents [M]. Changsha: Central South University of Technology Press, 1987: 177-185. (in Chinese)
[26] QIN Wen-qing WEI Qian, JIAO Fen, LI Ning, WANG Pei-pei, KE Li-fang. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena [J]. International Journal of Science and Technology, 2012, 22(3): 345-349.
[27] WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation [M]. Changsha: Hunan Science and Technology Press, 1988: 221-224. (in Chinese)
[28] CHENG J G. Research on a new collector of fine cassiterite-phosphorous ester [D]. Changsha: Central South College of Mining and Metallurgy, 1985: 72. (in Chinese)
[29] ASSIS S M, MONTENEGRO L C M, PERES A E C. Utilization of hydroxamates in minerals froth flotation [J]. Minerals engineering, 1996, 9(1): 103-114.
[30] QIU Xian-yang, CHENG De-ming, WANG Dian-zuo. Reation mechanism between Benzoylhydroxamic acid and scheelite [J]. Mining and Metallurgical Engineering, 2001, 21(3). (in Chinese)
[31] LU Yi-ping, ZHANG Ming-qiang, FENG Qi-ming, LONG Tao, OU Le-ming, ZHANG Guo-fan. Effect of sodium hexametaphosphate on separation of serpentine from pyrite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 208-213.
[32] JING Xu-ying, CHEN Shi-di, ME En-yun. Guide of the usage of infrared spectrum [M]. Tianjin: Tianjin Science and Technology Press, 1992: 363. (in Chinese)
[33] YIN Zhi-gang. The synthesis of alkyl carboxyl hydroxamic acids and their flotation properties to the aluminosilicate minerals [D]. Changsha: Central South University, 2010: 50. (in Chinese)
[34] QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong Adsorption mechanism of mixed salicylhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711-1717.
[35] LU Yong-quan, DENG Zhen-hua. Analysis of practical infrared spectrum [M]. Beijing: Electronic Industry Press, 1989: 132. (in Chinese)
[36] ZHU Yu-shuang, ZHU Jian-guang. The chemical principle of flotation reagents [M]. Changsha: Central South University of Technology Press, 1996: 117. (in Chinese).
烷基羟肟酸对细粒锡石的浮选及其溶液化学性质
王佩佩1,覃文庆1,任浏祎1,魏 茜1,刘瑞增1,杨聪仁1,衷水平2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 低品位矿综合利用国家重点实验室,上杭 364200
摘 要:采用烷基羟肟酸作为捕收剂对细粒锡石进行浮选,并对其溶液化学性质进行研究。结果表明,3种烷基羟肟酸在碱性条件下对细粒锡石有较好的捕收能力,且适宜的浮选pH随着捕收剂碳链的加长而升高。金属离子对锡石浮选的影响主要由矿浆pH决定,金属离子浓度对锡石浮选也有影响。计算基团电负性得出烷基羟肟酸作为锡石捕收剂的适合烷基碳链长度大约为7。由溶液的组分浓度对数分布图(lg c—pH)分析可知,捕收剂以分子-离子共吸附模式作用于矿物表面。动电位测试及红外光谱分析表明,吸附反应中涉及到电荷作用力、氢键力和络合作用力,其最终产物可以表示为锡石的O, O-五元环结构的螯合物。
关键词:细粒锡石;浮选;烷基羟肟酸;螯合物
(Edited by Hua YANG)
Foundation item: Project (2010CB630905) supported by the National Basic Research and Development Program of China; Project (51274255) supported by the National Natural Science Foundation of China; Project supported by the Foundation of State Key Laboratory of Comprehensive Utilization of Low-Grade Ores (Zijin Mining Group Co., Ltd), China
Corresponding author: Wen-qing QIN; Tel: +86-731-88830884; E-mail: qinwenqing369@126.com
DOI: 10.1016/S1003-6326(13)62662-X
Abstract: Solution chemistry and flotation of cassiterite in the presence of alkyl hydroxamic acid collector were investigated. Results show that the alkyl hydroxamic acid is able to float fine cassiterite very well at relatively alkaline conditions, and the pH range of better flotability broadens with the increase of chain length of the collector. Moreover, the metal cations influence the flotation mostly depending on the pH of the medium and to some extent on the concentration of metal cations. Theoretical calculation on the group electronegativity indicates that the alkyl chain length of about seven is of great superiority in the synthetization of hydroxamic acid collector for cassiterite flotation. A co-adsorption between molecules and ions onto cassiterite surface is considered according to the lg c—pH diagram analysis. In terms of zeta potential and infrared spectra, the main interaction involves electric charge interaction, hydrogen bonding interaction and complexation, with the proposed formation of an O, O-five membered rings chelate compound.


