
Preparation of Al(OH)3 by ion membrane electrolysis and precipitation of sodium aluminate solution with seeds
LI Yuan-gao(李元高)1, 2, CHEN Qi-yuan(陈启元)2, WANG Song-sen(王松森)2,
YIN Zhou-lan(尹周澜)2, ZHANG Ping-min(张平民)2
1. Department of Environmental Engineering, Xiamen University of Technology, Xiamen 361024, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 16 June 2007; accepted 30 October 2007
Abstract:
The preparation of Al(OH)3 by the ion exchange membrane electrolysis followed by the precipitation of sodium aluminate solution with seeds was made. During the process of ion membrane electrolysis, the sodium aluminate solution is rapidly acidified and the caustic ratio (aK) is decreased due to oxygen evolution in the anodic region. And the causticity of solution is increased due to hydrogen evolution in the cathode region, producing the high concentration of caustic soda solution. Regulating the acidity of the anodic solution by controlling the electric quantity in the electrolysis and subsequent decomposing the solution, Al(OH)3 could yield with very large rate and high efficiency. The experiments also indicate that the quality of aluminum hydroxide product is greatly affected by the impurity silicon.
Key words:
sodium aluminate solution; ion exchange membrane; electrolysis and precipitation with seeds; silicon;
1 Introduction
Sodium aluminate solution can be prepared with bauxite of different ratios of alumina to silica. The well-known Bayer process is simple and less energy consuming except the long precipitation time, low precipitation efficiency and mass ratio of seeds[1-3]. The speed of the carbonation precipitation of sodium aluminate solution is fast and the carbonation rate is high, but it’s a complex technological process with higher power consumption[4-8].
The ion-exchange membrane technology for caustic soda has been industrialized since middle of 1970s. It has a lot of advantages such as small investment, low energy consumption and product cost, high concentration and good quality of sodium hydroxide solution (40%-50%, mass fraction), and high-pure chlorine and hydrogen (99%)[9-10]. Now, the ion-exchange membrane technology has been applied in many fields of hydrometallurgy such as ore leaching, treatment of metal compounds, preparation of high purity metal and wastewater treatment due to the technology progress and economical benefit. But it has not been applied to produce Al(OH)3[12-15]. In this work, a new method for preparation of Al(OH)3 by ion exchange membrane electrolysis from sodium aluminate solution was studied to provide a new process for the manufacture of aluminum hydroxide and aluminum oxide.
2.1 Materials and reagents
Commercial sodium hydroxide(≥96.0%, supplied by Zhengzhou Research Institute of Light Metals, China) and pure sodium hydroxide(≥96.0%, Tianjin Jinhong Ltd., China) were used for preparing sodium aluminate solution, and sodium silicate as the silicon-bearing impurity added into the sodium aluminate solution was chosen.
2.2 Experimental devices and instruments
An ion exchange membrane electrolysis bath was specially designed and installed, as shown in Fig.1. Stainless steel plate and flat passivating titanium plate were used as the cathode and the anode, respectively. Nafion cation exchange membrane (Dupont Ltd.) was used as the ion membrane.
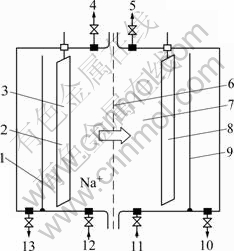
Fig.1 Schematic diagram of ion membrane electrolysis bath: 1 Baffle; 2 Anode; 3 Anode region; 4 Oxygen outlet; 5 Hydrogen outlet; 6 Ion membrane; 7 Cathode region; 8 Cathode; 9 Baffle; 10 Concentrated sodium hydroxide outlet; 11 Dilute sodium hydroxide inlet; 12 Anode solution inlet; 13 Acidifying anode solution outlet
A tank for precipitation made of stainless steel was also specially designed and assembled (Fig.2). The thermostatic bath (LH586-2, Shanghai Jingying Ltd.) and electronic constant speed mixer (JHS-1/90, Hangzhou instruct Motors Co. Ltd.) were adopted.
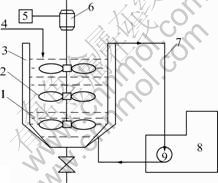
Fig.2 Schematic diagram of precipitation tank: 1 Materials of decomposition; 2 Paddle wheel; 3 Thermostatic water jacket; 4 Acidifying anode solution inlet; 5 Governor; 6 Motor; 7 Circulating thermostated water; 8 Thermostatic bath; 9 Water pump
The products of electrolysis and precipitation were determined by XRD (Japan’s Rigaku D/max 2550VB+18 kW, computer controlled X-ray diffractometer with Cu Kα radiation), and their size distribution was measured by laser particle sizer (Mastersizer 2000, Marlvern Co. England).
2.3.1 Ion membrane electrolysis
The sodium aluminate solution was electrolyzed intermittently in the electrolytic bath. Firstly, turn off all the electrolyte outlet valves, and send a certain amount of sodium aluminate solution (aK=1.3-3.0, containing sodium hydroxide of 180 g/L, mixed with impurity silicon or not) and a quantity of sodium hydroxide solution with concentration of 180 g/L into the anode region and the cathode region of the bath, respectively. Secondly, turn off all inlet valves for the electrolyte and switch on the gas outlet. Then the electrolysis started under the conditions of constant bath voltage of 3.0 V and initial current of 2.0 A/dm2, and electrolytic current and voltage were recorded every 5 min until the current declined rapidly, and electrolysis was ceased.
The solution of the anode region was acidified and oxygen evolution occurred during the process of ion membrane electrolysis. The anode reaction and transportation reaction of Na+ were as follows:
2H2O-4e→O2↑(Anode region)+4H+ (1)
4Na+(Anode region)→4Na+(Cathode region) (2)
At the same time, sodium hydroxide solution in cathode region was causticized and H2 was produced. The cathode reaction was as follows:
4H2O+4e→2H2↑(Cathode region)+4OH- (3)
A large amount of OH- produced in cathode region combined with Na+ transferred from the anode region through the ion membrane to form the sodium hydroxide.
As a result, similar to the ion membrane electrolysis of sodium chloride, higher concentration sodium aluminate solution could be obtained in cathode region after ion membrane electrolysis of sodium aluminate solution.
2.3.2 Precipitation of acidifying anode solution and separation of products
The acidified anode solution and a certain amount of aluminum hydroxide seeds were put into precipitation tank. Keeping the constant temperature under a certain stirring speed, the precipitation reaction took place as follows:
4H++![]() →4Al(OH)3↓(Precipitation tank)+4H2O (4)
→4Al(OH)3↓(Precipitation tank)+4H2O (4)
The ion membrane electrolysis and precipitation of sodium aluminate were carried out separately, which could avoid the direct precipitation of aluminum hydroxide in anode region, destroying the ion exchange membranes. According to Eqns.(1) and (4), the theoretical consumption of electric energy for production of 1 mol Al(OH)3 is calculated as W=UIt=UQ=UFne= 0.08 kW·h.
The total reaction of electrolysis and precipitation was as follows:
4NaAl(OH)4+2H2O→4Al(OH)3↓(Precipitation tank)+
4NaOH(Cathode region)+2H2↑(Cathode region)+O2↑(Anode region) (5)
After about 3 h of precipitation process, filter and wash the product cake with hot water. When pH of the washing solution was approximately 7.0, mix the washing solution and the filtrate, and measure the total volume, which would be used as anodic solution in the next electrolysis. After drying the product cakes at 100 ℃, it was weighted (to calculate the precipitation rate) and determined to be aluminum hydroxide.
2.3.3 Multi ion membrane electrolysis and precipitation
As mentioned above, after the primary ion membrane electrolysis and the precipitation of sodium aluminate solution, the filtrate and washing solution were recycled as the anodic solution for the next electrolysis and precipitation. That’s called secondary ion membrane electrolysis and precipitation. If the process is repeated for many times, a multi ion membrane electrolysis and precipitation of sodium aluminate solution can be carried out.
2.3.4 Analysis of products and cathode solution
The compositions of the products and cathode solution after electrolysis were determined. The silicon content in sodium aluminate solution and in solid product was analyzed by the silico-molybdate blue colorimetry. The particle size and the phase of the product were also detected.
3.1 Characteristics of bath voltage and current of ion membrane electrolysis at different temperatures
The initial concentration of sodium hydroxide was 217.26 g/L (αK=1.5) in anolyte and 180 g/L in catholyte. The temperatures of electrolytic experiments were 60, 65, 70 and 75 ℃, respectively. The characteristics of bath voltage and current of ion membrane electrolysis at different temperatures are shown in Fig.3.
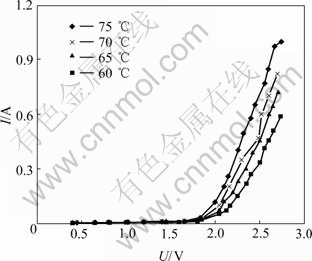
Fig.3 Characteristics of bath voltage and current of ion membrane electrolysis at different temperatures
According to Fig.3, the current increases suddenly when the bath voltage exceeds 1.8 V, and sharply rises with the increase of bath voltage. When the bath voltage is more than 1.8 V, the higher the temperature, the larger the current would be, because the conductance of the electrolyte increases with increasing the temperature. By considering the various factors, 70 ℃ is a better choice in the ion membrane electrolysis.
The initial concentration of aluminium hydroxide in anolyte was about 230 g/L (αK=1.5, 2.0 or 3.0), and that of sodium hydroxide in catholyte was 217.26 g/L. The characteristics of current and time of the ion membrane electrolysis at different αK are shown in Fig.4. It can be seen that the current decreases slowly at the initial stage of electrolysis and declines rapidly after a period of time. At the same time, the anolyte turns to turbid, yielding some fine solid crystal of aluminum hydroxide, because sodium aluminite is precipitated due to the gradually acidified anodic solution.
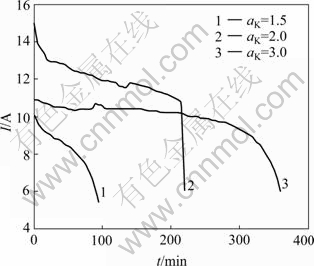
Fig.4 Characteristics of current and time of ion membrane electrolysis at different caustic ratios
According to the equation Q=ΣI?t, the electric quantity that makes the sodium aluminate solution with different αK continuously acidified by the electrolytic action produce the crystallite of the aluminum hydroxide can be obtained. The calculated results are listed in Table 1. This indicates that the higher the αK value, the more electric quantity will be consumed due to more NaOH in sodium aluminate solution.
Table 1 Relations between αK and electric quantity in ion membrane electrolysis of sodium aluminite

3.3 Changes in concentration of sodium hydroxide of cathode region during electrolysis
After a period of ion membrane electrolysis, the concentration change of sodium hydroxide in cathode is listed in Table 2. It is shown that the concentrations of three samples of sodium hydroxide solution are all increased, because a large amount of OH- produced in cathode region combines with Na+ transferred from anode region through ion membrane, to form sodium hydroxide.
Table 2 Concentration changes of sodium hydroxide of cathode region during ion membrane electrolysis
3.4 Characteristics of current and time of multi ion membrane electrolysis and precipitation
When sodium aluminite solution was electrolyzed and precipitated twice, the characteristics of current and time are shown in Fig.5. It can be concluded that the secondary electrolysis is faster than primary one. The reason is that the less electric quantity is enough for acidifying the electrolyte after primary electrolysis and precipitation owing to sharp decrease of the quantity of sodium aluminite and sodium hydroxide.
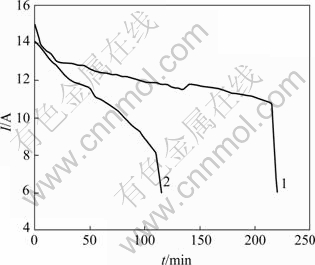
Fig.5 Characteristics of current and time of multi ion membrane electrolysis and precipitation: 1 Primary electrolysis and precipitation; 2 Secondary electrolysis and precipitation
3.5 Precipitation ratio in ion membrane electrolysis and precipitation
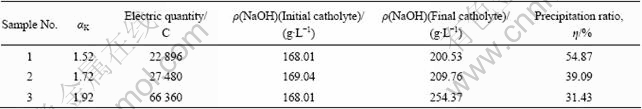
Precipitation ratios of sodium aluminite solution (initial concentrate of aluminum hydroxide is 230 g/L, αK=1.52, 1.72 and 1.92, respectively) in ion exchange membrane electrolysis and precipitation are listed in Table 3. According to Table 3, when the alumina concentration is the same, the supersaturation of the sodium aluminite solution increases with the decrease of αK. As a result, the electric quantity consumed for acidifying the electrolyte is less and precipitation ratio is improved distinctly, and visa versa.
Table 3 Precipitation ratios of sodium aluminite solution in ion exchange membrane electrolysis and precipitation
Precipitation ratios of sodium aluminite solution in multi ion membrane electrolysis and precipitation are listed in Table 4. It can be seen that although each of the primary or secondary electrolysis precipitation ratios is less than 50%, the total ratio could be up to 71.7%. The quantity of input electricity is positively correlated with precipitation ratio. Thus, if multiple cyclic electrolysis and precipitation are applied, the precipitation ratio of the solution could be greatly improved by controlling input electric quantity.
Table 4 Precipitation ratio of sodium aluminite solution and its electric quantity for acidification by ion exchange membrane electrolysis and precipitation
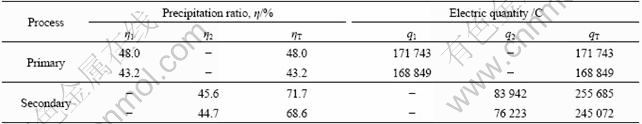
η1: Precipitation ratio of primary electrolysis and precipitation; η2: Precipitation ratio of secondary electrolysis and precipitation; ηT: Total precipitation ratio, ηT=η1+η2(1-η1).
3.6 Effect of silicon on product quality
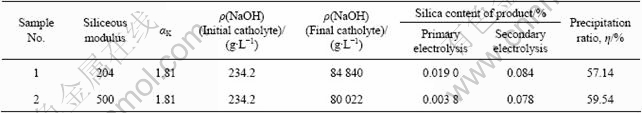
Silicon is the most important impurity in alkaline process of Al(OH)3 and Al2O3 production. Through adding sodium silicate, sodium aluminite solution with different siliceous modulus was electrolyzed and precipitated by ion membrane electrolysis. The silica contents in Al(OH)3 products are listed in Table 5. We could conclude that Al(OH)3 product from the solution with lower siliceous modulus has higher silica content. Silica content in the product by the primary electrolysis and precipitation is much less than that by the secondary one, which indicates that silica content in the product increases with the process of electrolysis and improvement of precipitation ratio. This fact is consistent with the industrial practice in the alkaline process of Al(OH)3 and Al2O3.
Table 5 Silica contents of products from sodium aluminite solution with different siliceous modulus by ion exchange membrane electrolysis and precipitation
The particle size distribution of the products from ion membrane electrolysis and precipitation with siliceous modulus of 204 and 500 is shown in Fig.6.
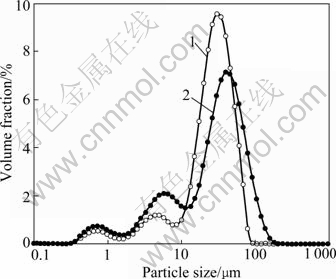
Fig.6 Particle size distribution of products from primary ion membrane electrolysis and precipitation of solution with different siliceous modulus: 1 Primary electrolysis with siliceous modulus of 204; 2 Primary electrolysis with siliceous modulus of 500
Fig.6 shows that silica content in sodium aluminate solution has a distinct effect on the particle size distribution of products. The larger the silica content, the smaller the average particle size of products, or the finer the particles. The conclusion can be drawn that the processes of nucleation, agglomeration and radial growth are all affected by silica contents in the solution.
Products from twice electrolysis and precipitation of the solution with siliceous modulus of 204 and 500 were characterized by XRD, and the results are shown in Fig.7. It is seen that all the products are detected to be monoclinic crystal of higher purity aluminum hydroxide, indicating that the low silica content doesn’t influence the crystal structure of the product.
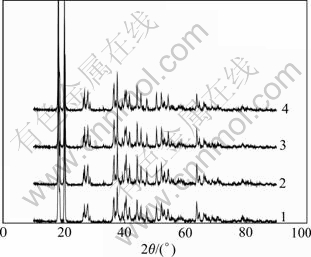
Fig.7 XRD patterns of products from ion exchange membrane electrolysis and precipitation of sodium aluminite solution with different siliceous modulus: 1 Primary electrolysis of solution with siliceous modulus of 204; 2 Secondary electrolysis of solution with siliceous modulus of 204; 3 Primary electrolysis of solution with siliceous modulus of 500; 4 Secondary electrolysis of solution with siliceous modulus of 500
4 Conclusions
1) The preparation of aluminum hydroxide by ion exchange membrane electrolysis and precipitation of sodium aluminite solution is feasible. This method has very large rate and high efficiency of precipitation. Sodium hydroxide solution with high concentration as well as by-product H2 and O2 of high purity could be obtained simultaneously when Al(OH)3 is produced.
2) The precipitation rate and efficiency could be controlled through regulating the input of electric quantity (electric current and electrolysis time) to acidify the sodium aluminite solution.
3) Sodium aluminate solution is acidified in anode region and the precipitation of solution in precipitation tank is followed. The two processes are separated, which could avoid the aluminum hydroxide from precipitating in anode region and destroying ion exchange membranes.
4) Quality and particle size distribution of the products are all affected by the silica content in sodium aluminate solution.
References
[1] YANG Chong-yu. Technology of alumina production [M]. Beijing: Metallurgy Industry Press, 1993. (in Chinese)
[2] DAMIEN R H, ROLAND I K, CLIVE A P, JOHN C T. A dynamic light scattering investigation of nucleation and growth in supersaturated alkaline sodium aluminate solutions (synthetic Bayer liquors) [J]. Colloids and Surfaces A: Physicochem Eng Aspects, 1999, 154: 343-352.
[3] SEYSSIECQ I, VEESLER S, BOISTELLE R, LAMERANT J M. Agglomeration of gibbsite Al(OH)3 crystals in Bayer liquors: Influence of the process parameters [J]. Chemical Engineering Science, 1998, 53(12): 2177-2185.
[4] EREMIN N I. Process and equipment in alumina production [M]. Moskwa: Metallurgy Press, 1980: 12-156.
[5] CLIVE A, PRESTIDGE, IGOR A. Cation effects during aggregation and agglomeration of gibbsite particles under synthetic Bayer crystallization conditions [J]. Journal of Crystal Growth, 2000, 209: 924-933.
[6] GU Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(S1): 91-97. (in Chinese)
[7] ZHANG Bao-qi, LOU Dong-min. New technology of add seeds carbonization in aluminiate solution [J]. Light Metals, 1999(12): 22. (in Chinese)
[8] LI Xiao-bin, LIU Xiang-ming, LIU Gui-hua, PENG Zhi-hong, LIU Ye-xiang. Study and application of intensified sintering process for alumina production [J]. The Chinese Journal of Nonferrous Metal, 2004, 14(6): 1031-1036. (in Chinese)
[9] XU Tong-wen. Ion exchange membranes: State of their development and perspective [J]. Journal of Membrane Science, 2005, 263: 1-29.
[10] RUKMA B, WALTER W, CAROL K. Hydration of freestanding Nafion membrane in proton and sodium ion exchanged forms probed by infrared spectroscopy [J]. Electrochimica Acta, 2007, 53(3): 1260-1265.
[11] CHENG Dian-bing. Technology evolution and prospect of caustic soda production about ion exchange membrane [J]. Chlor-Alkali Industry, 1996(2): 14-18. (in Chinese)
[12] VERONI B, SERGIO S F, RAINER G, NICO S, NORBERT S. Nanostructure of Nafion membrane material as a function of mechanical load studied by SAXS [J]. Polymer, 2003, 44: 4853-4861.
[13] TOSHIKATSU S. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions [J]. Journal of Membrane Science, 2001, 67: 1-31.
[14] LEE H J, MOON S H, TSAI S P. Effects of pulsed electric fields on membrane fouling in electrodialysis of NaCl solution containing humate [J]. Separation and Purification Technology, 2002, 27: 89-95.
[15] TANG Ling-yan, WANG Xun-zong. Technology of combining production of both the imported DAP and the domestic MAP installations [J]. Chemical Industry and Engineering Progress, 2002, 21(12): 912-915. (in Chinese)
Foundation item: Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: LI Yuan-gao; Tel: +86-592-6291056; E-mail: liyuangao202@sina.com.cn
(Edited by YANG Bing)



