DOI:10.19476/j.ysxb.1004.0609.2017.07.02
高强铝合金时效微结构演变与性能调控
张 勇1,李红萍1,康 唯2,张新明1
(1. 中南大学 材料科学与工程教育部重点实验室,长沙 410083;
2. 中国商用飞机有限公司,上海 200126)
摘 要:
综述铝合金时效过程中,用近代物理检测分析技术表征和确定的微结构从原子团簇、GP区、亚稳相到平衡相的结构、大小及形貌析出序列演变过程;析出微结构与基体形成的共格、半共格和非共格界面结构;界面上析出第二相;论述通过空位、位错、外应力及应变、淬火冷却速率、多级时效及微合金化等手段调控铝合金微结构及其演变原理,铝合金微结构与性能调控技术,铝合金时效调控技术与理论的发展;介绍铝合金时效与微合金化调控性能的一些实例,为改善或提升高强铝合金性能提供理论与技术参考。
关键词:
文章编号:1004-0609(2017)-07-1323-14 中图分类号:TG146.2 文献标志码:A
时效铝合金是指具有时效硬化能力的铝合金。常用时效铝合金包括Al-Cu-(Mg)(2xxx系),Al-Mg-Si (6xxx系)和Al-Zn-Mg-(Cu)(7xxx系)系列。随着铝合金的发展,发现一些其他合金元素也具有时效强化能力,例如Al-Cu-Li系、Al-Mg-Sc系等。这些合金元素单独添加或复合添加都能产生明显的时效强化能力,是由于合金元素的固溶度随温度-时间的变化而产生的纳米尺度的时效析出相能够有效阻碍位错的运动原因。时效相增加合金塑性变形的难度,也就是提高了合金的强度。
表1 一些常用元素在铝基体中的最大溶解度和温度[1]
Table 1 Maximum solid solubility and temperature for some traditional addition elements in aluminium[1]
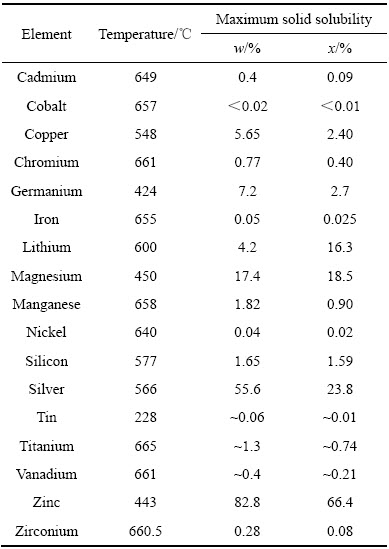
时效析出需同时满足过饱和固溶体(Supersaturated solid solution)产生的析出热力学/驱动力,并且需要克服热力学激活能能垒(Activation energy)。所以理论上只要合金化元素在铝合金基体中的固溶度随温度变化,则合金在低于国溶温度就可能产生“时效析出”反应。表1列出了一些常用元素在铝基体中的最大溶解度和温度[1]。
铝合金的调控时效析出包含固溶-淬火-时效过程。其中,固溶处理的目的是使合金元素能充分溶解到基体中。淬火过程是通过快速冷却使得高温固溶状态一般在室温下得以保持,形成过饱和固溶体。时效过程则是通过改变温度-时间参数使过饱和固溶体分解,形成细小弥散的析出相。过饱和固溶体的时效分解过程伴随着系统自由能的降低;析出相随温度-时间的变化而发生结构及成分的演变,进而形成新的亚稳相或稳定相。这种过程称为时效析出序列。时效过程又包含了微结构的形核-长大-粗化过程。通过精细的时效参数调控,铝合金材料的应用不仅可满足某项重要的服役性能,而且其他服役性能即综合性能也要能满足要求。这些综合服役性能包括强度、疲劳与韧性等力学性能,密度、电磁性等物理性能以及电化学腐蚀、应力腐蚀等化学性能。通过消除初生相和残余结晶相,调控微米、亚微米、纳米相的结构、尺度、数量与分布,甚至早期原子团簇中原子浓度和分布;调控相界/晶界结构,降低界面能;可以获得材料的某种特征微结构组织,达到各个微结构协同提高综合性能的目标。
以下综述铝合金时效过程中,用近代物理检测分析技术表征和确定的Al-Cu-(Mg)、Al-Mg-Si-(Cu)和Al-Zn-Mg-(Cu)铝合金的微观结构析出序列和演变规律,简述了借助空位、位错、外应力及应变、淬火冷却速率、多级时效及微合金化等手段调控铝合金微结构演变原理, 以及在此基础上发展的时效技术。
1 铝合金时效原理及微结构演变
1.1 Al-Cu-(Mg)系合金微结构析出演变
Al-Cu-Mg系是最早发现的时效强化铝合金。早在1906年,德国冶金学家Alfred Wilm和他的助手Fritz Jablonski发现了Al-Cu-Mg合金在室温下强度增加的现象[2-5]。图1所示为WILM等[5]发表的第一条铝合金时效硬化曲线。由图1可以看到,该合金的硬度在室温下从70HB增加到100HB。该系列合金在第二次世界大战中得到广泛的发展和应用。这种合金也被称为杜拉铝(Duralumin)或硬铝。第一个成功应用于飞机上的合金为2017合金(Al-4Cu-1.5Mg-0.5Mn,质量分数,%)。这种合金的屈服强度约为250 MPa。合金2014 (Al-(3.9~5)Cu-(0.2~0.8)Mg-(0.4~1.2)-Mn,质量分数,%)也成功应用到商业飞行器DC-3型飞机上,在T3状态下合金的屈服强度达到325 MPa[5]。
所有时效铝合金过饱和固溶体都会分解成不同结构的亚稳中间相,并最终演变成平衡相,并由此带来合金性能的一系列变化。
研究表明Al-Cu合金的析出序列为[6-7]:S.S.S.S→ GPI→GPII(θ″)→θ′→θ。
1) GP区
1938年左右,法国人Andrè Guninier在分析铝合金小角度衍射(Small angle X-ray scattering, SAXS)条纹时发现有一种在(100)面上的Cu原子偏聚会造成额外的X射线散射。与此同时,英国人George Preston在分析Al-Cu合金的Laue衍射斑点时,也发现在Laue斑点周围有被拉长的细条纹。他认为这些细小的条纹是由一种薄片状,在(100)面上聚集的Cu原子结构散射入射光束的结果[8]。他们俩人发现的这种原子偏聚区,被后人简称为GP区。现代高精度的多种表征手段都已证实了GP区的存在[6]。研究表明GPI区是一种厚度为单层原子,宽度为1~9 nm的片状结构[9]。
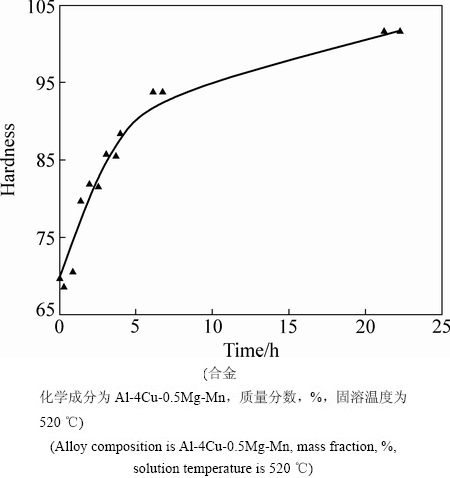
图1 WILM等发表的第一条铝合金时效硬化曲线[5]
Fig. 1 First age hardening curve developed by WILM et al[5]
2) θ″相
θ″相也被称为GPII区。与单层原子的GPI区不同,θ″是在(001)面上的双层原子结构,中间隔着三层铝原子[10-11]。图2中包含了θ″相的结构示意图,可以看到θ″相是与铝基体完全共格的界面。研究表明,θ″相多在Al-Cu二元合金的峰值时效状态下被发现,所以认为它是由GP1区演变而来的。
3) θ′相
θ′相的分子式为Al2Cu,它是一种体心正方结构(空间群I4/mcm,晶胞参数a=0.404 nm,c=0.580 nm)。θ′相与基体半共格,在(001)面上呈八角形片状。
4) θ相
θ相是平衡相,具有正方结构(空间群I4/mcm,晶胞参数a=0.607 nm,c=0.487 nm),有多种变体和形貌。θ相和基体完全不共格。当复合添加Cu和Mg后,Al-Cu-Mg合金的析出序列为:S.S.S.S→clusters→ S″/GPB→S′→S(Al2CuMg)。
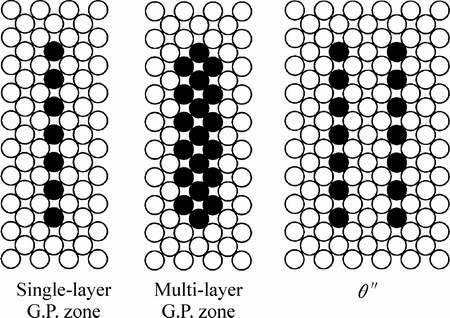
图2 GP区、多层GP区和θ″相的结构示意图[10-11]
Fig. 2 Schematic diagram showing crystal structure of single-layer G.P. zone, multi-Layer G.P. zone and θ″[10-11]
Al-Cu-Mg合金系列的代表合金是2014, 2024等合金。这些合金具有优异的损伤容限性能,所以广泛应用于现代商业飞机。一些中强Al-Cu-Mg合金,如2026等,也可应用于汽车车身板。
RINGER等[12-13]报道,Al-Cu-Mg系列时效开始会形成Cu-Mg原子团簇。这些原子团簇被认为是GPB区的前期阶段。这些Cu-Mg原子团簇可以使合金在淬火后迅速达到峰值时效T6状态的60%。
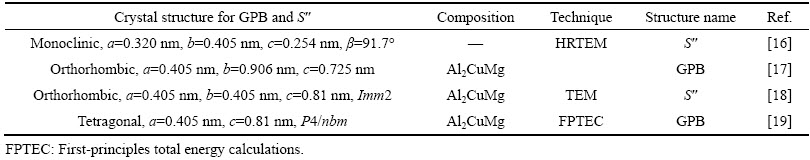
SILCOCK认为GPB区是一种共格的,杆状结构。BAGARYATSKY[14]开展的XRD工作表明,GPB区是一种在<100>方向上的短程有序结构。GPB的直径大约为1~2 nm,长度约为4~8 nm,依据冷却速率的不同而稍有变化。与Al-Cu系合金中的GP区不同,一般认为GPB区和S″相没有特定的晶体结构。表2列举了一些文献中提到的GPB和S″相的分子式和结构[15]。
5) S相
经过长时间的人工时效,Al-Cu-Mg合金中的S″相演变成S′和S相,后两者的结构与S″相的基本相同,但晶格参数有微小的差别。S′相与S相正斜结构,与铝基体分别共格与半共格。S′相的晶格参数a=0.404 nm,b=0.904 nm,c=0.720 nm,与S相的晶格参数a=0.400 nm,b=0.925 nm,c=0.718 nm相比,只存在微小差别,但原子的位置有明显不同。S相的微观形貌如图3所示[20]。
1.2 Al-Mg-Si-(Cu)系合金微结构析出演变
Al-Mg-Si-(Cu)系合金是一种中强度的铝合金。该系列合金具有优良的可焊性,耐蚀性和成型性。所以该系列合金广泛应用于建筑型材和汽车的车身板。Al-Mg-Si系列的时效析出序列为[21-25]:S.S.S.S→ clusters→GP zones→β″′→β′→β(Mg2Si)。
Al-Mg-Si系合金在固溶淬火后会形成大量的细小,无序的原子团簇结构。MURAYAMA等[26]通过3DAP手段分析认为:1) 固溶淬火后会分别形成富Mg和富Si的原子团簇;2) 在自然时效过程中,Mg原子、Si原子会聚集形成Mg-Si原子团簇;3) 在70 ℃时效时,会形成球形的GP区,这些GP区的成分和Mg-Si原子团簇相近。由于这些原子团簇的形成,导致Al-Mg-Si系合金在后续人工时效过程中不能达到设计的强度,因而近年来国内外开展了很多工作研究原子团簇形成的机理和结构。
1) GP区
一般认为,Al-Mg-Si系合金中有两种GP区: 1) MATSUDA等[27]发现的片条状GP区。这些片条状的GP区是单层或者多层原子,在70~150 ℃左右形成。他们会消耗Mg和Si原子,使得随后的人工时效不能达到强化效果;2) MAIROARA等[28]报道的球形的GP区。这种球形GP区大小只有1~3 nm,其晶胞参数和铝基体接近。
表2 同文献中的GPB和S″相的分子式和结构[15]
Table 2 Different stoichiometry and crystal structure of GPB and S″ from various references[15]
2) β″相
β″相是一种沿<100>方向生长的针状析出物,它与基体完全共格,是Al-Mg-Si系合金在峰值时效的主要析出相。β″是一种单斜结构,a=0.77 nm,b=0.67 nm,c=0.405 nm,a和c之间的夹角为75°。但是ANDERSEN等[29]运用高分辨电镜照片得出不同的结论:a=1.516 nm,b=0.405 nm,c=0.674 nm,β=105.3°。ANDERSEN等[29]运用3DAP手段测量出β″相的化学成分为Mg5Si6。之前人们大多认为β″相的成分接近平衡相β-Mg2Si,至少Mg/Si比应该接近1[29]。HASTING等[30]认为应考虑Al原子的替代作用,基于APT测量和ab initio计算,他们认为β″相的实际成分为Mg5Al2Si4。
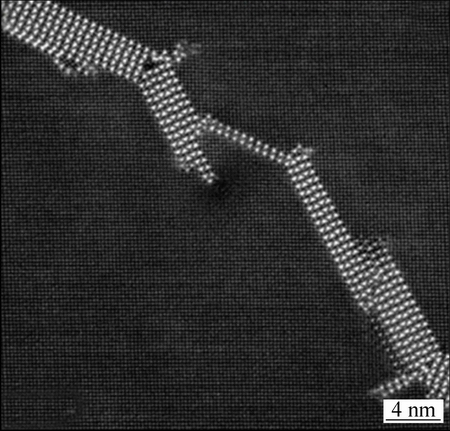
图3 Al-Cu-Mg合金中S相的高分辨图像[20]
Fig. 3 HAADF-STEM image of S-phase in Al-Cu-Mg alloy[20]
3) β′相
与β″相类似,β′相也是在<100>方向上生长的一种杆状析出相。但是β′相是一种六方结构:a=0.705 nm,c=0.405 nm。
Al-Mg-Si系合金中添加合金元素Cu可形成强度更高的Al-Mg-Si-Cu系合金。这也类似于在Al-Cu-Mg合金系中添加合金元素Si。Al-Mg-Si-Cu系合金的析出序列为:S.S.S.S→clusters→GP zones→β″→β′→ Q′→Q+Si。
可以看出,由于Cu元素的添加,Al-Mg-Si-Cu系合金中会形成新的Q相。但是大多数的文献报道峰值时效的Al-Mg-Si-Cu系合金包含大量的β″相和更加弥散的β′相,所以其强度会增加。Q′只在过时效状态才会被观察到,而这个状态的合金强度在缓慢下降。但是CHAKRABARTI等[31]在峰值时效状态下观察到了一种板条状的Q′先导相。他们认为这种板条状的Q′先导相对Al-Mg-Si-Cu系合金的强度具有明显的贡献。
Al-Mg-Si-(Cu)系合金中还有一些其他的非平衡相如B′,U1,U2,L,S和C相等。BANHART等[32]认为这些非平衡相都是由β″相向平衡相β相过度的中间产物。
1.3 Al-Zn-Mg-(Cu)系合金微结构析出演变
Al-Zn-Mg-(Cu)系的主要合金元素Zn、Mg和Cu在铝基体中的溶解度都很高,所以需要添加足够的含量才能使得人工时效时有足够的析出驱动力。该系列一般高强合金中的Zn含量有5%~7%(质量分数),Mg含量 1%~2%,Cu 含量1%~2%,合金的总含量约在10%左右。这大大超过了其他系列的合金含量。所以Al-Zn-Mg-(Cu)系合金的形核时效析出相的密度也超过其他铝合金系列,时效析出相η″的数量密度(Number density)在T6状态的约为(7~9)×1017 cm-3[33],析出相的体积分数(Volume fraction)在T6状态约为4%~5%。Al-Zn-Mg-(Cu)系的经典析出序列为:S.S.S.S→ GP I→ Dissolution,S.S.S.S→VRC→ GP II→η′→η (MgZn2)。
1) GP区
Al-Zn-Mg-(Cu)系合金的GP区一般在室温或者70~100 ℃左右形成[34]。它们与基体完全共格。HONO等[12]认为Al-Zn-Mg-(Cu)系合金中的GP区是富含Mg和Zn原子的球体,且Mg-Zn的比值约为1(摩尔比)。但是BUHA等[35]的3DAP数据表明GP区中除去Mg和Zn原子外也含有少量的Al原子。JIANG等[36]认为Al-Zn-Mg-(Cu)系列中存在两种GP区:GPI区和GPII区。SHA等[37]认为Al-Zn-Mg-(Cu)系中的GPI区最主要含有Zn原子和Mg原子,其Zn-Mg比值约为0.9。GPI区随着时效的发生会溶解到基体中,GPII区会逐渐转变成η′相。
2) η′相
η′相是Al-Zn-Mg-(Cu)系合金的主要强化相。虽然围绕η′相的研究有很多,但是对该相的晶体结构,取向和成分还存在争议。这可能是由于η′相的变体很多,能和基体形成不同的取向。据 等[38]和DEGISHER等[39]报道,η′相有多个变体,能和铝合金基体形成11种位向关系。另一方面,由于时效参数的不同也可能导致η′相的成分发生改变。但是一般认为η′相是六方结构,a=0.496 nm,c=1.403 nm,其惯析面是(111)面[40]。它与基体半共格,即在(111)面上共格,但是在厚度方向与基体不共格。MARIOARA等[41]发现η′相的外层原子结构与T1相(Al2CuLi)很相似。随后ZHANG等[42]在Al-Zn-Mg-(Cu)系合金中发现了与T1相似的单层原子结构,被命名为Y相。一般默认η′相的化学成分为MgZn2,这与η相的化学成分一样。表3一些针对η'相的化学成分的研究。通过这些研究可以发现大多数的Zn与Mg比值都小于2。有一些研究也表明η'相中含有50%以上的Al原子。
等[38]和DEGISHER等[39]报道,η′相有多个变体,能和铝合金基体形成11种位向关系。另一方面,由于时效参数的不同也可能导致η′相的成分发生改变。但是一般认为η′相是六方结构,a=0.496 nm,c=1.403 nm,其惯析面是(111)面[40]。它与基体半共格,即在(111)面上共格,但是在厚度方向与基体不共格。MARIOARA等[41]发现η′相的外层原子结构与T1相(Al2CuLi)很相似。随后ZHANG等[42]在Al-Zn-Mg-(Cu)系合金中发现了与T1相似的单层原子结构,被命名为Y相。一般默认η′相的化学成分为MgZn2,这与η相的化学成分一样。表3一些针对η'相的化学成分的研究。通过这些研究可以发现大多数的Zn与Mg比值都小于2。有一些研究也表明η'相中含有50%以上的Al原子。
表3 η′相的化学成分
Table 3 Chemical composition for η′ precipitates
3) 平衡相η
平衡相η(MgZn2)是六方结构,空间族群P63/mmc,a=0.5221 nm,c=0.8567 nm。据报道η相与基体有9种位向关系[18]。XU等[47]认为η相中的Zn原子能够被Al原子和Cu取代,所以η相的分子式应为Mg3Zn3Cu3Al。
2 时效过程微结构演变的调控
上述铝合金时效微结构可以根据性能要求,引入空位、位错、外应力/应变,通过淬火诱导、多级时效和微合金化等手段进行调控。
2.1 空位调控
空位浓度决定了原子在时效时的扩散速率。依据经典的原子扩散理论,固溶原子在固溶体中的扩散通过与周围的空位交换位置完成。所以通过引入高浓度空位能够加速时效析出的过程。铝合金中有两种空位,一种是平衡空位,一种是淬火空位。平衡空位是合金的固有属性,其浓度只与温度相关。铝合金的空位浓度在25 ℃为1×1011~6×1012 cm-3。另一种是淬火空位(Quenched-in vacancy)。铝合金中的淬火空位浓度与固溶温度和淬火速率相关。FALAHATI等[48-49]研究了Al-Mg-Si系合金中的空位浓度与冷却速率的关系。结果表明,当从固溶温度以10 K/s冷下来时,空位浓度约为3×1019,但是当合金的冷却速度降为0.1 K/s的时候,合金的空位浓度约为12×1014。FISCHER等[50]认为淬火空位会发生湮灭,形成不同的陷阱-位错。MARCEAU等[51]报道了在Al-Cu-Mg系合金中,淬火后,淬火空位迅速合并形成位错环,结果如图4所示。这些位错环有利于Mg原子和Cu原子扩散并形成S相。在Al-Cu-Mg系中,这是一种常见的S相非均匀形核的机制。在Al-Zn-Mg-(Cu)系合金中,淬火后会马上形成空位团簇(Vacancy rich clusters),这种空位团簇可以看成为时效析出相提供非均匀形核的质点。但是也有人指出原子团簇可能在高空位浓度条件下更稳定。所以使得这些原子团簇能吸引更多的固溶原子从而发生长大。
固溶原子-空位的结合能在运用Monte Carlo方法计算过饱和固溶体的分解过程中很重要。一些文献中应用赝势理论和第一性原理计算得出的数值常常被用于此类模拟过程。
空位的扩散速率很快,当他们扩散到晶界附件时会形成空位阱。当晶界附件的空位浓度降低到不能够帮助固溶原子扩散,从而在时效过程中会形成无沉淀析出区/带(Precipitate-free zones)。
2.2 位错调控
Al-Cu合金往往通过一定的塑性变形引入不同量的位错密度后进行时效,细化组织,提高析出密度,从而提高强度[52],结果如图5所示。固溶淬火后冷变形的合金经自然时效、人工时效后,分别被称为T3、T8状态,根据形变量的大小,又分别称为T3x、T8x状态。在实际中,2024(T3、T351、T361、T81、T861),2324-T39,2219(T3、T37、T87、T851),2519-T87等合金牌号状态均已大量生产。
2.3 外应力/应变调控
时效在一定的外应力/外应变诱导下同时进行,改变析出第二相的取向, 从而调控合金的性能,这个过程称为应变时效(Stress assisted ageing)。ETO等[53]最早报道了Al-Cu合金中,外应力的同时引入使得合金的析出位向发生了改变。
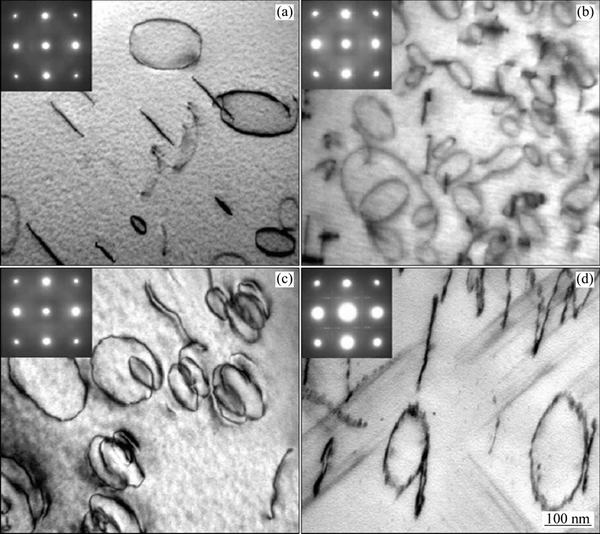
图4 Al-1.1Cu-1.7Mg(x, %) 合金的位错环和位错环上非均匀形核的TEM像[51]
Fig. 4 TEM images of dislocation loop and heterogeneous nucleation in alloy Al-1.1Cu-1.7Mg (x, %)[51]
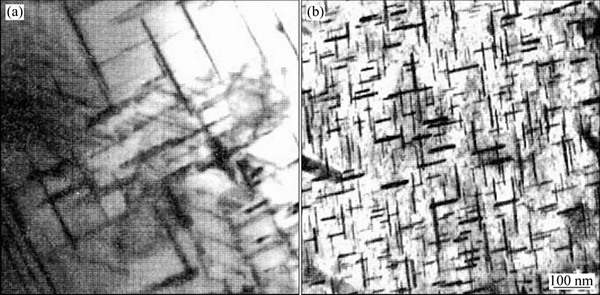
图5 2519铝合金经形变时效后TEM像[52]
Fig. 5 TEM images of aged alloy 2519 with no deformation during ageing (a) and deformation (b)[52]
DECHAMPS等[54]研究了7010合金在时效过程中加入不同的变形量,来研究合金在应变时效过程中的动态析出过程。结果表明应变时效能够加快时效析出过程,析出粒子变细(见图7(a))。加大应变量/加快应变速率能使得时效析出更容易。图7(b)表明,在同时加上变形后,时效析出相的体积分数比未加入变形时效果要高出许多。
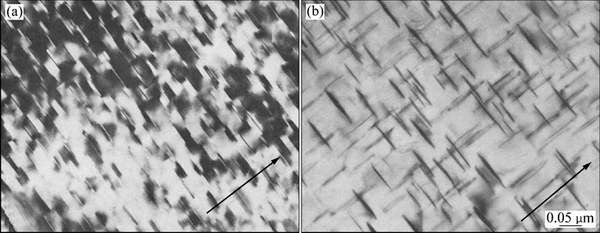
图6 应力时效(压力 73.5 MPa)后在443 K自由时效32 h及无压力状态下在443 K应力时效32 h后θ′析出相的TEM像[53]
Fig. 6 TEM images of θ′ precipitates in stress assisted ageing at 443K for 5 min (a) and aged at 443 K without external stress (b)[53]
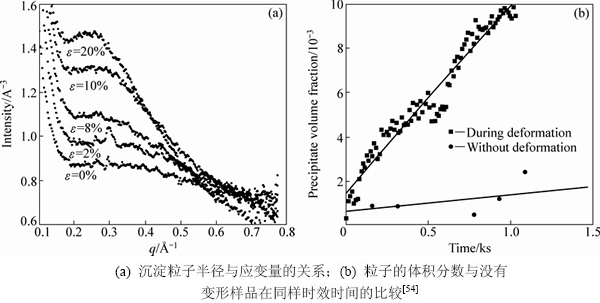
图7 样品经不同应变拉伸后采集的X射线散射宽度曲线
Fig. 7 X-ray scattering intensity changes with different deformations (a) and comparison of volume fraction of age hardening precipitates with/without deformation for Al-Zn-Mg alloys aged at same condition (b)[54]
2.4 淬火冷却速率调控
铝合金的固溶-淬火过程影响后续的时效过程。在固溶度一定的情况下,合金越快冷却,则能获得最大的过饱和固溶度和空位,有利调控性能。但是当合金冷却速率过慢时,则会生成淬火诱导析出相。淬火诱导析出相一般是粗大的粒子,与基体不共格,它们消耗了时效时所必须的固溶原子,在后续时效过程中,合金的强化能力得到了弱化。为了避免淬火诱导相,需要设法尽快冷却合金。但是这样会使得合金的内应力增加,特别是对于厚截面大构件材料,在后续加工过程中会发生翘曲,尺寸不均匀甚至产生裂纹。所以如何避免淬火诱导析出相的形成,在厚截面尺寸淬火处理中显得尤为重要。铝合金Al-Cu-Mg系,Al-Mg-Si系和Al-Zn-Mg-Cu系合金均存在淬火敏感性问题[55-57]。在实际生产中Al-Zn-Mg-Cu系的淬火敏感性问题更加突出,因为该系合金多应用于厚截面大型结构件。
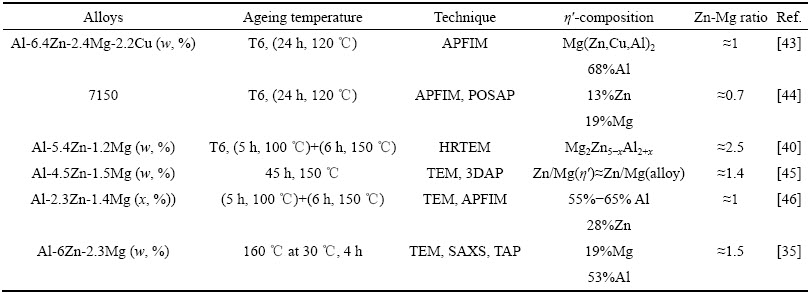
研究表明,Al-Mg-Si系合金在慢速淬火过程中至少存在两种淬火诱导析出相[57]。Al-Zn-Mg-Cu系至少有三种淬火诱导析出相,如图8所示,其中新发现的Y相由于其特殊的晶体结构还具有强化效果[42]。
AA7150合金的组成如下:Si 0.02,Fe 0.05,Cu 2.04,Mn 0.04,Mg 2.15,Zn 6.33,Zr 0.12,Cr <0.01,Ti 0.01;固溶处理为(460 ℃,1 h)+(480 ℃, 1 h)。
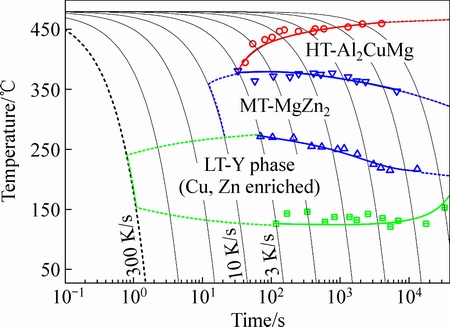
图8 7150合金的连续冷却析出相图[57]
Fig. 8 Continuous cooling precipitation (CCP) diagram for alloy 7150[57]
2.5 多级时效调控
借助不同时效过程中微结构的形成与演变,进行单级与不同形式的多级时效,调控铝合金的性能,在这方面形成了许多创新思路,并创立和发展了系列时效制备技术。时效过程中根据硬度(或强度)与温度(或时间)由小增大和下降,经由峰值的变化规律,合金可调控为不同的欠时效、峰值时效和不同的过时效状态。若合金需要满足耐蚀性能、韧性等要求,一般需采用双级或多级时效,或断续时效等技术进行调控[58-62]。
峰值时效使合金具有最高强度。人们为了获得最高强度建立了峰值时效制度,材料的时效状态被称为T6。此时,时效析出相粒子的大小与分布可同时阻碍位错切过和绕过,强化效果最佳。7178-T6,7079-T6,7075-T6,7050-T6等合金材料为峰值强度状态。并且,随时效温度的降低,合金中析出相更加细小弥散,强度会更高。但是,应用表明,T6态7xxx系铝合金往往存在严重应力腐蚀开裂问题。
过时效提高抗应力腐蚀性能。合金的过时效状态被称为T7状态。根据结构设计对强度和抗应力腐蚀性能的要求,可采用不同的T7过时效状态,如T76、T74、T73等。对于强度由高到低的顺序为T6、T76、T74、T73,而对于抗应力腐蚀性能则顺序相反。过时效一般采用双级时效工艺,根据性能要求,调控GP区的数量、体积分数以及亚稳态相的数量达到目标。如图9所示,7449铝合金在120 ℃、160 ℃双级时效过程中的微结构演变用小角度X射线散射测量结果进行了直观的表征。
由图9可见,时效过程中沉淀粒子的体积分数、密度、半径大小与长大速度与时间的关系表征得非常清楚,为性能的设计和调控奠定了基础。
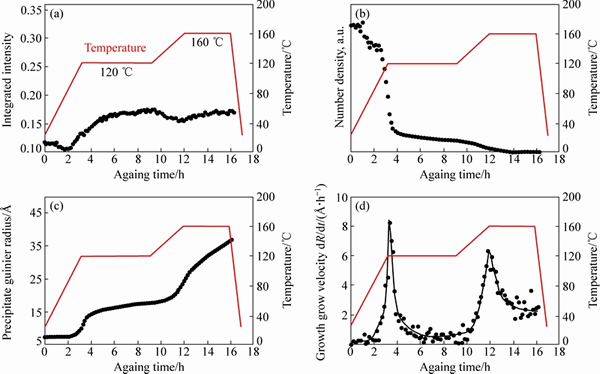
图9 采用小角度X射线散射测量7449铝合金双级时效过程中的沉淀粒子体积分数、密度、半径与长大速度与时间温度的关系[63]
Fig. 9 Volume fraction (a), number density (b), precipitate radius (c) and growth velocity (d) of age hardening precipitates in alloy 7449 during two-step ageing[63]
合金经过时效后,一方面T6状态的连续分布晶界相变成T7状态的断续分布,有利切断腐蚀电流;另一方面促使晶内、晶界之间元素的扩散,降低晶界与基体间的电极电位差,减小腐蚀倾向。合金抗应力腐蚀性能提高,但晶内第二相已变得粗大,合金强度下降[64]。合金状态可根据强度与耐腐蚀性能等要求进行调控。过时效的铝合金材料7075-T73,7050-T73,7050-T74已大量用做飞机关键结构件。
回归再时效使合金高强和高抗应力腐蚀。如上所述,虽然过时效可提高抗应力腐蚀性能,但合金的强度一般降低10%~15%。为了改善抗应力腐蚀性能,而又尽量保持高强度,1973年CINA等[65]发明了三级时效制度,称为回归再时效(Retrogression and re-ageing,RRA)。合金通过回归处理,回溶第一级时效中小于临界尺寸的微结构,尔后再时效,使晶界上形成T7状态不连续分布相,而晶内形成T6状态弥散分布的高密度亚稳相,因而合金的强度高,耐蚀性好[66]。冯迪等[67]研究7055铝合金经RRA热处理后的显微组织,结果表明晶内第二相细小高密度分布,晶界断续分布,是典型的高强抗应力腐蚀组织特征。经三级时效处理后的铝合金材料7150-T7752,7150-T7751,7055-T7751已大量用做飞机的关键结构件。
断续时效使合金高强高韧。为了使基体中的原子充分析出对材料的服役性能作贡献,在第一级时效后不是升温回归处理,而是采取快速冷却,形成高浓度空位和一定饱和浓度的溶质原子,紧接着高温时效,进行二次析出。此技术称之断续时效(Interrupted ageing)。利用第一次高温时效后的淬火空位进行时效,调控原子团簇或GP区的浓度与大小,使合金进一步强化;若继续高温时效,低温时效中形成的微结构进行演变、长大,提高合金的韧性。如表4所列,铝合金经断续时效后,与T6状态相比,不仅强度相当或提高,而且韧性显著提高。
2.6 微合金化调控
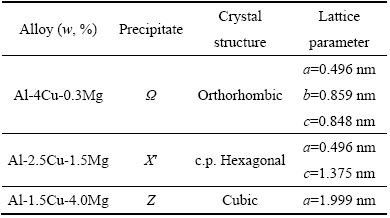
在原有铝合金系列中,添加不同微量元素,可以形成新的析出相,从而调控合金的性能。如表5所列,在不同Cu-Mg比值的Al-Cu-Mg合金中添加0.4%Ag,形成不同的新相,其中Ω相在高温下稳定,有利于提高合金的耐热性能。在Al-Cu系中添加微量Cd、In、Sn可改变合金的时效硬化特性。由于这些元素有利于与空位反应,并容易吸附在析出相/基体界面上,降低析出相所需界面能,易形成小的(约5 nm)原子团簇,发生非均质形核,降低沉淀相形成温度,研究表明,Al-4Cu-0.05In与Al-4Cu合金相比,θ′相沉淀温度降低了85 ℃;并且提高析出相密度和合金的硬化能力。
表4 一些2xxx,6xxx,7xxx铝合金T6 、T6I4 与T6I6 状态性能的比较[68]
Table 4 Mechanical properties of some commercial 2xxx, 6xxx and 7xxx aluminium alloys in different tempers[68]
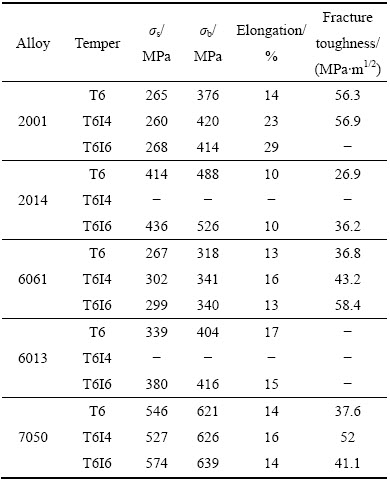
表5 在Al-Cu-Mg 三元合金中添加 0.4%Ag 所形成的析出相[11, 45, 69-71]
Table 5 New phases in Al-Cu-Mg ternary system with different compositions by adding 0.4% (mole fraction) Ag respectively[11, 45, 69-71]
添加微量过渡簇元素,如Cr、Mn、Ti、Zr等,可通过控制基体组织和界面析出调控性能[72]。这些元素与铝形成弥散相,一方面阻止再结晶,控制晶界结构;另一方面可根据弥散相与基体界面的结构,控制淬火过程中界面的析出,降低合金的淬火敏感性[73-75]。研究表明,以Zr、Sc共格/半共格相界面替代Cr、Mn非共格相界面,铝合金材料的淬火敏感性降低,材料的截面可加厚,沿厚向性能的均匀性提高。但共格/半共格界面通过再结晶会向非共格界面转化。如图10所示的7150铝合金在空冷和水淬条件下Al3Zr弥散相的分布情况。由于再结晶的发生,导致Al3Zr粒子与基体形成非共格相界面[73],缓慢冷却过程中这种界面有利于平衡相的析出,提高了合金的淬火敏感性。但回复组织中的Al3Zr粒子仍与基体共格,有利于降低淬火敏感性。
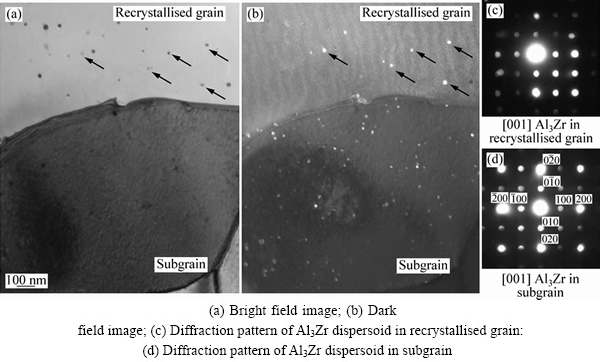
图10 Al3Zr弥散相的TEM像和电子衍射谱[73]
Fig. 13 Al3Zr dispersoids showing same crystal structure in recrystallized grain and subgrain[73]
3 结语
系统地回顾了3类常用时效热处理铝合金的典型时效析出序列和时效析出相结构,阐述了空位、位错、相界面晶体缺陷以及外应力/应变和合金化元素调控时效析出相的基本原理,获得以下结论。
1) 铝合金淬火、时效处理是调控组织性能的重要技术途径。性能的调控涉及强度、耐蚀、疲劳和韧性等,时效技术包括峰值时效、过时效、多级时效、断续时效等。
2) 淬火、时效技术的发展,需设法控制淬火冷却速率,抑制平衡相析出;时效需设法增加位错密度、空位浓度,大幅提高具有多临界尺度GP区的密度,最大限度降低溶质原子的浓度,增大析出量,促使析出的细化、均匀化和亚稳化。
3) 添加微合金化元素降低时效亚稳相形核表面能,有利时效析出,或促使新相形成;添加过渡簇元素,控制再结晶晶界和相界析出。
REFERENCES
[1] POLMEAR I J. Light alloys: metallurgy of the light metals[M]. 4th ed, Melbourne: Elsevier, 2006.
[2] DUPARE O H, WILM Alfred. The beginnings of duralumin[J]. Z Metallkd, 2005, 96(4): 398-404.
[3] WILM A. Physical-metallurgical alccminium-magnesium alloys[J]. Z Metallkd, 1911, 8(8): 225-227.
[4] WILM A. Methods for application of aluminum-magnesium alloys[P]. DE 244554, 1909.
[5] POLMEAR I J. Aluminium alloys—A century of age hardening[J]. Mater Forum, 2004, 28: 1-13.
[6] KARLIK M, JOUFFREY B. High resolution electron microscopy study of Guinier-Preston (GP1) zones in Al-Cu based alloys[J]. Acta Mater, 1997, 45(8): 3251-3263.
[7] HU S Y, BASKER M I, STAN M, CHEN L Q. Atomistic calculations of interfacial energies, nucleus shape and size of θ′ precipitates in Al-Cu alloys[J]. Acta Mater, 2006, 54(18): 4699-4707.
[8] PRESTON G D. The diffraction of X-rays by an age-hardening alloy of aluminium and copper. The structure of an intermediate phase[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1938, 26(178): 855-871.
[9] KARLIK M, JOUFFREY B, BELLIOT S. The copper content of Guinier-Preston (GP1) zones in Al-1.84 at.% Cu alloy[J]. Acta Mater, 1998, 46(5): 1817-1825.
[10] HONO K, SATOH T, HIRANO K I. Evidence of multi-layer GP zones in Al-1·7at.%,Cu alloy[J]. Philosophical Magazine A, 1986, 53(4): 495-504.
[11] RINGER S P, HONO K, POLMEAR I J, SAKURAI T. Nucleation of precipitates in aged AlCuMg(Ag) alloys with high Cu:Mg ratios[J]. Acta Mater, 1996, 44(5): 1883-1898.
[12] HONO K, RINGER S P. Microstructural evolution and age hardening in aluminium alloys: atom probe field-ion microscopy and transmission electron microscopy studies[J]. Mater Charact, 2000, 44: 101-131.
[13] RINGER S P, CARAHER S K, POLMEAR I J. Response to comments on cluster hardening in an aged Al-Cu-Mg alloy[J]. Scr Mater, 1998, 39(11): 1559-1567.
[14] BAGARYATSKY Y A. Understand a nano-structure precipitate using X-ray scattering[J]. Dokl A Kad Nauk SSSR, 1952, 87: 559-562.
[15] WANG S C, STARINK M J. The assessment of GPB2/S″ structures in Al-Cu-Mg alloys[J]. Mater Sci Eng, A, 2004, 386(1/2): 156-163.
[16] CHARAI A, WALTHER T, ALFONSO C, ZAHRA A M, ZAHRA C Y. Coexistence of clusters, GPB zones, S″-, S′- and S-phases in an Al-0.9% Cu-1.4% Mg alloy[J]. Acta Mater, 2000, 48(10): 2751-2764.
[17] MONDOLFO L F. Metallography of aluminum alloys[M]. New York: John Willey & Sons, 1943.
[18] CUISIAT F, DUVAL P, GRAF R. Study of the early stage of decomposition of Al-Cu-Mg alloy[J]. Scripta Metallurgica, 1984, 18(10): 1051-1056.
[19] WOLVERTON C. Crystal structure and stability of complex precipitate phases in Al-Cu-Mg-(Si) and Al-Zn-Mg alloys[J]. Acta Mater, 2001, 49: 3129-3124.
[20] RALSTON K D, BIRBILIS N, WEYLAND M, HUTCHINSON C R. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al-Cu-Mg alloys[J]. Acta Mater, 2010, 58(18): 5941-5948.
[21] RINGER S P,  K. Microstructural evolution and age hardening in aluminium alloys: atom probe field-ion microscopy and transmission electron microscopy studies[J]. Mater Charact, 2000, 44(1/2): 101-131.
K. Microstructural evolution and age hardening in aluminium alloys: atom probe field-ion microscopy and transmission electron microscopy studies[J]. Mater Charact, 2000, 44(1/2): 101-131.
[22] POGATSCHER S, ANTREKOWITSCH H, WERINOS M, MOSZNER F, GERSTL S S A, FRANCIS M F, CURTIN W A,  J F, UGGOWITZER P J. Diffusion on demand to control precipitation aging: application to Al-Mg-Si alloys[J]. Phys Rev Lett, 2014, 112(22): 225701.
J F, UGGOWITZER P J. Diffusion on demand to control precipitation aging: application to Al-Mg-Si alloys[J]. Phys Rev Lett, 2014, 112(22): 225701.
[23] MARIOARA C D, ANDERSEN S J, ZANDBERGEN H W, HOLMESTAD R. The influence of alloy composition on precipitates of the Al-Mg-Si system[J]. Metallurgical and Materials Transactions A, 2005, 36(3): 691-702.
[24] WANG X, ESMAEILI S, LLOYD D J. The sequence of precipitation in the Al-Mg-Si-Cu alloy AA6111[J]. Metallurgical and Materials Transactions A, 2006, 37(9): 2691-2699.
[25] POGATSCHER S, ANTREKOWITSCH H, LEITNER H, EBNER T, UGGOWITZER P J. Mechanisms controlling the artificial aging of Al-Mg-Si alloys[J]. Acta Mater, 2011, 59(9): 3352-3363.
[26] MURAYAMA M,  K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys[J]. Acta Mater, 1999, 47(5): 1537-1548.
K. Pre-precipitate clusters and precipitation processes in Al-Mg-Si alloys[J]. Acta Mater, 1999, 47(5): 1537-1548.
[27] MATSUDA K, KAWABATA T, UETANI Y, SATO T, KAMIO A, IKENO S. HRTEM observation of G.P. zones and metastable phase in Al-Mg-Si alloys[J]. Mater Sci Forum, 2000, 331/337: 989-994.
[28] MARIOARA C D, ANDERSEN S J, JANSEN J, ZANDBERGEN H W. The influence of temperature and storage time at RT on nucleation of the β″ phase in a 6082 Al-Mg-Si alloy[J]. Acta Mater, 2003, 51(3): 789-796.
[29] ANDERSEN S J, ZANDBERGEN H W, JANSEN J,  C, TUNDAL U, REISO O. The crystal structure of the β″ phase in Al-Mg-Si alloys[J]. Acta Mater, 1998, 46(9): 3283-3298.
C, TUNDAL U, REISO O. The crystal structure of the β″ phase in Al-Mg-Si alloys[J]. Acta Mater, 1998, 46(9): 3283-3298.
[30] HASTING H S,  A G, ANDERSEN S J, VISSERS R, WALMSLEY J C, MARIOARA C D, DANOIX F, LEFEBVRE W, HOLMESTAD R. Composition of β″ precipitates in Al-Mg-Si alloys by atom probe tomography and first principles calculations[J]. J Appl Phys, 2009, 106(12): 123527.
A G, ANDERSEN S J, VISSERS R, WALMSLEY J C, MARIOARA C D, DANOIX F, LEFEBVRE W, HOLMESTAD R. Composition of β″ precipitates in Al-Mg-Si alloys by atom probe tomography and first principles calculations[J]. J Appl Phys, 2009, 106(12): 123527.
[31] CHAKRABARTI D J, LAUGHLIN D E. Phase relations and precipitation in Al-Mg-Si alloys with Cu additions[J]. Progress in Materials Science, 2004, 49(3/4): 389-410.
[32] BANHART J, CHANG C S T, LIANG Z, WANDERKA N, LAY M D H, HILL A J. Natural aging in Al-Mg-Si alloys—A process of unexpected complexity[J]. Advanced Engineering Materials, 2010, 12(7): 559-571.
[33] DESCHAMPS A, BIGOT A, LIVET F, AUGER P, BRECHET Y, BLAVETTE D. A comparative study of precipitate composition and volume fraction in an Al-Zn-Mg alloy using tomographic atom probe and small-angle X-ray scattering[J]. Philosophical Magazine A, 2001, 81(10): 2391-2414.
[34] SCHMUCK P A C, DANOIX F, BLAVETTE D. Quantitative analysis of GP zones formed at room temperature in a 7150 Al-based alloy[J]. Appl Surf Sci, 1995, 87/88: 228-233.
[35] BUHA J, LUMLEY R N, CROSKY A G. Secondary ageing in an aluminium alloy 7050[J]. Mater Sci Eng A, 2008, 492: 1-10.
[36] JIANG X J, TAFTO J, NOBLE B, HOLME B, WATERLOO G. Differential scanning calorimetry and electron diffraction investigation on low-temperature aging in Al-Zn-Mg alloys[J]. Metallurgical and Materials Transactions A, 2000, 31(2): 339-348.
[37] SHA G, CEREZO A. Early-stage Precipitation in Al-Zn-Mg-Cu alloy (7050)[J]. Acta Mater, 2004, 52: 4503-4516.
[38]  H,
H,  I, LENDVAI J. Decomposition processes in Al-Zn-Mg alloys[J]. J Mater Sci, 1983, 18(8): 2215-2240.
I, LENDVAI J. Decomposition processes in Al-Zn-Mg alloys[J]. J Mater Sci, 1983, 18(8): 2215-2240.
[39] DEGISHER H P, LACOM W, ZAHRA A, ZAHRA C Y. Decomposition processes in an Al-5%Zn-1%Mg alloy[J]. Z Metallkd, 1980, 71: 231-238.
[40] LI X Z, HANSEN V,  J, WALLENBERG L R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al-Zn-Mg alloys[J]. Acta Mater, 1999, 47(9): 2651-2659.
J, WALLENBERG L R. HREM study and structure modeling of the η′ phase, the hardening precipitates in commercial Al-Zn-Mg alloys[J]. Acta Mater, 1999, 47(9): 2651-2659.
[41] MARIOARA C D, LEFEBVRE W, ANDERSEN S, FRIIS J. Atomic structure of hardening precipitates in an Al-Mg-Zn-Cu alloy determined by HAADF-STEM and first-principles calculations: relation to η-MgZn2[J]. J Mater Sci, 2013, 48(10): 3638-3651.
[42] ZHANG Y, WEYLAND M, MILKEREIT B, REICH M, ROMETSCH P A. Precipitation of a new platelet phase during the quenching of an Al-Zn-Mg-Cu alloy[J]. Scientific Reports, 2016. doi: 1038/srep23109.
[43] BRENNER S S, KOWALIK J, HUA M J. FIM/atom probe analysis of a heat treated 7150 aluminum alloy[J]. Surf Sci, 1991, 246(1): 210-217.
[44] WARREN P J, GROVENOR C R M, CROMPTON J S. Field-ion microscope/atom-probe analysis of the effect of RRA heat treatment on the matrix strengthening precipitates in alloy Al-7150[J]. Surf Sci, 1992, 266(1): 342-349.
[45] MALONEY S K, HONO K, POLMEAR I J, RINGER S P. The chemistry of precipitates in an aged Al-2.1Zn-1,7Mg at.% alloy[J]. Scr Mater, 1999, 41(10): 1031-1038.
[46] STILLER K, WARREN P J, HANSEN V, ANGENETE J,  J. Investigation of precipitation in an Al-Zn-Mg alloy after two-step ageing treatment at 100° and 150 ℃[J]. Mater Sci Eng A, 1999, 270: 55-63.
J. Investigation of precipitation in an Al-Zn-Mg alloy after two-step ageing treatment at 100° and 150 ℃[J]. Mater Sci Eng A, 1999, 270: 55-63.
[47] FANG X, SONG M, LI K, DU Y, ZHAO D, JIANG C, ZHANG H. Effect of Cu and Al on the crystal structure and composition of h (MgZn2) phase in over-aged Al-Zn-Mg-Cu alloys[J]. J Mater Sci, 2012, 47: 5419-5427.
[48] FALAHATI A, LANG P, KOZESCHNIK E. Precipitation in Al-alloy 6016-the role of excess vacancies[J]. Mater Sci Forum, 2011, 706/709: 317-322.
[49] LANG P, FALAHATI A, AHMADI M R, WARCZOK P, POVODEN-KARADENIZ E, KOZESCHNIK E, RADIS R. Modeling the influence of cooling rate on the precipitate evolution in Al-Mg-Si (Cu) alloys[J]. Mater Sci Technol, 2011, 16/20: 284-291.
[50] FISCHER F D, SVOBODA J, APPEL F, KOZESCHNIK E. Modeling of excess vacancy annihilation at different types of sinks[J]. Acta Mater, 2011, 59(9): 3463-3472.
[51] MARCEAU R K W, SHA G, LUMLEY R N, RINGER S P. Evolution of solute clustering in Al-Cu-Mg alloys during secondary ageing[J]. Acta Mater, 2010, 58(5): 1795-1805.
[52] 李慧中, 张新明, 陈明安, 周卓平, 龚敏如. 预变形对2519铝合金组织与力学性能的影响[J]. 中国有色金属学报, 2004, 14(12): 1990-1994.
LI Hui-zhong, ZHANG Xin-ming, CHEN Ming-an, ZHOU Zhou-ping, GONG Ming-ru. Effect of pre-deformation on microstructures and mechanical properties of 2519 aluminum alloy[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(12): 1990-1994.
[53] ETO T, SATO A, MORI T. Stress-oriented precipitation of G.P. Zones and θ′ in an Al-Cu alloy[J]. Acta Metallurgica, 1987, 26(3): 499-508.
[54] DESCHAMPS A, BLEY F, LIVET F, FABREGUE D, DAVID L. In-situ small-angle X-ray scattering study of dynamic precipitation in an Al-Zn-Mg-Cu alloy[J]. Philos Mag, 2003, 83(6): 677-692.
[55] ZHANG X M, LIU W, LIU S D, ZHOU M. Effect of Processing Parameters on Quench Sensitivity of an AA7050 sheet[J]. Mater Sci Eng A, 2011, 528: 795-802.
[56] MILKEREIT B, WANDERKA N, SCHICK C, KESSLER O. Continuous cooling precipitation diagrams of Al-Mg-Si alloys[J]. Mater Sci Eng A, 2012, 550: 87-96.
[57] ZHANG Y, MILKEREIT B, KESSLER O, SCHICK C, ROMETSCH P. Development of continuous cooling precipitation diagrams for aluminium alloys AA7150 and AA7020[J]. J Alloys Compd, 2014, 584: 581-589.
[58] 李 海, 郑子樵, 王芝秀. 含银7055铝合金回归再时效过程中得组织与性能变化[J]. 稀有金属材料与工程, 2004, 33(7): 718-722.
LI H, ZHENG Z Q, WANG Z X. Retrogression and reaging of Ag-containing 7055 Al alloy[J]. Rare Metal Materials and Engineering, 2004, 33(7): 718-722
[59] 谢优华, 杨守杰, 戴圣龙, 陆 政. 锆元素在铝合金中的应用[J]. 航空材料学报, 2002, 22(4): 56-61.
XIE Y H, YANG S J, DAI S L, LU Z. Application of element Zr in aluminum alloys[J]. Journal of Aeronautical Materials, 2002, 22(4): 56-61.
[60] 刘 刚, 张国君, 丁向东, 孙 军, 陈康华. 含有不同尺度量级第二相的高强铝合金断裂韧性模型[J]. 中国有色金属学报, 2002, 12(4): 706-713.
LIU Gang, ZHANG Guo-jun, DING Xiang-dong, SUN Jun, CHENG Kang-hua. A model for fracture toughness of high strength aluminum alloys containing second particles of various sized scales[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(4): 706-713.
[61] 刘 刚, 丁向东, 孙 军, 陈康华. 具有盘状析出相铝合金的时效强化模型[J]. 中国有色金属学报, 2001, 11(3): 337-347.
LIU Gang, DING Xiang-dong, SUN Jun, CHENG Kang-hua. A model for age strengthening of plate-like-precipitate-containing Al alloys[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3): 337-347.
[62] 伍尚华, 卢 宜. LY12合金在拉伸变形中的微观结构[J]. 中国有色金属学报, 1994, 4(4): 87-90.
WU Shang-hua, LU Yi. The microstructure changes during tensile deformation of alloy LY12[J]. The Chinese Journal of Nonferrous Metals, 1994, 4(4): 87-90.
[63] FRIBOURG G,  Y, DESCHAMPS A, SIMAR A. Microstructure-based modelling of isotropic and kinematic strain hardening in a precipitation-hardened aluminium alloy[J]. Acta Mater, 2011, 59(9): 3621-3635.
Y, DESCHAMPS A, SIMAR A. Microstructure-based modelling of isotropic and kinematic strain hardening in a precipitation-hardened aluminium alloy[J]. Acta Mater, 2011, 59(9): 3621-3635.
[64] 张 勇, 张新明, 刘胜胆, 欧 军, 徐 敏, 钟奇鸣. 时效工艺对1933铝合金锻件腐蚀性能的影响[J]. 中国有色金属学报, 2011, 21(7): 1527-1534.
ZHANG Yong, ZHANG Xin-ming, LIU Sheng-dan, OU Jun, XU Min, ZHONG Qi-ming. Effects of aging treatment on corrosion properties of 1933 aluminum alloy forging[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1527-1534.
[65] WILM A. Hardening effect from aluminium-magnesium alloys[P]. DE 2444554, 1909.
[66] FENG D, ZHANG X M, LIU S D, WANG T, WU Z Z, GUO Y W. The effect of pre-ageing temperature and retrogression heating rate on the microstructure and properties of AA7055[J]. Mater Sci Eng A, 2013, 588: 34-42.
[67] 冯 迪, 张新明, 刘胜胆. 非等温回归再时效对7055铝合金中厚板的厚向组织及性能均匀性的影响[J]. 有色金属学报, 2015, 25(11): 3000-3010.
FENG Di, ZHANG Xin-ming, LIU Sheng-dan. Effect of non-isothermal retrogression and re-ageing on through-thickness homogeneity of microstructure and properties in 7055 aluminum alloy medium thick plate[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(11): 3000-3010.
[68] LUMLEY R N, POLMEAR I J, MORTON A J. Temper developments using secondary ageing[J]. Mater Forum, 2004, 28: 85-95.
[69] MUDDLE B C, POLMEAR I J. The Precipitate phase in Al-Cu-Mg-Ag alloys[J]. Acta Mater, 1989, 3: 777-789.
[70] POLMEAR I J. Tensile properties of modified aluminium-zinc-magnesium alloys containing silver[J]. Journal of the Institute of Metals, 1966, 94: 36-37.
[71] VIETZ J T, SARGANT K R, POLMEAR I J. The influence of small additions of silver on the ageing of aluminium alloys: further observations on Al-Zn-Mg alloys[J]. Journal of the Institute of Metals, 1964, 92: 327-333.
[72] 李培跃, 熊柏青, 张永安, 李志辉, 朱宝宏, 王 峰, 刘红伟. 淬火介质对7050铝合金末端淬特性的影响[J]. 中国有色金属学报, 2011, 21(5): 961-967.
LI Pei-yue, XIONG Bai-qing, ZHANG Yong-an, LI Zhi-hui, ZHU Bao-hang, WANG Feng, LIU Hong-wei. Effect of quenching media on Jominy end quench behavior of 7050 Al alloy[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 961-967.
[73] ZHANG Y, BETTLES C, ROMETSCH P A. Effect of recrystallisation on Al3Zr dispersoid behaviour in thick plates of aluminium alloy AA7150[J]. J Mater Sci, 2014, 49: 1709-1715.
[74] 刘胜胆, 李承波, 欧阳惠, 邓运来, 张新明, 刘星兴. 超高强7000系铝合金的淬火敏感性[J]. 中国有色金属学报, 2013, 22(4): 927-938.
LIU Sheng-dan, LI Cheng-bo, OUYANG Hui, DENG Yun-lai, ZHANG Xing-ming, LIU Xing-xing. Quench sensitivity of ultrahigh strength 7000 series aluminum alloys[J]. The Chinese Journal of Nonferrous Metals, 2013, 22(4): 927-938.
[75] 刘胜胆, 张新明, 游江海, 黄振宝, 张 翀, 张小艳, 7055铝合金的TTP曲线及其应用[J]. 中国有色金属学报, 2006, 16(12): 2034-2039.
LIU Sheng-dan, ZHANG Xing-ming, YOU Jiang-hai, HUANG Zhen-bao, ZHANG Chong, ZHANG Xiao-yan. TTP curve of 7055 alum inum alloy and its application[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(12): 2034-2039.
Aging microstructure evolution in high strength aluminum alloys and performance controlling
ZHANG Yong1, LI Hong-ping1, KANG Wei2, ZHANG Xin-ming1
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China
2. Commercial Aircraft Corporation of China, Shanghai 200126, China)
Abstract: The ageing precipitates evolution determined and described with the modern physics test/analysis technology in high strength aluminum alloys was reviewed, which includes microstructure, size, distribution and morphology of clusters of atoms, Guinier-Preston (GP) zones, intermediate metastable precipitates and equilibrium phases, which can form and evolve during ageing due to spontaneous reducing of the system free-energy. The precipitates on the coherent/half-coherent dispersoid interfaces were compared with those on the incoherent interfaces. The microstructures can be adjusted with vacancy, dislocation, stress, strain, quench-cooling rate, multi-ageing and micro-alloying, the microstructure evolution principles were elucidated, and some ageing controlling technologies were shown. Some achievements were demonstrated in the improvement in the ageing and microalloying of high strength aluminum alloys as well.
Key words: aluminum alloy; ageing; microstructure; interface; property
Foundation item: Project (152612) supported by the Chinese and International Post-Doctor Exchange Project; Project (2012CB619500) supported by the National Basic Research Program of China; Project (2013DFG51890) supported by the China-Australia Jointing Research Project for Light Metals
Received date: 2016-12-20; Accepted date: 2017-02-20
Corresponding author: ZHANG Xin-ming; Tel: +86-731-88830265; E-mail: xmzhang@csu.edu.cn
(编辑 龙怀中)
基金项目:中国博士后国际交流引进计划资助项目(152612);国家重点基础研究发展计划资助项目(2012CB619500);中澳国际合作资助项目(2013DFG51890)
收稿日期:2016-12-20;修订日期:2017-02-20
通信作者:张新明,教授,博士;电话:0731-88830265;E-mail:xmzhang@csu.edu.cn
摘 要:综述铝合金时效过程中,用近代物理检测分析技术表征和确定的微结构从原子团簇、GP区、亚稳相到平衡相的结构、大小及形貌析出序列演变过程;析出微结构与基体形成的共格、半共格和非共格界面结构;界面上析出第二相;论述通过空位、位错、外应力及应变、淬火冷却速率、多级时效及微合金化等手段调控铝合金微结构及其演变原理,铝合金微结构与性能调控技术,铝合金时效调控技术与理论的发展;介绍铝合金时效与微合金化调控性能的一些实例,为改善或提升高强铝合金性能提供理论与技术参考。
[1] POLMEAR I J. Light alloys: metallurgy of the light metals[M]. 4th ed, Melbourne: Elsevier, 2006.
[2] DUPARE O H, WILM Alfred. The beginnings of duralumin[J]. Z Metallkd, 2005, 96(4): 398-404.
[3] WILM A. Physical-metallurgical alccminium-magnesium alloys[J]. Z Metallkd, 1911, 8(8): 225-227.
[4] WILM A. Methods for application of aluminum-magnesium alloys[P]. DE 244554, 1909.
[5] POLMEAR I J. Aluminium alloys—A century of age hardening[J]. Mater Forum, 2004, 28: 1-13.
[17] MONDOLFO L F. Metallography of aluminum alloys[M]. New York: John Willey & Sons, 1943.
[52] 李慧中, 张新明, 陈明安, 周卓平, 龚敏如. 预变形对2519铝合金组织与力学性能的影响[J]. 中国有色金属学报, 2004, 14(12): 1990-1994.
[58] 李 海, 郑子樵, 王芝秀. 含银7055铝合金回归再时效过程中得组织与性能变化[J]. 稀有金属材料与工程, 2004, 33(7): 718-722.
[59] 谢优华, 杨守杰, 戴圣龙, 陆 政. 锆元素在铝合金中的应用[J]. 航空材料学报, 2002, 22(4): 56-61.
[60] 刘 刚, 张国君, 丁向东, 孙 军, 陈康华. 含有不同尺度量级第二相的高强铝合金断裂韧性模型[J]. 中国有色金属学报, 2002, 12(4): 706-713.
[61] 刘 刚, 丁向东, 孙 军, 陈康华. 具有盘状析出相铝合金的时效强化模型[J]. 中国有色金属学报, 2001, 11(3): 337-347.
[62] 伍尚华, 卢 宜. LY12合金在拉伸变形中的微观结构[J]. 中国有色金属学报, 1994, 4(4): 87-90.
[64] 张 勇, 张新明, 刘胜胆, 欧 军, 徐 敏, 钟奇鸣. 时效工艺对1933铝合金锻件腐蚀性能的影响[J]. 中国有色金属学报, 2011, 21(7): 1527-1534.
[65] WILM A. Hardening effect from aluminium-magnesium alloys[P]. DE 2444554, 1909.
[67] 冯 迪, 张新明, 刘胜胆. 非等温回归再时效对7055铝合金中厚板的厚向组织及性能均匀性的影响[J]. 有色金属学报, 2015, 25(11): 3000-3010.
[72] 李培跃, 熊柏青, 张永安, 李志辉, 朱宝宏, 王 峰, 刘红伟. 淬火介质对7050铝合金末端淬特性的影响[J]. 中国有色金属学报, 2011, 21(5): 961-967.
[74] 刘胜胆, 李承波, 欧阳惠, 邓运来, 张新明, 刘星兴. 超高强7000系铝合金的淬火敏感性[J]. 中国有色金属学报, 2013, 22(4): 927-938.
[75] 刘胜胆, 张新明, 游江海, 黄振宝, 张 翀, 张小艳, 7055铝合金的TTP曲线及其应用[J]. 中国有色金属学报, 2006, 16(12): 2034-2039.


