
J. Cent. South Univ. (2019) 26: 2688-2703
DOI: https://doi.org/10.1007/s11771-019-4206-4
Characterization of carbon fibers recovered through mechanochemical-enhanced recycling of waste carbon fiber reinforced plastics
NZIOKA Antony Mutua1, ALUNDA Bernard Ouma2, YAN Cao-zheng(鄢曹政)3, SIM Ye-Jin4,KIM Myung-Gyun5, YOON Bok-Young1, KIM Young-Ju4
1. R & D Institute, Silla Entech Company, Daegu 41566, Korea;
2. Department of Mechanical Engineering, Kyungpook National University, Daegu 41566, Korea;
3. Department of Biochemical and Environmental Engineering, Hanjiang Normal University,Shiyan 442000, China;
4. Department of Environmental and Energy Engineering, Kyungpook National University,Daegu 41566, Korea;
5. Department of Health Environment, Daegu Health College, Daegu 41453, Korea
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
In this study, we present the characterization of the carbon fibers recovered from the mechanochemical-enhanced recycling of carbon fiber reinforced fibers. The objectives of the study were to investigate the effect of our modified recycling method on the interfacial properties of recovered fibers. The reinforced plastics were recycled; the recycling efficiency was determined and the recovered fibers were sized using 1 wt% and 3 wt% concentration of (3-aminopropyl)triethoxysilane. We characterized the morphologies utilizing the electron spectroscopy for chemical analysis (ESCA), atomic force microscopy (AFM), FTIR-attenuated total reflection (ATR) spectroscopy and scanning electron microscopy (SEM). Although the surface of the fibers had no cracks, there was evidence of contaminations which affected the interfacial properties and the quality of the fibers. Results showed that the trends in the recovered and virgin fibers were similar with an increase in sizing concentration. The results highlighted the perspectives of increasing the quality of recovered fibers after the recycling process.
Key words:
Cite this article as:
NZIOKA Antony Mutua, ALUNDA Bernard Ouma, YAN Cao-zheng, SIM Ye-Jin, KIM Myung-Gyun, YOON Bok-Young, KIM Young-Ju. Characterization of carbon fibers recovered through mechanochemical-mechanochemical-enhanced recycling of waste carbon fiber reinforced plastics [J]. Journal of Central South University, 2019, 26(10): 2688-2703.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4206-41 Introduction
Several researchers have proposed and developed the chemical recycling process of the waste carbon fiber reinforced plastics (CFRP) as one of the viable and environmentally friendly recycling processes. This process uses known and environmentally friendly solvents (e.g., water, methanol, benzyl alcohol, etc.) in different process conditions to produce recyclable products [1-6]. The conventional chemical recycling process involves the breaking down of high molecular weight polymeric materials to low molecular weight monomers as well as producing carbon fibers with minimal or negligible damage to the mechanical properties [3]. From the multitude of researches reported that are related to chemical recycling/depolymerization process, NAKAGAWA et al [3] and ZHAO et al [4] showed that depolymerization process of FRP at the normal pressure using benzyl alcohol might be more efficient and sustainable. Notwithstanding the cost of solvent/reagent used and the hazardous gases generated as reported by SUN et al [7], previous research has proven otherwise.
On the contrary, subcritical/supercritical recycling process requires a significant amount of energy to maintain the necessary pressure and the process conditions of the solvent [8-11]. The process is costly, and several researchers have noted that higher operating temperatures might have an adverse effect on the tensile strength and the quality of the fibers (i.e., depolymerized product). The fact is evident from the by-products (e.g., char, hazardous fumes) generated and reported in other publications [12, 13].
In our research, we sought to recycle the waste FRP from decommissioned ship/boats using a modified version of the chemical recycling process/es that utilizes benzyl alcohol. This process, relative to other processes (e.g., NAKAGAWA et al [3] and ZHAO et al [4]), uses the mechanochemical principle to enhance the depolymerization and separation process of the depolymerization products. Depolymerization process reported by ZHAO et al [4] and NAKAGAWA et al [3] utilized high solvent temperatures to depolymerize the waste FRP: these authors did not use the force of synthesis (i.e., mechanochemical process) to intensify the depolymerization reaction. We concluded that the high solvent temperature influences the depolymerization reaction and the utilization of mechanochemical processes is inherent in the intensification of the process as well as reduce the overall amount of energy required to maintain the high temperature necessary for the depolymerization process [14].
The overall concept of this modified process is to produce recyclable and reusable products (i.e., fibers and resin) of high quality within a treatment period that might be justified from the techno-economic point of view. The concept of our process hinges on the idea that the 4th industrial revolution, just like any other industrial revolution, will require more resources which are limited in nature. In his opinion blog entitled “Waste industry must prepare for fourth industrial revolution”, MAVROPOULOS [15] highlighted that the fourth industrial revolution would provide unprecedented opportunities, efficient utilization of resources, exponential increase in consumption and generation of waste. Furthermore, other researchers found that human beings tend to consume and generate more waste when they are aware that the material will be recycled [16-18]. The exponential growth in consumption and generation of waste coupled with the “unpredictable human behavior” related to CATLIN et al [16] research findings will require an adequate supply of limited resources of which recycling is one of the options that we may utilize.
Several researchers have focused on different analyses to attest the quality of recycled fibers: these analyses range from the mechanical and interfacial characteristics of the recycled fibers (glass and carbon fiber) under different conditions [3, 10, 19-21]. Other research results related to other methods of recycling/disposal other than chemical recycling have focused on the treatment of woven cloth recovered from mechanical separation of GFRP recovered from the end-of-life ships [22]. We also recognize the research findings by OKAJIMA et al [11] showing the mechanical and chemical characterization of recovered fibers. In this particular research, the authors utilized the electron spectroscopy for chemical analysis (ESCA) technique to determine the chemical composition on the surface of the fibers. This method, relative to the technique used by AKONDA et al [23] can also be used to understand the interfacial adhesion properties of sized fibers sized with coupling agents. Several authors highlighted the applicability of ESCA and atomic force microscopy (AFM) by analyzing the interfacial performance of fibers based on different conditions [24, 25]. Research highlighting the interfacial properties of the sized fibers recovered through chemical recycling under normal pressure has not been presented hitherto. According to WEHLAK et al [26], the interfacial properties, which are essential in determining the long-term stability of an adhesive joint, is little known and hidden for our understanding. Therefore, these research findings will provide additional knowledge on the interfacial properties of the carbon fibers.
The objective of this research is to analyze the effect of our modified process on the interfacial properties of the recovered carbon fiber. The objectives will be achieved by observing the interaction between the surfaces of the carbon fibers obtained from our modified process and coupling agent used in this study. We will use the electron spectroscopy for chemical analysis (ESCA), atomic force microscopy (AFM), Fourier transforms infrared spectroscopy (FTIR) and scanning electron microscopy techniques to achieve our objectives. This paper will cover the material and experimental procedures used in the analysis, results and the discussion thereof.
2 Materials and methods
2.1 Material recovery procedure
Figure 1 shows the diagram of the apparatus for the depolymerization process and the material recovery and surface analysis workflow diagram. The sample material used in this study was waste CFRP obtained from the marine structures, Korea. SHENOI et al [27] provided detailed information about the properties of the materials and the manufacturing processes thereof similar to our sample material. The solvent used in this study is reagent-grade benzyl alcohol (Boiling point=205 °C, Formula weight=108.14, ρ=1.043-1.048 g/mL) and was supplied by Duksan Pure Chemicals, Korea.
The depolymerization reactor consists of modified water-cooled ultrasound probe attached to an ultrasonic generator(Sonics VC–750) and a laboratory mixer (IKA Eurostar 40 digital) equipped with a turbine impeller. The combination of ultrasound cavitation and turbulent mixing processes facilitated by the ultrasound probe and the laboratory mixer were considered to be our mechanochemical process for this study. High temperature required for the process was facilitated using a laboratory heater. We prepared the sample by cutting into small pieces (30 mm×30 mm×7 mm), inspected and removed undesirable impurities from the sample’s surface. We immersed the samples into the solvent (initial temperature of 100 °C) for 20 min followed by the depolymerization process coupled with the combination of ultrasound cavitation and turbulent mixing. The solvent/CFRP ratio (V/V) was 20:1. The depolymerization process was conducted for a period of 30-60 min and the initial temperature was 100 °C. Later on, the products generated were separated and the liquid product filtered out using a vacuum filtration unit. The remaining solid portion (carbon fibers and insoluble solid) was poured into beakers containing ethanol (Duksan Pure Chemicals) and mixed. We separated the carbon fibers from the remaining insoluble solids and calculated the effectiveness of the recycling process. The effectiveness was calculated using the following expression used in other publications [7, 28]:
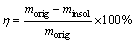 (1)
(1)
where morig and minsol represent the initial mass of the FRP before depolymerization and the mass of insoluble solids after depolymerization and washing process, respectively. Later on, the fibers were suspended in acetone (Duksan Pure Chemicals) as proposed by GARDNER et al [29].
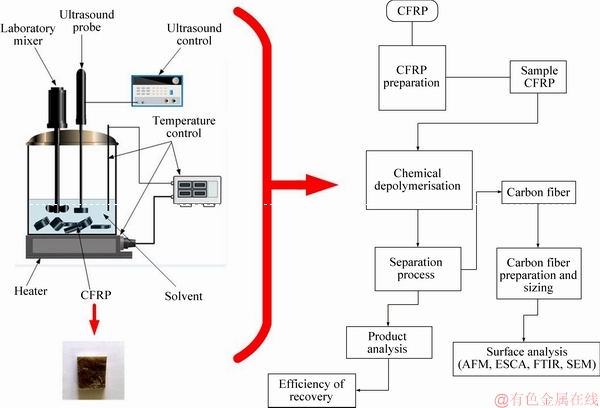
Figure 1 Experimental workflow diagram for material recovery process from carbon fiber reinforced plastics
2.2 Fiber sizing preparation
Intensive research has been innate in the development of a variety of coupling/sizing agents of which organosilanes are one of them. Organosilanes have been used to diversify the surface characteristics of fibers depending on the level of oxidation of the respective fibers. Application of sizing procedure was conducted based on experimental procedures presented in previous publications [30-33]. We used (3-aminopropyl)triethoxysilane (Sigma-Aldrich, USA) to coat the unsized recovered carbon fibers and for simplification purpose, the abbreviation (APTES) was used to signify (3-aminopropyl) triethoxysilane. Some researchers have deemed essential to study the interfacial properties at different concentrations [34-36]. Therefore, we prepared 1 wt% and 3 wt% APTES solution byhydrolyzingan equivalent amount of the APTES in deionized water at room temperature. The solutions were stored at room temperature for 1 h. The unsized recovered carbon fibers that were suspended in acetone were removed and washed several times with deionized water after which they were dried at room temperature for 24 h. Before sizing, the fibers were pretreated using the protocol proposed by NIE [33]. Based on this procedure, approximately 2.62 g of KMnO4 was added into 250 mL of 0.5 mol/L H2SO4 aqueous solution, and the resulting solution was thoroughly mixed using a magnetic stirrer. About 0.5 g of the recovered fibers were dipped into the resulting H2SO4/KMnO4 solution and sonicated for 3 h at room temperature. The fibers were then filtered out and spiked with concentrated HCl. The fibers were washed with deionized water several times. Afterward, the pretreated unsized fibers were equally divided based on the classification (Table 1) and requirements for different analysis and then suspended in 1 wt% and 3 wt% APTES solution for 15 min. The samples were removed and dried in a vacuum oven (Jeio Tech OV – 11/12) for 6 h at a temperature of 50 °C. Part of the samples sized in 1 wt% and 3 wt% APTES solution were immersed in water (50 °C) and acetone (room temperature) for 24 h after which they were dried again in the vacuum oven (Jeio Tech OV–11/12) for 2 h. For this experimental study, the samples were classified with abbreviations shown in Table 1. The overall goal of the recycling process is to obtain recovered fibers with the characteristics equivalent to those of the virgin fibers, and thus the spectra from the virgin fibers were obtained for comparison. The virgin fibers were subjected to similar sizing procedures explained in this section.
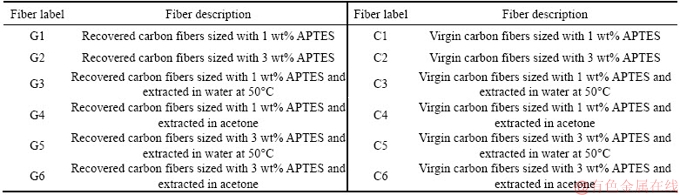
Table 1 Classification of samples used in experiment based on sizing and extraction processes thereafter
2.3 Analytical instrument procedures
Atomic force microscope (Model XE-70) from Park Instruments was used to characterize the surface of the sized and extracted carbon fibersrecovered from our modified recycling method. This analytical equipment was equipped with TESP cantilevers (Bruker Nano) operated at room temperature in air using non-contact mode and at the scan speed of 0.4 Hz for a scan area of approximately 10 μm×10 μm. We obtained 256× 256 pixel topography from the AFM. For each sample, several topographic measurements were made from the 3-4 test strips. The number of measurements increased if the significant difference between the measured surface roughness and the mean value was characteristic. The XEI version 4.3.0 software from Park systems was used to process the topographic images and analyze the surface roughness. ESCA was carried out in Quantera SXM from ULVAC-PHI Inc., USA. This ESCA equipment was equipped with a monochromatic Al-Kα X-ray source and a <9 μm focused beam. The fiber tows were mounted on the sample holder using a double-sided adhesive tape and placed into the vacuum chamber of the analytical equipment where they were analyzed at a contact angle of 45°. The resulting C 1s and O 1s peaks were corrected using linear and/or Tougaard background and fitted using Lorentzian–Gaussian functions. Additional analysis of the chemical structure was carried out using FTIR spectrometer (Perkin Elmer Frontier) equipped with a universal-type single reflection attenuated total reflectance crystal (diamond crystal) at room temperature. Several scans were made at a scanning speed of 1 cm-1 and spectral range between 4000 and 600 cm-1. In addition to the aforementioned analyses, SEM analyses for all the samples that were sized and extracted using water (50 °C) and acetone were carried out using FE-SEM (Hitachi SU8200). SEM images were used to supplement the research results from our study.
3 Experimental results
3.1 Efficiency of depolymerization process
Experimental results revealed that the ultrasound cavitation process was inherent in the increase in the solvent temperature from 100 °C to approximately 170 °C during the specified depolymerization period. The solvent temperature increased due to the high energy emission from the collapse of bubbles created by the ultrasound equipment [37]. Figure 2(a) illustrates the rate of change in solvent temperature, the recycling efficiency with the depolymerization time and the products generated from the recycling process. The efficiency of recycling increased with an increase in the solvent temperature and the swelling process of the waste CFRP was characterized during the depolymerization period. Figure 2(b) shows the swelling phenomena and the swelling ratio observed and reported in our previous publication [38]. The effectiveness of the recycling process, (η); increased as the rate of increase in the solvent temperature reduced. During the reaction period of 10-20 min, the significant debonding process had taken place and the resulting product was in the form of thin layers of wet recovered fibers.
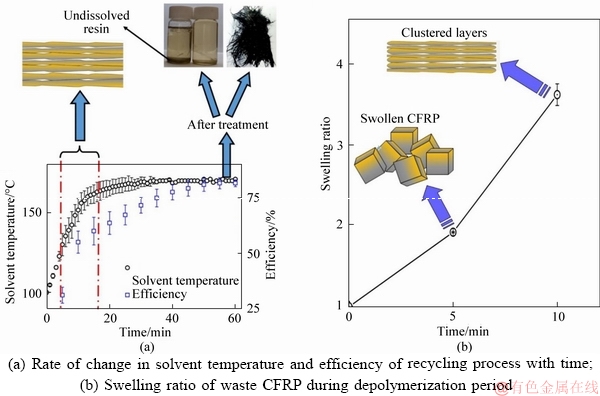
Figure 2 Dependence of recycling efficiency with time:(Note: swelling ratios were reported in Ref. [38])
The debonding process, which was observed and reported in our previous research publication [38], led to the creation of layers that could be easily dismantled thanks to the turbulent mixer included in the reactor. Similar characteristics were also observed in our previous study where we recycled glass fiber reinforced fiber using a similar method to recover the glass fibers and a mixture of resin and solvent [39].
3.2 ESCA analysis
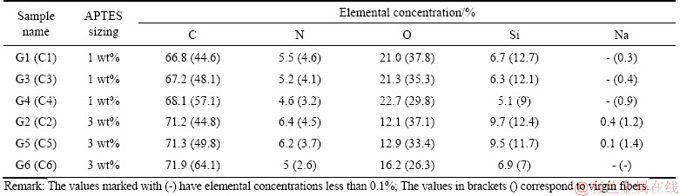
Hitherto there are many studies conducted related to the surface characterization of the carbon fiber surface using the angle-resolved ESCA: the carbon fibers studied were either coated or treated under different conditions. The results of some of these studies will be cited in this study for comparison purposes. Figure 3 shows the ESCA wide spectra of the recovered carbon fibers samples G1-G2. Additional spin-orbital splittings (Ca 2s,N 1s, etc.) were detected at 3% APTES concentration relative to 1% APTES concentration. Table 2 shows the elemental composition of the fibers’ surfaces from the ESCA analysis of the recovered (G1-G6) and virgin (C1-C6) fibers. From Figure 3 and Table 2, the difference in the wide spectra and the elemental composition between samples sized with APTES at different concentrations were characteristic.
The carbon content was higher than the rest of the elements followed by oxygen and silicon when the APTES concentration was 1 wt% (Table 2). When the concentration of the APTES was increased, the carbon, nitrogen and silicon concentrations increased while the oxygen concentration decreased. Virgin fibers exhibited a small change in the concentration of the elements investigated (<1% difference).
As the fibers were subjected to the extraction the changes were evident in all samples (G1-G6) recovered after recycling. Although the carbon concentration of the virgin fibers (C1-C6) increased during the extraction process using water and acetone, the concentration of the rest of the elements reduced at different rates. LIU et al [40] investigated the effect of hot water and acetone extraction and on the atomic concentration of fibers coated with an organosilane. The authors observed changes in the atomic concentration on the surface of the fibers and that the differences were more evident after acetone extraction: the changes in the atomic concentration were apparent in this experimental study. After acetone extraction process using water and acetone, carbon and oxygen content increased while the silicon content decreased. Figures 4 and 5 show the C 1s and O 1s spectra respectively for the recovered carbon fibers samples G1 and G2 while Tables 3 and 4 show the peak area of the C 1s and O 1s spectra for samples G1-G6 and C1-C6.
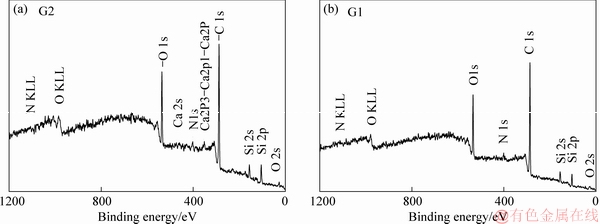
Figure 3 ESCA wide spectra of recovered carbon fibers samples G2 (a) and G1 (b)
Table 2 Elemental concentration of virgin and recovered carbon fibers sized with APTES
From the C 1s spectra, it was observed that the C—C bond was dominant in samples G1 and C1 and that as the concentration of the APTES sizing increased, a shift in the C 1s peak was observed (see Figure 6). The changes in the peak area of C—C, C—O—C and C=O bonds were characteristic. The changes in the peak areas are presented in Table 3. The peak area of the C—C functional group increased when the sized samples were subjected to extraction using water at 50 °C and acetone. There was a marginal increase (approximately 0.5%) in the peak area when samples G1 and G2 were extracted using acetone, and that the increase was smaller as compared to the virgin fibers (1%-2% ) as shown in Table 3. The C—O—C and C=O functional groups showed varying results. From the O 1s spectra, a shift in the O 1s peak, similar to that observed for C 1s peak was characteristics (see Table 4). Likewise, the C—O functional group exhibited a greater peak area than the rest of the functional groups investigated (C—OH and chemisorbed oxygen).
As the concentration of the APTES sizing increased, the shift in the peak resulted in an increase in the C—OH peak area and a significant decrease in the C—O peak area. Even though the spectra obtained from the recovered fibers has C—OH peak is higher than the rest of the functional groups (i.e., C—O and chemisorbed oxygen), the increase in the same (i.e., C—OH) after an increase in the concentration of the APTES sizing was characteristic. A similar trend was observed with the chemisorbed oxygen. As the recovered fibers were subjected to the extraction process, the C—O and chemisorbed oxygen functional groups peak area increased while the C—OH functional groups decreased. A similar trend was observed in the virgin fibers.
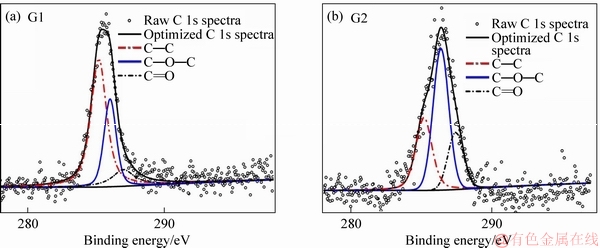
Figure 4 C 1s spectra of recovered fibers sized samples G1 and G2
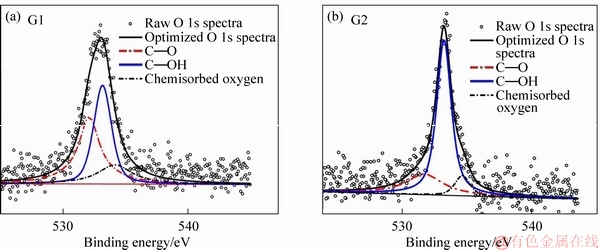
Figure 5 O 1s spectra of recovered fibers sized samples G1 and G2
Table 3 Data for C 1s peak of the recovered fibers sample G1-G6 and C1-C6
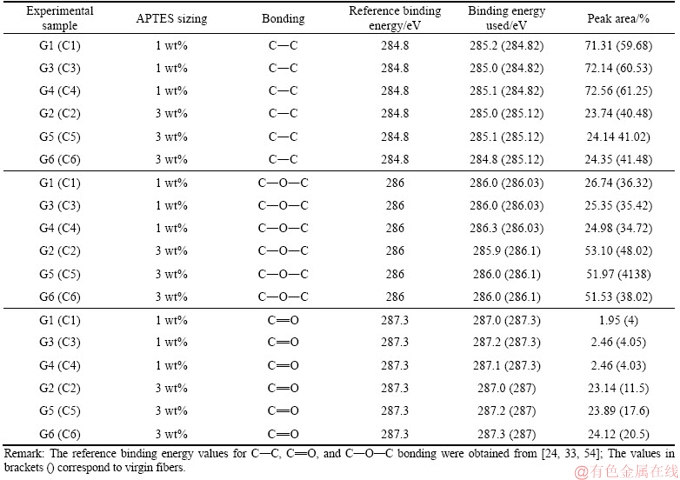
Table 4 Data for O 1s spectra of carbon fibers sample G1-G6 and C1-C6
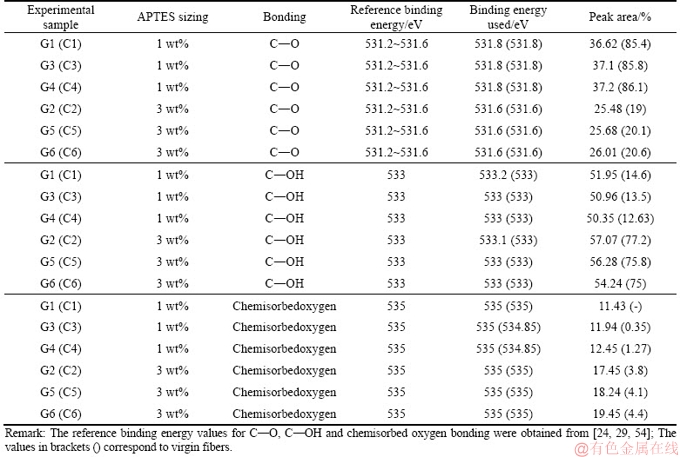
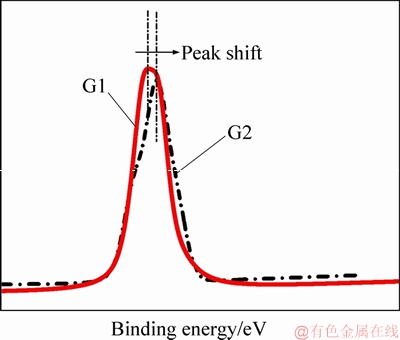
Figure 6 Representation on shift in C1s peak between sample G1 and G2
3.3 FTIR-ATR analysis
Figures 7(a) and (b) show the FTIR-ATR spectra of the recovered carbon fibers G1-G6 while Figures 7(c) and (d) show the FTIR-ATR spectra of the virgin fibers. From the FTIR-ATR spectra, the recovered carbon fiber samples G1-G6 exhibited different levels of stretching across the spectra. One of the noticeable vibrations were in diapason of 3500-4000 cm-1 where the stretching was visible after sizing with APTES and decreased in the course of the extraction process. This trend was evident for all pretreated virgin and recovered fibers: these forms of vibrations were characteristic of the stretch related to the amide N—H and/or OH functional groups. NIE [33] observed broadband of OH group from 3000-3600 cm-1. It is possible that the vibrations noticed in the spectra correspond to the OH function group and the corresponding vibrations diminished with the extraction process: the vibrations were not evident when the fiber was extracted using acetone. There were broad bands in the range from 800-1280 cm-1 which corresponded to the different levels of oxidation of the fibers, and the broad bands were exhibited on both the recovered and virgin fibers [33]. At 1700-1750 cm-1, there was peak which could correspond to the C=O stretch which increased when the sized fibers (i.e., recovered fibers) were subjected to the extraction process using hot water (50 °C) and acetone. The C=O stretching was more evident in the recovered fibers which were extracted using acetonethan in other fibers. Even though the instrument did not identify this stretching, there was evidence of similar stretching in virgin fibers especially when the concentration of the APTES increased to 3% (Figure 7(d)).
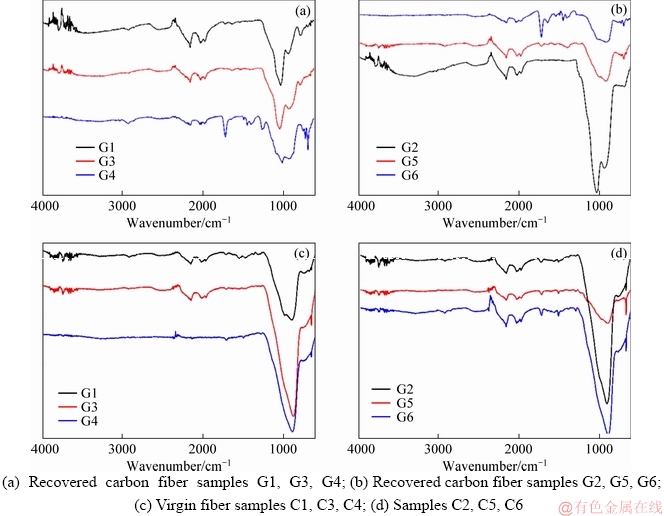
Figure 7 FTIR-ATR spectra of sized carbon fiber samples:
3.4 Surface morphology
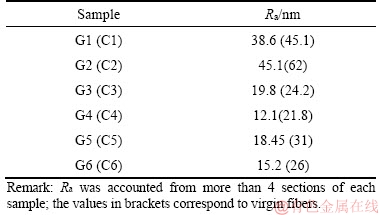
The XYZ scale was used to transform the 3D images obtained from the atomic force microscope and are shown in Figure 8. Table 4 shows the surface roughness of the samples G1–G6. From the scans, it was evident that the sizing used created a smooth coating on the surface of the recovered fibers. Sample G2 exhibited an even smoother surface with additional bubble-like protrusions of the APTES sizing. Sample G2 had greater surface roughness than G1, and this was attributed to the concentration of the APTES sizing (see Table 4). NIE et al [41] and ZAKIR et al [42] indicated that the application of sizing or coupling agents on fibers was inherent in increasing the surface roughness of the fibers. Therefore, an increase in the concentration of APTES might have increased the surface roughness of the recovered fibers.
As the samples were subjected to extraction using water at 50 °C, part of the APTES sizing was eliminated from the surface of the recovered fibers (samples G3 and G5) and that considerable impurities protruded above the surface of the APTES sizing. Likewise, the contaminants coated with APTES sizing were seen on samples G3 and G5. However, sample G5 exhibited minor impurity protrusions than sample G3. The surface roughnesses of samples G3 and G5 are shown in Table 4. When sample G4 and G6 were subjected to acetone extraction, the impurity protrusions were more than those in samples G3 and G5. However, the presence of impurities coated with APTES sizing was more evident in sample G6 than in G4. The values of the surface roughness provided in Table 4 highlights the surface roughness of sample G4 and G6.
Figure 9 shows the SEM scans obtained for samples G1-G6 and sample G2 exhibited a thick layer of APTES coating than the sample G1. Also, the samples G1 and G2 showed a smooth surface as compared to unsized surfaces. As the samples G3 and G5 were subjected to extraction using hot water, the layer of the APTES sizing was reduced, and presence of impurities was evident in sample G3 while the sample G5 exhibited minimal or negligible impurity protrusion on the surface. These surface topographies corroborated with the results obtained using AFM.
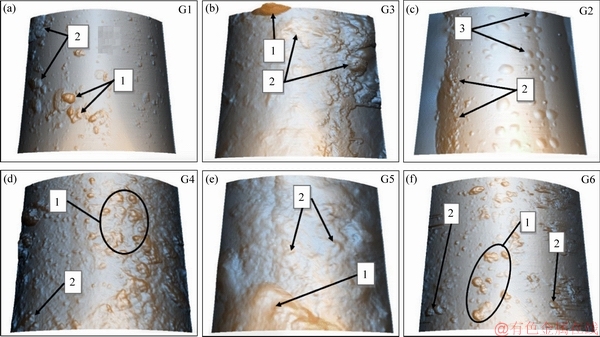
Figure 8 Surface morphology of recovered carbon fibers obtained using AFM: (Area 1-Impurities; 2-Impurities coated with APTES sizing; 3-APTES sizing)
Table 4 Surface roughness of recovered and virgin carbon fibers sized with APTES
4 Discussion
The results of this study indicated that the recovered fibers exhibited similar interphase properties as the virgin fibers. However, the results presented above put into question the quality of the recovered fibers. Extensive discussion by THOMASON et al [13] indicated that the quality of the fibers was affected by the thermal recovery/ recycling process. Presence of impurities in the fibers could also impede the fibers’ quality and the surface chemistry. For example, LIU et al [40] observed a significant increase in the concentration of oxygen during the analysis and attributed the carbonaceous contamination. These contaminants were covered with a thicker layer when the concentration of APTES increased from 1% to 3% (see Figure 9). Thus, at lower APTES concentration (i.e., 1 wt%), the concentration of elemental oxygen was higher than at 3 wt%.
There was a shift in the spectra with an increase in APTES concentration which was observed in both the recovered and virgin fibers as mentioned before. Usually, a change in the spectra is an indication of the change in the chemical state associated with the respective chemical bonding or the addition and/or removal of the functional group [43, 44]. From the spectroscopic studies conducted by PROCTOR and SHERWOOD [45], it was evident that the shift in question might have been caused by the changes in the surface chemistry of the fibers associated/bonded with carbon as presented in the previous section. Thus, this highlighted that the recovered fibers exhibited similar interphase properties (after recovery) as the virgin fibers irrespective of the quality of the recovered fibers. This tendency (i.e., spectra shift) observed in the recovered fibers and confirmed in the virgin fibers indicates the possibility of reutilizing these fibers after improving the quality. Even though the changes in the oxygen concentration after an increase in the APTES was characteristic, the trend could be associated with the increase in carbon content at 3% APTES [45]. Thus as the carbon concentration increased, the oxygen concentration also increased.
C—C peak area, representing hydroxyl or phenolic compounds, might have reduced due to the increase C—O—C and C=O functional groups. Pretreatment process using H2SO4/KMnO4 solution could have improved the surface thus creating additional linkage for the C—O—C and C=O functional groups. QIAN et al [46] reported that oxidation-like pretreatment processes are inherent in the increase in the oxygen-containing functional groups. MUNOZ-VELEZ et al [47] also reported a rise in oxygen concentration after pretreatment (oxidation) process. The varying results exhibited by C—O—C and C=O functional groups could also be attributed to the quality of the fibers or on the narrow bands characterized by the oxygen atom in the O 1s spectra.
As the sized fibers were subjected to the extraction process, there were significant changes in the elemental concentrations and spectral areas. Usually, the extraction process/es are characterized by the erosion/peeling-out of the sizing [39, 40, 48] as shown in the SEM scans (see Figures 6 and 7). As a result, the oxygen-containing functional groups were extracted from the surface of the carbon fibers: the respective peak areas reduced during the extraction process (Table 3). The peeling-off effect of the oxygen-containing functional groups was evident with the hydroxyl group which was reduced when the fibers were subjected to the extraction process (Figure 5).
Oligomers with weak bonds, physisorbed to the surface and soluble in water, could have been removed during the extraction process using hot water while other stable sizing polymers (including silane) were removed during the extraction process using acetone. METWALI et al [49] reported that loosely bounded physisorbed compounds were easily removed with water/alcohols while stronger chemicals (e.g., acids) could be used to remove chemisorbed functional groups such as silane molecules. The erosion of the sizing might take place depending on the nature ofthe sizing/coating on the surface of the recovered fibers. THOMASON et al [50] indicated that the ESCA equipment detects photoelectrons from the emitted from the sizing or the “variable volume” of the fiber surface below the sizing: these electrons are inherent in characterizing the different elements. The sizing might have been inhomogeneous relative to the surface and that the erosion process was not even across the fiber surface: inhomogeneous erosion phenomenon was discussed by THOMASON et al [50]. Likewise, JING [51] and SCHULTZ et al [52] have highlighted the importance of quality on the interfacial and mechanical properties of the fiber or fiber-based composites. Surface roughness is the essential property for assessing the interfacial adhesion of fibers and reinforced plastics in general as well as the resulting mechanical properties of materials [53]. Usually, an increase in the surface roughness will promote adhesion, guarantee excellent interaction between fibers and the resin matrix: this property is essential since carbon fibers have been known to exhibit poor interfacial characteristics. Therefore, we concluded that the surface roughness increased because of the chemical modification and the APTES sizing applied. The extraction process contributed to the decrease of the surface roughness by 50%-70% for both the recovered and virgin fibers. Such a reduction (i.e., 50%-70%) could have untold effects on the interfacial characteristics of the reinforced composite, and we will consider this factor in the ongoing research.
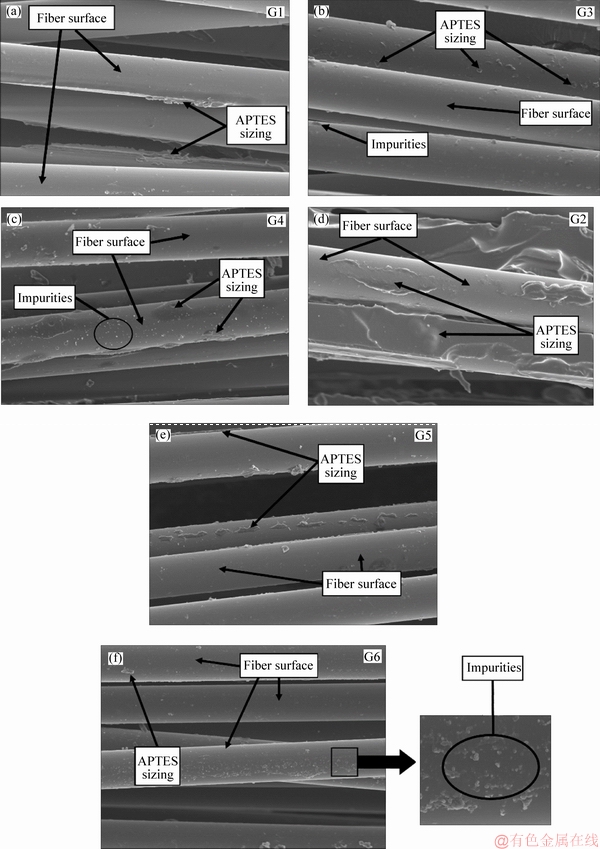
Figure 9 SEM images of recovered fibers samples G1-G6
5 Conclusions
This research focused on the interfacial characteristics inherent in the manufacturing process of the reinforced composites. We utilized ESCA, AFM, FTIR, and SEM to describe the interfacial properties thereof and we compared with virgin fibers. From the experimental results, the following conclusions were made:
1) Proposed recycling revealed higher recycling efficiency for less than 1 h, thanks to the mechanochemical process used.
2) Mechanism of the depolymerization process revealed the significance of the combination of the mechanochemical process.
3) ESCA analysis showed the changes in the elemental concentration at different APTES concentrations and extraction processes thereof.
4) Changes in concentration of the APTES sizing led to shifts in the peak on the O 1s and C 1s spectra.
5) The C—C bond was dominant in samples G1, G3 and G4 and this dominance diminished in samples G2, G5 and G6 when subjected to a higher concentration of APTES sizing due to increasing in the oxygen-based functional groups.
6) The extraction process using water and acetone removed loosely-linked physisorbed and chemisorbed functional groups respectively.
7) FTIR-ATR analysis showed vibrations associated with the OH functional groups and broad bands related to the different level of oxidation.
8) Surface morphology of samples G1 and G2 exhibited a smooth surface covered with APTES sizing and that the surface characteristics changed when samples were subjected to extraction. Impurity protrusions were more evident in samples G3-G6 after extraction using water and acetone than in samples G1-G2.
9) The surface roughness of the fibers reduced by 50%-70% after extraction.
A combination of ESCA, AFM, FTIR, and SEM provided a set of results inherent to describe the interfacial properties of the fibers recovered through chemical recycling and a comparison of the same with virgin fibers. The recycling process in this study did not encompass CFRP with different geometric forms manufacturing using different methods. Our future work will focus on the various waste FRP materials produced after the decommissioning process of structures such as wind turbines (after exploitation process), marine and/or aerospace structures. In addition, our ongoing research highlighted the importance of researching on the parameters such as impurities/ contamination among other parameters that might affect the interfacial properties and the quality of products recovered. Moreover, future research will focus on better treatment methods to reduce impurities on the surface of the fibers while increasing the quality of the recovered fibers.
Acknowledgment
The authors wish to acknowledge the Brain Korea 21 Plus Program (Republic of Korea) for the support they have provided. The authors in part recognize the technical assistance provided by the Microscopy and Instrumentation Laboratory, Kyungpook National University (Republic of Korea).
References
[1] HEDLUND-ASTROM A. Model for end of life treatment of polymer composite materials [D]. Stockholm: Royal Institute of Technology, 2005.
[2] HEDLUND-ASTROM A, LUTTROPP C, REINOLDSSON P. Outline of guidelines for recycling and recovery for FRP composites [C]// HORWVATH I, XIROUCHAKIS P. 5th International Symposium on Tools and Methods for Concurrent Engineering (TMCE 2004). Lausanne, Switzerland: Millpress, 2004: 563-572.
[3] NAKAGAWA M, KASUGA K, AOYAGI K, ISHIHARA K, IKEDA Y. CFRP recycling technology using depolymerization under ordinary pressure [C]// KIM H, WHISLER D, CHEN Z, BISAGNI C, KAWAI M, KRUEGER R. 29th Technical Conference of the American Society for Composites. California, USA: Destech Publisher, 2014: 929-947.
[4] ZHAO C, SHITIAN M, MA W, QIAN X, ZHANG J, CHEN X. Methods for recovering carbon fiber from carbon- fiber-reinforced polymer (CFRP) composites: PCT Patent, WO/2014/179939 [P]. 2014.
[5] HENRY L, SCHNELLER A, DOERFLER J, MUELLER W M, AYMONIER C, HORN S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near- and supercritical solvolysis [J]. Polymer Degradation and Stability, 2016, 133: 264-274. DOI: 10.1016/j.polymdegradstab.2016.09.002.
[6] KIM K W, LEE H M, AN J H, CHUNG D C, AN K H, KIM B J. Recycling and characterization of carbon fibers from carbon fiber reinforced epoxy matrix composites by a novel super-heated-steam method [J]. Journal of Environmental Management, 2017, 203(3): 872-879. DOI: 10.1016/ j.jenvman.2017.05.015.
[7] SUN H F, GUO G, MEMON S A, XU W, ZHANG Q, ZHU J H, XING F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method [J]. Composites: Part A, 2015, 78: 10-17. DOI: 10.1016/ j.compositesa.2015.07.015.
[8] CHENG H, HUANG H, LIU Z, ZHANG J. Reaction kinetics of CFRP degradation in supercritical fluids [J]. Journals of Polymers and the Environment, 2018, 26: 2153-2165. DOI: 10.1007/s10924-017-1114-2.
[9] DAUGUET M, MANTAUX O, PERRY N, ZHAO Y F. Recycling of CFRP for high value applications: Effect of sizing removal and environmental analysis of the supercritical fluid solvolysis [J]. Procedia CIRP, 2015, 29: 734-739. DOI: 10.1016/j.procir.2015.02.064.
[10] ONWUDILI J A, YILDIRIR E, WILLIAMS P T. Catalytic hydrothermal degradation of carbon reinforced plastic wastes for carbon fibre and chemical feedstock recovery [J]. Waste and Biomass Valorization, 2013, 4(1): 87-93. DOI: 10.1007/s12649-013-9204-4.
[11] OKAJIMA I, SAKO T. Recycling of carbon fiber-reinforced plastic using supercritical and subcritical fluids [J]. Journal of Material Cycles and Waste Management, 2017, 19(1): 15-20. DOI: 10.1007/s10163-015-0412-9.
[12] OLIVEUX G, DANDY L O, LEEKE G A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties [J]. Progress in Materials Science, 2015, 72: 61-99. DOI: 10.1016/j.pmatsci. 2015.01.004.
[13] THOMASON J, JENKINS P, YANG L. Glass fibre strength—A review with relation to composite recycling [J]. Fibers, 2016, 4(2): 18. DOI: 10.3390/fib4020018.
[14] NZIOKA A M, KIM Y J. Mechanochemical-enhanced chemical depolymerisation of glass-based fibre reinforced plastics from end-of-life-boats and ships [C]// CINAR O. 3rd International Conference on Engineering and Natural Sciences. Budapest, ICENS, 2017: 315.
[15] MAVROPOULOS A. ISWA blog: Waste industry must prepare for 4th industrial revolution [EB/OL] [2017-06-20]. https://waste-management-world.com/a/iswa-blog-waste-industry-must-prepare-for-4th-industrial-revolution.
[16] CATLIN J R, WANG Y. Recycling gone bad: When the option to recycle increases resource consumption [J]. Journal of Consumer Psychology, 2013, 23(1): 122-127: DOI: 10.1016/j.jcps.2012.04.001.
[17] SUN M, TRUDEL R. The effect of recycling versus trashing on consumption: theory and experimental evidence [J]. Journal of Marketing Research, 2017, 54(2): 293-305. DOI: 10.1509/jmr.15.0574.
[18] TRUDEL R. The behavioral economics of recycling [EB/OL] [2017-06-20]. https://hbr.org/2016/10/the-behavior al-economics-of-recycling.
[19] YAMADA K, TOMONAGA F, KAMIMURA A. Improved preparation of recycled polymers in chemical recycling of fiber-reinforced plastics and molding of test product using recycled polymers [J]. Journal of Material Cycles and Waste Management, 2010, 12(3): 271-274. DOI: 10.1007/ s10163-010-0296-7.
[20] CHENG H, HUANG H, ZHANG J, JING D. Degradation of carbon fiber-reinforced polymer using supercritical fluids [J]. Fibers and Polymers, 2017, 18: 795-805. DOI: 10.1007/ s12221-017-1151-4.
[21] KNIGHT C C, ZENG C, ZHANG C, LIANG R. Fabrication and properties of composites utilizing reclaimed woven carbon fiber by sub-critical and supercritical water recycling [J]. Materials Chemistry and Physics, 2015, 149: 317-323. DOI: 10.1016/j.matchemphys.2014.10.023.
[22] LEE S H, KIM Y S, YOON K Y. A study on the chemical treatments suitable for the simple mechanical manipulation during the recycling process of FRP waste from ships [J]. Journal of the Korean Society for Marine Environmental Engineering, 2009,12(1): 55-59.
[23] AKONDA M H, STEFANOVA M, POTLURI P, SHAH D. Mechanical properties of recycled carbon fibre/polyeter thermoplastic tape composites [J]. Journal of Composite Materials, 2016, 51(8): 2655-2663. DOI: 10.1177/ 0021998316672091.
[24] YANG Y, ZHAO Y, LI Y, DONG Q, CHEN D. Effect of sizing on the interfacial shear strength of carbon fiber/epoxy resin monofilament composite [J]. Journal of Wuhan University of Technology: Material Science Edition, 2014, 29(3): 483-487. DOI: 10.1007/s11595-014-0944-1.
[25] WANG X X, LI M, WU Q, GU Y, LI Y,WANG S, ZHANG Z. Influence of surface state on moisture sensitivity of carbon fiber and its composite interfacial properties [J]. Journal of Wuhan University of Technology: Material Science Edition, 2016, 31(4): 757-764. DOI: 10.1007/s11595-016-1442-4.
[26] WEHLACK C, POSSART W. Chemical structure formation and morphology in ultrathin polyurethane films on metals [C]// Adhesion Current Research and Applications. Weinheim: Wiley, 2005: 71-88.
[27] SHENOI R A, WELLICOME J F. Composite materials in maritime structures [M]. vol. 2 Cambridge: Cambridge University Press, 1993.
[28] LIU Yu-yan, SHAN Guo-hua, MENG Li-hua. Recycling of carbon fibre reinforced composites using water in subcritical conditions [J]. Material Science and Engineering A, 2009, 520(1, 2): 179-183. DOI:10.1016/j.msea.2009.05.030.
[29] GARDNER S D, SINGAMSETTY C S K, BOOTH G L, HE G R. Surface characterization of carbon fibers using angle- resolved XPS and ISS [J]. Carbon, 1995, 33(5): 587-595.
[30] YU B, JIANG Z, TANG X, YUE C Y, YANG J. Enhanced interphase between epoxy matrix and carbon fiber with carbon nanotube-modified silane coating [J]. Composites Science and Technology, 2014, 99: 131-140. DOI: 10.1016/ j.compscitech.2014.05.021.
[31] BATTLESON K A. Surface characterization of pan-based carbon fibers using XPS, SIMS and AFM [D]. Bozeman: Montana State University, 1998.
[32] VELASCO-SANTOS C, MARTíNEZ-HERNANDEZ A L, CASTANO V M. Silanization of carbon nanotubes: Surface modification and polymer nanocomposites [C]// Carbon Nanotubes-polymer Nanocomposites. Rijeka: Intech Publisher, 2011: 252-280.
[33] NIE Y. Surface silanization of carbon nanofibers and nanotubes for altering the properties of epoxy composites [D]. Berlin: Bundesanstalt für Materialforschung und- prüfung, 2012.
[34] WIJEWARDANE S. The role of CNT and CNT/composites for the development of clean energy [C]// Handbook of Polymer Nanocomposites: Processing, Performance and Application. Volume B: Carbon Nanotube-Based Polymer Composites. Berlin: Springer, 2015: 543-576.
[35] ZHANG R L, HUANG Y D, LI N, LIU L, SU D. Effect of the concentration of the sizing agent on the carbon fibers surface and interface properties of its composites [J]. Journal of Applied Polymer Science, 2012, 125(1): 425-432. DOI: 10.1002/app.35616.
[36] FEUILLADE V, BERGERET A, QUANTIN J C, CRESPY A. Relationships between the glass fibre sizing composition and the surface quality of sheet moulding compounds (SMC) body panels [J]. Composites Science and Technology, 2006, 66(1): 115-127. DOI: 10.1016/j.compscitech.2005.05.009.
[37] ENSMINGER D, STULEN F B. Ultrasonics: Data, equations, and their practical uses [M]. Boca Raton: CRC Press, 2009.
[38] NZIOKA A M, YAN C Z, KIM M G, SIM Y J, LEE C S, KIM Y J. Improvement of the chemical recycling process of waste carbon fibre reinforced plastics using mechanochemical process: Influence of process parameters [J]. Waste Management and Research, 2018, 36(10): 952- 964. DOI: 10.1177/0734242X18790351
[39] NZIOKA A M, KIM Y J. Surface analysis of glass fibres using XPS and AFM: Case study of glass fibres recovered from the glass fibre reinforced polymer using chemical recycling [J]. Journal of Physics: Conference Series, 2018, 953(1). DOI: 10.1088/1742-6596/953/1/012012.
[40] LIU X, THOMASON J L, JONES F R. XPS and AFM study of interaction of organosilane and sizing with E-glass fibre surface [J]. Journal of Adhesion, 2008, 84(4): 322-338. DOI: 10.1080/00218460802004386.
[41] NIE Wen-zhong. The effect of coupling agents on the mechanical propertiesof carbon fiber-reinforced polyimide composites [J]. Journal of Thermoplastic Composite Materials, 2014, 28(11): 1572-1582. DOI: 10.1177/ 0892705714535794.
[42] ZAKIR M, ASHRAF U, TIAN T, HAN A, QIAO W, JIN X, ZHANG M, TSOI J K, MATINLINNA J P. The role of silane coupling agents and universal primers in durable adhesion to dental restorative materials—A review [J]. Current Oral Health Reports, 2016, 3(3): 244-253. DOI: 10.1007/ s40496-016-0108-9.
[43] KREBS F C. Lifetime and stability studies [C]// Polymer Photovoltaics: A Practical Approach. Bellingham: Spie Press, 2008.
[44] AKOVALI G. The interfacial interactions in polymeric composites [M]. Dordrecht: Kluwer Academic, 1992.
[45] PROCTOR A, SHERWOOD P M A. X-ray photoelectron spectroscopic studies of carbon fibre surfaces. I. carbon fibre spectra and the effects of heat treatment [J]. Journal of Electron Spectroscopy and Related Phenomena, 1982, 27(1): 39-56. DOI: 10.1016/0368-2048(82)85051-2.
[46] QIAN X, CHEN L, HUANG J, WANG W, GUAN J. Effect of carbon fiber surface chemistry on the interfacial properties of carbon fibers/epoxy resin composites [J]. Journal of Reinforced Plastics and Composites, 2012, 32(6): 393-401. DOI: 10.1177/0731684412468369.
[47] MUNOZ-VELEZ M F, VALADEZ-GONZALEZ A, HERRERA-FRANCO P J. Effect of fiber surface treatment on the incorporation of carbon nanotubes and on the micromechanical properties of a single-carbon fiber-epoxy matrix composite [J]. Express Polymer Letters, 2017, 11(9): 704-718. DOI: 10.3144/expresspolymlett.2017.68.
[48] PETERSEN H N, KUSANO Y, BR NSTED P, ALMDAL K. Preliminary characterization of glass fiber sizing [C]// MADSEN B, LILHOLT H, KUSANO Y, FESTER S, RALPH B. 34th Ris
NSTED P, ALMDAL K. Preliminary characterization of glass fiber sizing [C]// MADSEN B, LILHOLT H, KUSANO Y, FESTER S, RALPH B. 34th Ris International Symposium on Materials Science. Ris
International Symposium on Materials Science. Ris : DTU, 2013: 333-340.
: DTU, 2013: 333-340.
[49] METWALLI E, HAINES D, BECKER O, CONZONE S, PANTANO C G. Surface characterizations of mono-, di-, and tri-aminosilane treated glass substrates [J]. Journal of Colloid and Interface Science, 2006, 298(2): 825-831. DOI: 10.1016/j.jcis.2006.03.045.
[50] THOMASON J L, DWIGHT D W. XPS analysis of the coverage and composition of coatings on glass fibres [J]. Journal of Adhesion Science and Technology, 2000, 14(5): 745-764. DOI: 10.1163/156856100742852.
[51] JING Z. Different surface treatments of carbon fibers and their influence on the interfacial properties of carbon fiber/epoxy composites [D]. Paris: Ecole Centrale Paris, 2012.
[52] SCHULTZ J, LAVIELLE L, MARTIN C. The role of the interface in carbon fibre-epoxy composites [J]. Journal of Adhesion, 1987, 23(1): 45-60. DOI: 10.1080/0021846 8708080469.
[53] SONG W, GU A, LIANG G, YUAN L. Effect of the surface roughness on interfacial properties of carbon fibers reinforced epoxy resin composites [J]. Applied Surface Science, 2011, 257(9): 4069-4074. DOI: 10.1016/j.apsusc. 2010.11.177.
(Edited by HE Yun-bin)
中文导读
机械化学法回收废弃碳纤维增强塑料的表征
摘要:在本研究中,我们介绍了机械化学强化回收碳纤维的特性。本研究的目的是探讨改良的再生方法对再生纤维界面性能的影响。对增强塑料进行了回收,测定了其回收效率,并用浓度分别为1%和3% 的(3-氨丙基)三乙氧基硅烷对回收纤维进行了施胶。利用化学分析电子光谱(ESCA)、原子力显微镜(AFM)、红外衰减全反射(ATR)光谱和扫描电子显微镜(SEM)对其形貌进行了表征。虽然纤维表面没有裂纹,但有污染的迹象,影响了纤维的界面性能和质量。结果表明,随着施胶浓度的增加,再生纤维和原生纤维的变化趋势相似。回收纤维具有很好的前景。
关键词:可回收碳纤维;纤维增强塑料;机械化学过程;界面质量;表面形态
Foundation item: Project(S2598445) supported by the Project for Cooperative R&D between Industry, Academy and Research Institute Funded by the Korea Ministry of SME and Startups in 2018
Received date: 2018-06-02; Accepted date: 2019-01-26
Corresponding author: KIM Young-Ju, PhD, Professor; Tel: +82-0539506585; E-mail: yjukim@knu.ac.kr; ORCID: 0000-0002- 6853-1444
Abstract: In this study, we present the characterization of the carbon fibers recovered from the mechanochemical-enhanced recycling of carbon fiber reinforced fibers. The objectives of the study were to investigate the effect of our modified recycling method on the interfacial properties of recovered fibers. The reinforced plastics were recycled; the recycling efficiency was determined and the recovered fibers were sized using 1 wt% and 3 wt% concentration of (3-aminopropyl)triethoxysilane. We characterized the morphologies utilizing the electron spectroscopy for chemical analysis (ESCA), atomic force microscopy (AFM), FTIR-attenuated total reflection (ATR) spectroscopy and scanning electron microscopy (SEM). Although the surface of the fibers had no cracks, there was evidence of contaminations which affected the interfacial properties and the quality of the fibers. Results showed that the trends in the recovered and virgin fibers were similar with an increase in sizing concentration. The results highlighted the perspectives of increasing the quality of recovered fibers after the recycling process.


