J. Cent. South Univ. (2016) 23: 1890-1898
DOI: 10.1007/s11771-016-3244-4
Influence of hydrogen-enriched atmosphere under coke oven gas injection on reduction swelling behaviors of oxidized pellet
LONG Hong-ming(龙红明)1, WANG Hong-tao(王宏涛)2, DI Zhan-xia(狄瞻霞)1,
CHUN Tie-jun(春铁军)1, LIU Zheng-gen(柳政根)2
1. School of Metallurgical Engineering, Anhui University of Technology, Ma’anshan 243002, China;
2. School of Metallurgy, Northeastern University, Shenyang 110819, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
It is of great importance to elucidate reduction swelling behaviors and reaction mechanism of oxidized pellet in hydrogen-enriched atmosphere under coke oven gas injection. In this work, the effects of hydrogen concentration in N2-CO-H2 atmosphere with unchanged CO content on reduction swelling behaviors of oxidized pellet at 1173 K were studied, to clarify the mechanism of hydrogen-enriched reduction and exclude the influences of CO. Then, the reduction swelling behaviors of oxidized pellet at 1173 K in actual atmosphere under coke oven gas (COG) injection, got from the simulation results of multi-fluid blast furnace model, were investigated. The results show that with the concentration of hydrogen increasing in N2-CO-H2 gas from 2% to 18%, the reduction swelling index of pellet decreases from 10.12% to 5.57% while the reduction ratio of pellet increases obviously from 39.85% to 69.58%. In addition, with COG injection rate increasing from 0 to 152.34 m3/t, the reduction swelling index of pellet decreases slightly from 10.71% to 9.54% while the reduction ratio of pellet is increased from 31.57% to 36.39%. The microstructures of pellet are transformed from the platy structure to the flocculent structure.
Key words:
1 Introduction
Currently, reduction of CO2 emission discharged from steel enterprise is one of the remarkably urgent issues for the remission of global greenhouse effect and sustainable development of iron and steel industry, which accounts for about 15% of the whole CO2 emission in China [1]. During the long period in the future, the traditional long process, namely blast furnace to basic oxygen furnace process, is the dominant route of steel production worldwide [2]. Blast furnace is the main source of CO2 emission accounting for 70% of the whole iron and steel enterprise [3]. Therefore, blast furnace ironmaking is the pivotal process for CO2 emission reduction in the chain of steel production.
Coke oven gas (COG) injecting into blast furnace is considered as one of the effective measures for reducing CO2 emission [4-6]. Since the concentration of inner- furnace hydrogen is enhanced with COG injection, the variations of reduction behaviors of blast furnace burdens are of great important. Pellet is one of the main ironmaking burdens, the reduction and swelling behaviors of which are critical to blast furnace smelting. Some researches [7-14] on the reduction behaviors of pellet reduced by hydrogen- enriched gas have been made. KEMPPAINEN et al [10] studied the effects of different hydrogen contents on the reduction behaviors of olivine pellet and found that pellets under H2-H2O-CO-CO2 atmosphere have a higher reduction rate at lower temperatures. KASHIHARA et al [11] investigated the effects of coke mixing with hydrogen bearing materials on the reduction behaviors of iron ore by means of experiment and a two-dimensional mathematical simulation model. The results show that hydrogen bearing materials increase the hydrogen reduction ratio and decrease the direct reduction ratio. MOUSA et al [12] researched the isothermal reduction behaviors of pellets with simulated original COG, reformed COG, original natural gas and reformed natural gas at 700-980 °C and found that the reduction degree of pellets reduced by reformed COG is highest at experimental temperature range. LI et al [13] reported the pellet reduction in different contents of hydrogen and found that hydrogen can improve reduction degree of pellet. ZUO et al [14] studied the influences of H2-N2, CO-N2 and H2-CO on the reduction behaviors of pellets at 1173 K and found that the reduction degree increases with the increase of the ratio of H2 or CO in the gas mixture, but the reduction with hydrogen is faster than that with CO.
However, most of the researches only aim at the effects of different hydrogen contents on the reduction behaviors of pellets. Simultaneously, the reduction swelling behaviors of pellet in reasonable atmosphere corresponding to COG injection haven’t been reported so far. Therefore, in order to clarify the mechanism of hydrogen-enriched reduction and exclude the influences of other gas, especially CO, the effects of enhanced hydrogen concentration in N2-CO-H2 atmosphere with CO unchanged content, on the reduction swelling behaviors of oxidized pellet at 1173 K were studied first. Simultaneously, the oxidized pellet reduced at 1173 K in actual atmosphere with COG injection calculated by multi-fluid blast furnace model was studied in order to make the reduction swelling behaviors of pellet under COG injection clear. Through this work, the reduction mechanisms and the reduction swelling behaviors of oxidized pellet corresponding to COG injection are clarified, which could provide technical-theoretical support for the practical application of COG injection.
2 Experimental
2.1 Raw materials
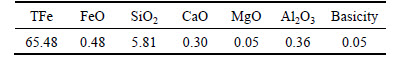
Experimental material includes the oxidized pellet prepared under laboratory condition, the composition is listed in Table 1. The total iron grade and the MgO content of pellet are 65.48% and 0.05%, respectively. The binary basicity (CaO/SiO2) of pellet is 0.05. The size of pellet is 10-12.5 mm.
Table 1 Chemical composition of pellet used in experiments (Mass fraction, %)
2.2 Experimental scheme
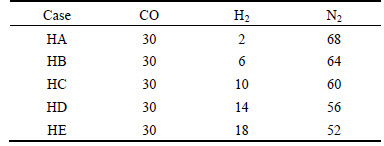
The influences of the single variation of hydrogen content in N2-CO-H2 gas on reduction swelling behaviors of pellet at 1173 K are investigated. The composition of N2-CO-H2 gas is listed in Table 2. The concentration of CO is kept constant at 30%. The contents of H2 are 2%, 6%, 10%, 14% and 18%, respectively, which are specified as HA, HB, HC, HDand HE, respectively.
Table 2 Composition of reducing gas (volume fraction, %)
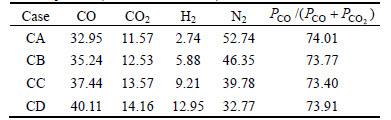
Then, the effects of simulation atmosphere with COG injection into blast furnace on reduction swelling behaviors of pellet were studied. The composition of simulation gas is listed in Table 3, which is calculated by means of multi-fluid blast furnace model [15-16] at 1173 K. The gas concentration (CO, CO2, H2 and N2) is the average value of isothermal concentration at 1173 K, as shown in Fig. 1 [17]. And the COG injection rates are 0, 58.89, 110.08 and 152.34 m3/t, respectively. For convenient description, the above four cases are specified as CA, CB, CC and CD, respectively.
Table 3 Composition of simulation hydrogen-enriched gas with COG injection (Volume fraction, %)
Eighteen pellets randomly collected were put in the furnace basket with three layers, as shown in Fig. 2. The temperature of the pellets was measured by utilizing a thermocouple inserted into the furnace from the top. The pellets were heated to 1173 K in N2 stream with a flow rate of 5 L/min. When the temperature of pellets reaches to 1173 K, the flow rate of N2 stream was adjusted to 15 L/min and kept for 30 min. Subsequently, the gas was changed to a reducing gas with a flow of 15 L/min, and the reduction experiment was carried out for 60 min. After experiment, the reducing gas was changed to N2 again, and then reduced sample was cooled down to room temperature. The reduction swelling index (RSI, Irs) was calculated from the volume change before and after reduction by
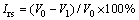 (1)
(1)
where V0 and V1 are the volumes of the sample before and after reaction, respectively.
The reduction ratio (Rr) was calculated by
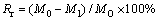 (2)
(2)
where M0 and M1 are the masses of the samples before and after reduction, respectively; MO is the total mass of oxygen coursed by iron oxide in the pellet.
3 Results and discussion
3.1 Reduction swelling behaviors of pellet in N2-CO- H2 gas
The effects of the single variation of hydrogen content in N2-CO-H2 gas on the RSI and the reduction ratio of pellet are shown in Figs. 3 and 4, respectively. With the concentration of CO unchanged, when the content of hydrogen increases from 2% to 18%, RSI of pellet decreases obviously from 10.12% to 5.57% and the reduction ratio of pellet increases obviously from 39.85% to 69.58%. Therefore, the increasing of hydrogen content in N2-CO-H2 gas can improve the reduction and swelling behaviors of pellet.
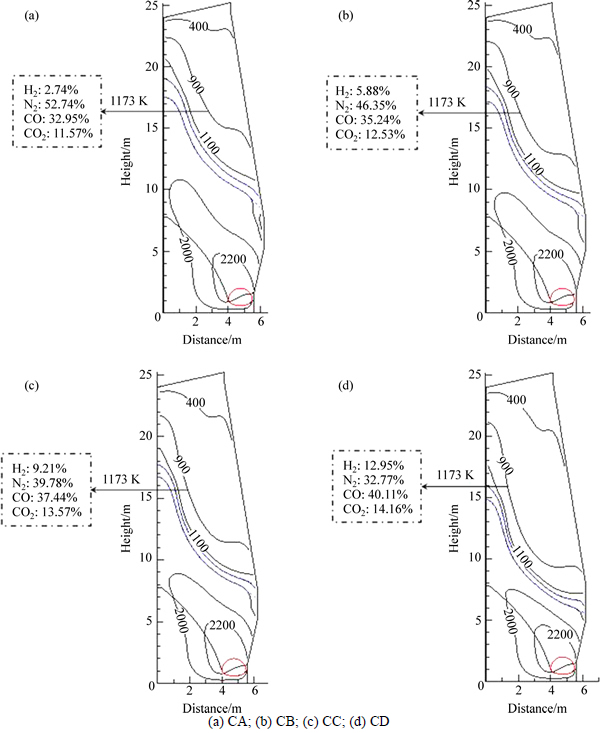
Fig. 1 Gas composition at 1173 K under COG injection (volume fraction):
The profile of reduced pellets is shown in Fig. 5. As seen from Fig. 5, it is shown that with the content of hydrogen increasing in N2-CO-CO2 gas, the reduction is enhanced from the surface to the center of pellet, which is mainly due to the reduction ability of gas phase accelerated with the increase of hydrogen content.
Reduction process of pellet can be divided into several stages, namely early stage (Fe2O3→Fe3O4), middle stage (Fe3O4→FeOx) and final stage (FeOx→Fe). Reduction swelling behaviors of pellet mainly happen in the early stage and middle stage of reduction process with the reduction ratio of pellet less than 33.3%. In the early stage and middle stage of reduction process, iron oxide is reduced according to following order: Fe2O3 → Fe3O4→FeOx (wustite), which results in pellet remarkably swelling, especially in the stage of Fe2O3 to Fe3O4. When the reduction ratio of pellet is more than 33.3%, FeOx reducing to Fe is the main reaction in the reduction of pellet. In this process, pellet swelling doesn’t happen basically. However, swelling extents decrease in the reduction process of FeOx to Fe compared with the former process (Fe2O3 to Fe3O4) [18].
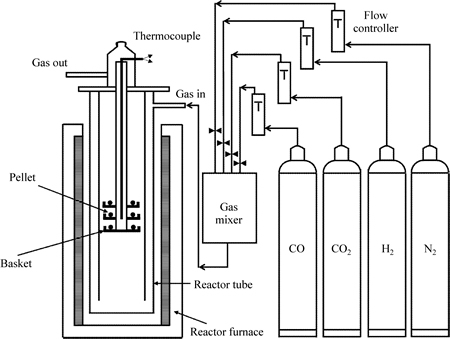
Fig. 2 Schematic diagram of experimental apparatus
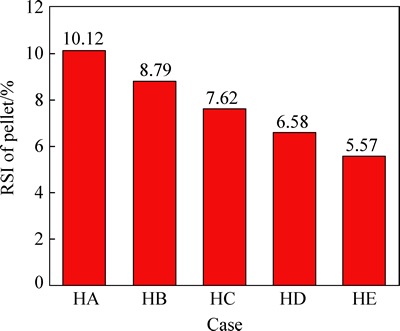
Fig. 3 Effects of hydrogen content variation in N2-CO-H2 gas on RSI of pellet
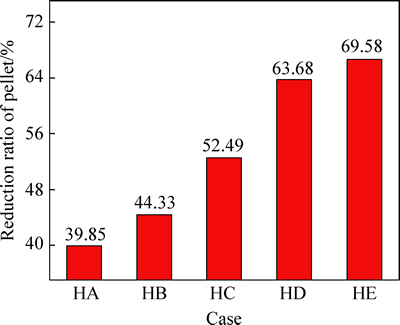
Fig. 4 Effects of hydrogen content variation in N2-CO-H2 gas on reduction ratio of pellet
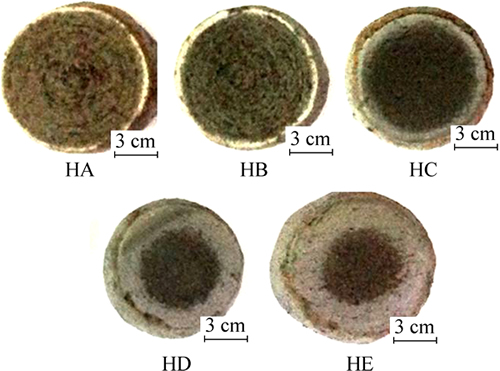
Fig. 5 Profile of reduced pellets under reducing gas with different hydrogen contents
Diffusion coefficient of hydrogen is high and its reduction kinetics condition is good at high temperature. Figure 6 shows the predominance area diagram of iron oxides reduced by CO/CO2 and H2/H2O. When the temperature is less than 1083 K, the reduction ability of H2 is lower than that of CO. When the temperature is more than 1083 K, the above result is adverse and hydrogen-enriched reduction is preponderant [19-20]. In addition, the reduction product of H2 is water, which is clean and beneficial for CO2 emission reduction. With increasing the content of hydrogen, the precipitation rate of metal iron crystal would be accelerated. A number of metal iron crystal would precipitate and fill among the crystal nucleus nucleated early, which benefits to form more crystal nucleus. As the interaction force of iron crystal nucleus is stronger, and the iron crystal nucleus aggregate closely, the swelling of pellet takes place difficultly. In other words, the volume of pellet decreases slightly in the stage of FeOx to Fe compared with that of Fe2O3 to Fe3O4. In this work, the reduction degrees of pellets are all higher than 33.3% according to Fig. 3, which indicates that the reduction of pellet mainly happens in the stage of FeOx to Fe and that reduction degree of pellet is enhanced increasingly with the hydrogen content increasing.
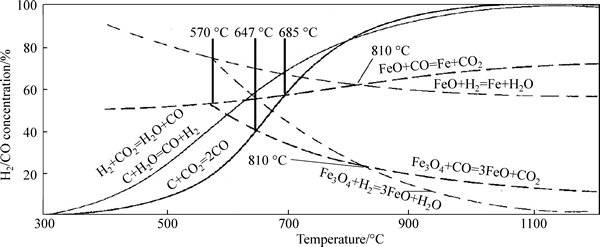
Fig. 6 Predominance area diagram of iron oxides reduced by CO/CO2 and H2/H2O
The above discussions can be conducted by the microstructure of the pellets after reduction. Figure 7 shows the scanning electron microscopy (SEM) photos of the central section of pellets after reduction. Four phases exist in pellet after reduction, namely iron oxide phase, slag phase containing little iron oxide (indexed as slag-io), slag phase and Fe phase. With increasing content of hydrogen, the reduction degree of pellet center is enhanced and the structure of pellet center is transformed from platy structure to flocculent structure. In addition, Fe2O3 phase in pellet center decreases while Fe increases gradually. As to pellet surface, the reduction is evident and the amount of Fe increases with the increase of hydrogen content. Therefore, the volume of pellet after reduction decreases with the content of hydrogen increasing and the reduction swelling behavior of pellet is improved.
3.2 Reduction swelling behaviors of pellet in actual atmosphere under COG injection
Figures 8 and 9 show the variations of RSI and reduction ratio of pellet in simulation reducing atmosphere corresponding to different COG injection rates, respectively. With COG injection rate increasing from 0 to 152.34 m3/t, RSI of pellet decreases slightly from 10.71% to 9.54% and the reduction ratio of pellet increases from 31.57% to 36.39%.
The profile of reduced pellets with different COG injection rates is shown in Fig. 10. With the increase of COG injection rates, the reduction of the pellet outer layer is enhanced while that of pellet center layer is changed slightly.
In order to explain the influence mechanisms of COG injection on RSI and the reduction ratio of pellets, microstructure analysis of pellet after reduction is carried out and SEM photos of the central section of pellets are shown in Fig. 11. Iron oxide phase, slag phase with little iron oxide (slag-io), slag phase and Fe phase are generated in pellet after reduction, which is identical to the pellet reduced in N2-CO-H2 gas as described in chapter 3.1. As seen from Fig. 11, the center structures of pellets after reduction are seldom different while some changes exist in pellet outer layer. With the increase of COG injection rate, the amount of platy structure in pellet outer layer after reduction decreases while that of flocculent structure increases gradually as well as Fe particle. With Fe particle generating in pellets inner, interaction force of metal iron crystal is enhanced, resulting in decreasing RSI of pellet. In addition, the concentrations of CO, CO2 and H2 in reducing gas all increase with the increasing COG injection rate. Simultaneously, the partial pressure of CO in reducing gas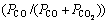 is kept constant at about 74% under the condition of only considering CO and CO2, which indicates that the reduction action of CO in pellet reduction is basically the same with different COG injection rates. Since the concentration of hydrogen increases from 2.75% to 12.75%, the reduction effect of hydrogen is enhanced, which results in the increasing reduction ratio of pellet and decreasing RSI of pellet.
is kept constant at about 74% under the condition of only considering CO and CO2, which indicates that the reduction action of CO in pellet reduction is basically the same with different COG injection rates. Since the concentration of hydrogen increases from 2.75% to 12.75%, the reduction effect of hydrogen is enhanced, which results in the increasing reduction ratio of pellet and decreasing RSI of pellet.
The balance composition of simulated gas with COG injection is calculated by FactSage 6.4 package at 1173 K, as shown in Table 4. With the initial concentrations of CO, CO2, H2 and N2, the balance concentrations of CO and H2O both increase while those of CO2 and H2 decrease and that of N2 is unchanged. In addition, with the increase of COG injection rate, the balance concentrations of CO, CO2, H2 and H2O all increase while that of N2 decreases.
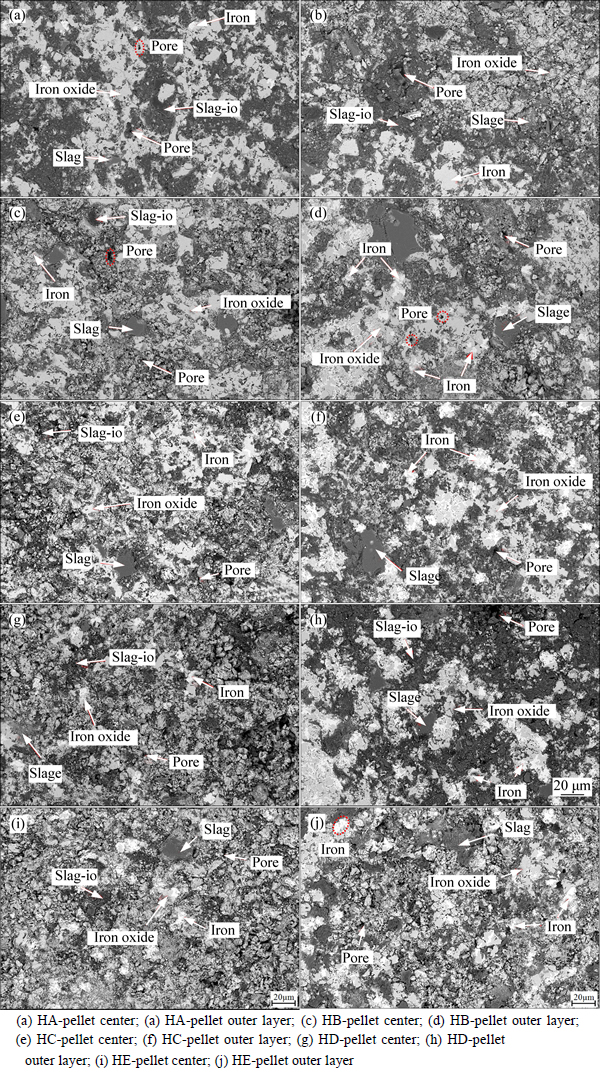
Fig. 7 SEM images of central section of pellets after reduction in N2-CO-H2 gas:
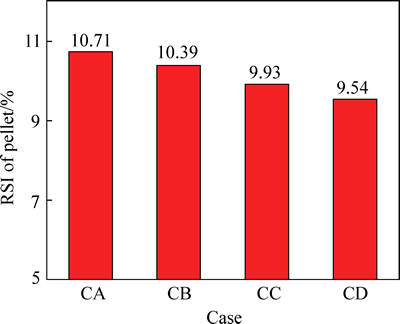
Fig. 8 Effects of COG injection rate on RSI of pellet
When CO, CO2, H2 and N2 exist in the system simultaneously with unbalance, some reactions could happen as follows [21]:
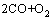 =2CO2,
=2CO2, 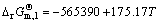 J/mol (3)
J/mol (3)
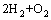 =2H2O,
=2H2O, 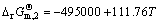 J/mol (4)
J/mol (4)
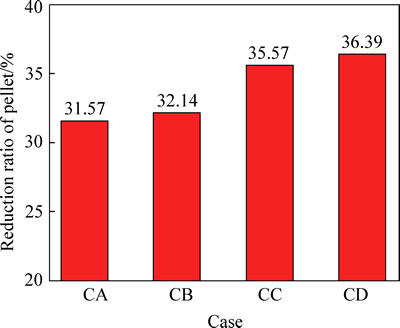
Fig. 9 Effects of COG injection rate on reduction ratio of pellet

Fig. 10 Profile of reduced pellets under simulated gas with different COG injection rates:
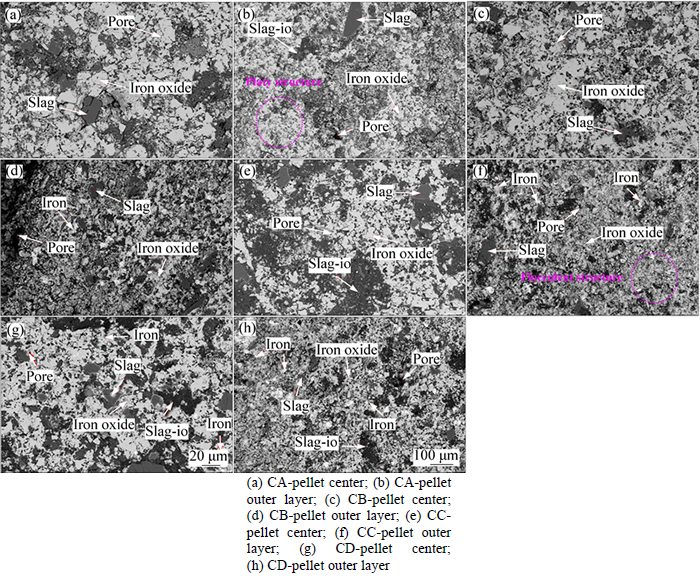
Fig. 11 SEM images of central section of pellets after reduction with different COG injection rates:
Table 4 Balance composition of simulated gas with COG injection (Volume fraction, %)
Water gas reaction can be obtained by
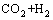 =
=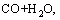
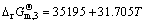 J/mol (5)
J/mol (5)
The oxygen potential of the balanced system consisting of the above gas varies due to the contents of the reducing components in the system is changed, which is calculated by
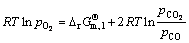
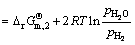 (6)
(6)
Through the calculation, the oxygen potential of the system is shown in Fig. 12. When the temperature is 1173 K, with COG injection rate increasing from 0 to 152.34 m3/t, the oxygen potential of the system decreases slightly from -382.16 kJ/mol to -386.58 kJ/mol, which indicates that the reduction ability of the system is enhanced and that the reduction condition of oxidized pellet is improved. Therefore, the reduction ratio of pellet increases.
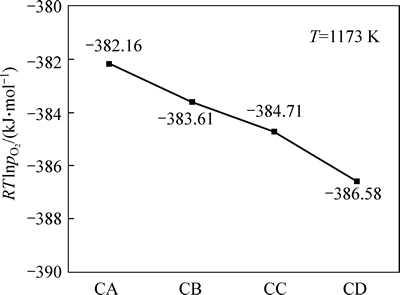
Fig. 12 Effects of COG injection rate on oxygen potential of system
4 Conclusions
1) Reduction swelling behaviors of oxide pellet in hydrogen-enriched atmosphere under blast furnace ironmaking operation with COG injection are experimentally investigated. Under N2-CO-H2 atmosphere, with the concentration of hydrogen increasing from 2% to 18% and CO content unchanged, RSI value of pellet decreases from 10.12% to 5.57% and the reduction ratio of pellet increases from 39.85% to 69.58%. Hydrogen-enriched reduction is beneficial for improving the reduction swelling behavior of pellet.
2) Under COG injection, with COG injection rate increasing from 0 to 152.34 m3/t, RSI of pellet decreases slightly from 10.71% to 9.54% and the reduction ratio of pellet increases from 31.57% to 36.39%. The microstructure of pellet is transformed from the platy structure to the flocculent structure. COG injection could improve reduction swelling behavior of pellet instead of worsening.
3) The results of pellet reduction under N2-CO-H2 atmosphere and under COG injection both indicate that the reduction swelling behavior of pellet could be improved by hydrogen-enriched reduction with COG injection.
References
[1] SHIGERU U. Preface to the special issue on “Development of technologies for the low carbon ironmaking” [J]. ISIJ International, 2015, 55(6): 1145-1148.
[2] WANG C, RYMAN C, DAHL J. Potential CO2 emission reduction for BF-BOF steelmaking based on optimized use of ferrous burden materials [J]. International Journal of Greenhouse Gas Control, 2009, 3(1): 29-38.
[3] XU Kuang-di. Low carbon economy and iron and steel industry [J]. Iron and Steel, 2010, 45(3): 1-12.
[4] GUO Tong-lai, LIU Zheng-gen, CHU Man-sheng. Numerical simulation of blast furnace raceway with coke oven injection [J]. Journal of Northeastern University (Natural Science), 2012, 33(7): 987-991. (in Chinese)
[5] GUO Tong-lai, CHU Man-sheng, LIU Zheng-gen, WANG Zhao-cai, TANG Jue, FU Xiao-jiao. Energy analysis on blast furnace with coke oven gas injection [J]. Journal of Central South University: Science and Technology, 2013, 44(8): 3108-3114. (in Chinese)
[6] MADDALENA F L, TERZA R R, SOBEK T F, MYKLEBUST K L. Coke oven gas injection into blast furnaces [J]. Ironmaking and Steelmaking, 1995, 22(10): 93-99.
[7] TATEO U, TSUNEHISA N, HIDEKI O, HIROKAZU K. Effective use of hydrogen in gaseous reduction of iron ore agglomerates with H2-CO [C]// 5th International Congress on the Science and Technology of Ironmaking-ICSTI. Shanghai, China: ICSTI, 2009: 1179-1184.
[8] DANG Jie, CHOU K, HU Xiao-jun, ZHANG Guo-hua. Reduction kinetics of metal oxides by hydrogen [J]. Steel Research International, 2013, 84(6): 526-533.
[9] KEMPPAINEN A, ALATARVAS T, ILJANA M, HAAPAKANGAS J, MATTILA O, PAANAENE T. Water-gas shift reaction in an olivine pellet layer in the upper part of blast furnace shaft [J]. ISIJ International, 2014, 54(4): 801-809.
[10] KEMPPAINEN A, MATTILA O, HEIKKINEN E P, PAANAENE T, FABRITIUS T. Effect of H2-H2O on the reduction of olivine pellets in CO-CO2 gas [J]. ISIJ International, 2012, 52(11): 1973-1978.
[11] KASHIHARA Y, SAWA Y, SATO M. Effect of hydrogen addition on reduction behavior of ore layer mixed with coke [J]. Tetsu-to-Hagané, 2014, 100(2): 312-318.
[12] MOUSA E A, BABICH A, SENK D. Reduction behavior of iron ore pellets with simulated coke oven gas and natural gas [J]. Steel Research International, 2013, 84(11): 1085-1097.
[13] LI Xiang-wei, CHEN Lin-kun, WANG Wei. Kinetics of rich hydrogen reduction of pellet [J]. Journal of Materials and Metallurgy, 2013, 12(4): 241-245. (in Chinese)
[14] ZUO Xiao-jian, WANG Jing-song, AN Xiu-wei, SHE Xue-feng, XUE Qing-guo. Reduction behaviors of pellets under different reducing potentials [J]. Journal of Iron and Steel Research International, 2013, 20(12): 12-18.
[15] AUSTIN P R, NOGAMI H, YAGI J. A mathematical model for blast furnace reaction analysis based on the four fluid model [J]. ISIJ International, 1997, 37(8): 748-755.
[16] CHU Man-sheng, WANG Hong-tao, LIU Zheng-gen, TANG Jue. Research progress on mathematical modeling of blast furnace ironmaking process [J]. Iron and Steel, 2014, 49(11): 1-8. (in Chinese)
[17] CHU Man-sheng, GUO Tong-lai, LIU Zheng-gen, XUE Xiang-xin, YAGI J. Numerical analysis on blast furnace low CO2 emission operation with coke oven gas injection [C]// 6th International Congress on the Science and Technology of Ironmaking-ICSTI, ABM, Rio de Janeiro, Brazil: ICSTI, 2012, (2): 992-1004.
[18] QI Yuan-hong, ZHOU Yu-sheng, CAI Ai-ping. Reduction swelling of pellets and its mechanism [J]. Iron and Steel, 1996, 31(2): 1-5. (in Chinese)
[19] CHU Man-sheng, NOGAMI H, YAGI J. Numerical analysis on injection of hydrogen bearing materials into blast furnace [J]. ISIJ International, 2004, 44(5): 801-808.
[20] SHEN Feng-man, JIANG Xin, WEI Guo, ZHENG Hai-yan, MU Lin. Effect of hydrogen-enriched gas on reducibility and reduction- disintegration of sinters [J]. China Metallurgy, 2014, 24(1): 2-10.
[21] HUANG Xi-hu. Iron and steel metallurgy principle [M]. Beijing: Metallurgical Industry Press, 2007: 56-78.
(Edited by FANG Jing-hua)
Foundation item: Project(51404005) supported by the National Natural Science Foundation of China
Received date: 2015-08-24; Accepted date: 2015-12-27
Corresponding author: DI Zhan-xia, Lecture, PhD; Tel: +86-18055576233; E-mail: dzx2012@ahut.edu.cn
Abstract: It is of great importance to elucidate reduction swelling behaviors and reaction mechanism of oxidized pellet in hydrogen-enriched atmosphere under coke oven gas injection. In this work, the effects of hydrogen concentration in N2-CO-H2 atmosphere with unchanged CO content on reduction swelling behaviors of oxidized pellet at 1173 K were studied, to clarify the mechanism of hydrogen-enriched reduction and exclude the influences of CO. Then, the reduction swelling behaviors of oxidized pellet at 1173 K in actual atmosphere under coke oven gas (COG) injection, got from the simulation results of multi-fluid blast furnace model, were investigated. The results show that with the concentration of hydrogen increasing in N2-CO-H2 gas from 2% to 18%, the reduction swelling index of pellet decreases from 10.12% to 5.57% while the reduction ratio of pellet increases obviously from 39.85% to 69.58%. In addition, with COG injection rate increasing from 0 to 152.34 m3/t, the reduction swelling index of pellet decreases slightly from 10.71% to 9.54% while the reduction ratio of pellet is increased from 31.57% to 36.39%. The microstructures of pellet are transformed from the platy structure to the flocculent structure.


