Trans. Nonferrous Met. Soc. China 25(2015) 4207-4215
Response surface methodology for optimizing adsorption performance of gel-type weak acid resin for Eu(III)
Ting CHEN1, Bin LI1, Lei FANG2, De-sui CHEN1, Wen-bin XU1, Chun-hua XIONG1
1. Department of Applied Chemistry, Zhejiang Gongshang University, Hangzhou 310018, China;
2. Department of Food Science and Human Nutrition, University of Florida, Bldg 475 Newell Drive, Gainesville, FL 32611, USA
Received 9 January 2015; accepted 7 June 2015
Abstract:
The conditions relating to enhanced adsorption procedure of earth element europium (Eu) onto gel-type weak acid resin (110-H) in aqueous solution were optimized by means of the response surface methodology (RSM), which proved that 110-H owned satisfactory adsorption capacity (346.85 mg/g) in optimum conditions, belonging to one of the high adsorption capacity materials. Then, the adsorption and desorption behaviors were investigated by batch studies. The adsorption performance showed high agreement with the Lagergren-first-order model and Langmuir isotherm with thermodynamic adsorption parameters of ΔH=36.1 kJ/mol and ΔS=200 J/(mol·K). Desorption study revealed that 110-H could be effectively eluted by a low concentration of HCl solution (0.1 mol/L) to regenerate and reuse. Finally, the 110-H and Eu(III) loaded 110-H were characterized by IR spectroscopy and scanning electron microscope (SEM) to analyze the mechanism of adsorption, which proved to be chemisorbed.
Key words:
Eu( III); response surface methodology; adsorption; gel-type weak acid resin;
1 Introduction
As essential raw materials and strategic resources, nonferrous metals play such important roles in national economy and defense that China is actively promoting the development of nonferrous metals industry and increasing nonferrous metals’ strategic reserve [1]. Since being highly relevant to human activity in various aspects, rare earth elements were widely studied recently, including La [2], Ce [3], Er [4] and so on. Europium (Eu) is a very active metal among the rare earth metals, which means that a small amount of europium may greatly enhance the properties of metals [5]. With a special electronic configuration, europium ion has excellent luminescent properties, especially as a sensitizer and the activator. Its unique fluorescence is widely used in industrial production [6,7], as well as agriculture, medical and biological research, which demonstrates the application potential of Eu[8,9]. Many efforts have been made to separate and enrich metals, such as chemical precipitation [10], ion exchange [11], membrane separation [12], electrochemical deposition [13], and extraction chromatography [14]. However, most of these traditional separation and enrichment methods have several shortcomings, including small capacity, high cost, and difficult treatment on sludge made after experiment.
Compared with other materials, the resin has some advantages because of its plasticity and recoverability. At the same time, many resins have already been put into industrialized production with a low cost. As an ion exchange resin, 110-H has outstanding features in rare earth separation or purification including low labor intensity, less pollution and simple operation, etc [15]. Moreover,it contains functional groups (—COOH), which has a proton that can be exchanged with cations, and its oxygen atoms can be chelated with some metal ions [16]. Its principal characteristics include great chemical and physical stabilities, high exchange capacity, and good ability for regeneration [17].
Considering above, this work is aiming at the adsorption performance and mechanism of the europium on 110-H in HAc-NaAc system. The highlight of this work is the design for optimal experiment condition, excellent adsorption capacity for Eu(III) [18] and outstanding capacity for regeneration and reuse of 110-H compared with previous researches. Therefore, it is expected to become an effective way of europium enrichment, applicable to the theoretical basis of hydrometallurgy and analytical chemistry.
2 Experimental
2.1 Apparatus
Shimadzu UV-2550 UV–visible spectrophotometer, AL204 electronic balance, Mettler Toledo delta 320 pH meter, DSHZ-300A temperature constant shaking machine, Nicolet 380 FT-IR spectrophotometer and HITACHI S-3000N scanning electron microscope (SEM) were used in the characterization.
2.2 Materials
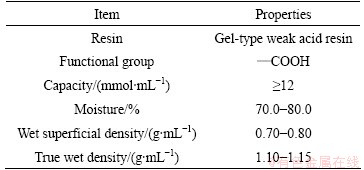
The 110-H resin was provided by Nankai University of China. And the properties are shown in Table 1. Standard solution (1.0 mg/mL) of europium was prepared from Eu2O3. HAc-NaAc buffer solution with pH=5.5-6.5 and HNO3-(HOCH2CH2)3N buffer solutions with pH=7.2 were prepared from the NaAc, HAc, HNO3, (HOCH2CH2)3N solution, respectively. All other chemicals were of analytical grade and purified water was used.
Table 1 General description and properties of 110-H resin
2.3 Experimental design for RSM study
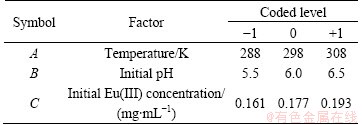
RSM is a common way to optimize experiment conditions. So, it was chosen to optimize the adsorption conditions of 110-H resin for Eu(III) from aqueous solutions in this study. Factor combinations were acquired by the application of a Box–Behnken design (BBD) using software Design-Expert 8.0.4. In the research, three process parameters, namely temperature, initial pH and initial Eu(III) concentration, were selected as the independent variables on the adsorption. The levels and independent variables are shown in Table 2.
Table 2 Experimental design of adsorption of 110-H resin for Eu(III)
2.4 Static adsorption and desorption tests
The static Eu(III) adsorption tests on 110-H were performed. A accurately weighed amount of treated 110-H was added into a conical flask, which was filled with a desired volume of HAc-NaAc solution at pH 6. One day later, a required amount of standard solution of Eu(III) was put in the flask and then shaken in a shaker at a constant temperature. The upper layer of clear solution was taken for analysis until adsorption equilibrium was reached.
Besides, desorption programs were performed. After static adsorption, the Eu(III)-loaded resin was separated from aqueous phase, washed by pH 6 buffer solution, and then shaken with HCl eluants of different concentrations. The concentrations of Eu(III) ion in the desorption solution were analyzed by UV–visible spectrophotometer.
2.5 Dynamic adsorption and desorption tests
The dynamic adsorption curve was applied to experimental data to predict the breakthrough curve and to determine the characteristic parameters of the column useful for process design.
Dynamic adsorption and desorption experiments were carried out on glass columns (d3 mm × 30 cm) wet-packed with 0.15 g (dry mass) of the selected resin. Sample solution flowed through the glass column and the Eu(III) solutions at the outlet of the column were collected at regular time intervals and the concentration of Eu(III) was measured using a UV–visible spectrophotometer at 573 nm [19]. While reaching adsorptive equilibrium, the adsorbate laden columns were washed firstly by pH 6.0 buffer solution, then eluted by 0.1 mol/L HCl solution with a flow rate of 0.167 mL/min. The concentrations of Eu(III) ion in the desorption solution were determined by UV–visible spectrophotometer.
2.6 Analytical method
A 25 mL colorimetric tube was filled with an adequate amount of adsorbed europium ion solution, 10 mL of HNO3-(HOCH2CH2)3N buffer, and 1 mL of 0.1% azo arsine-I chromogenic agent. Then, the mixture was diluted by adding purified water to the tube mark. After shaking the tube, the absorbency was determined in a 1 cm colorimetric vessel at wavelength of 573 nm and compared with the blank test. The concentration of diluent of Eu(III) was determined by UV–visible spectrophotometer. The adsorption ratio (E), adsorption amount (Q) and distribution coefficient (D) were calculated by
 (1)
(1)
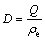 (2)
(2)
 (3)
(3)
where ρo is the initial concentration of Eu(III) in solution, ρe is equilibrium concentration of Eu(III) in solution, V is total volume of solution, m is mass of 110-H dry resin [20,21].
3 Results and discussion
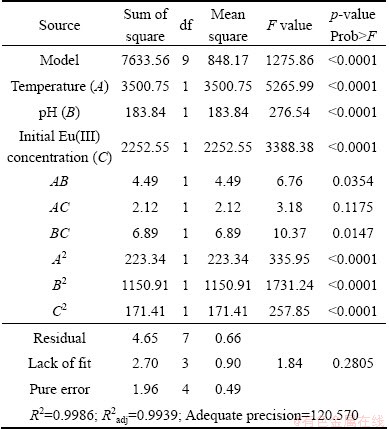
3.1 Analysis of variance (ANOVA) and development of regression model equation
Totally, a series of 17 runs were taken to analyze the experimental error for optimizing the three individual variables at BBD. The ANOVA (Table 3) was used to investigate the significance of the process parameters on the adsorption, which demonstrated whether the model developed was meaningful or not. As shown in Table 3, The Model F-value of 1275.86 implies that the model is useful. The possibility that a “Model F-value” largely occurred due to noise is only 0.01%. The determination coefficient (R2=0.9986) and adjusted coefficient (R2adj=0.9939) firstly demonstrate the adequacy of the model under these experimental conditions. The signal to noise ratio, which is desirable if greater than 4, was measured with adequate precision to be 120.570. Moreover, the “Lack of Fit F-value of 1.84 implies that the model is not significant relative to the pure error, which is desirable. Values of “Prob>F” less than 0.05 indicate that the model terms are significant. Values greater than 0.1 indicate that the model terms are not significant. Therefore, in this case, A, B, C, AB, BC, A2, B2, C2 are significant model terms.
Table 3 ANOVA results of response surface quadratic model according to adsorption capacity of 110-H for Eu (III)
From above, the model has an adequate precision. To analyze the conversion rate, the mathematical model developed by the software Design-Expert 8.0.4 is shown as follows in terms of coded factors:
y=323.46+20.92A+4.79B+16.78C-1.06AB-0.73AC-1.31BC-7.28A2-16.53B2-6.38C2
3.2 Optimization of adsorption conditions
RSM was used to investigate the effects of the three variables, namely temperature, initial PH and initial Eu(III) concentration on the adsorption capacity, and the results are shown in Fig. 1. These three plots show effects of two variables on the conversion rate while the other variable is kept at the level of zero. The 3D response surface plots clearly show the interaction between each variable, and then obtain the optimal values of the variables. From the RSM optimization, the optimal adsorption conditions of 110-H resin for absorbing Eu(III) from aqueous solutions are temperature of 308 K, initial pH of 6.04, and initial Eu(III) concentration of 0.19 mg/mL, and the predicted adsorption capacity (Q) reaches 346.85 mg/g.
In order to demonstrate the above mathematical model, three batch experiments of the adsorption for Eu(III) on 110-H resin were implemented under the predicted optimum conditions. The mean value of adsorption capacity in real experiments was 344.71 mg/g, which was in close agreement with the predicted adsorption capacity, which indicated that the developed model was adequate for predicting the adsorption conditions of 110-H resin for Eu(III) from aqueous solutions.
3.3 Adsorption kinetics
Experiments of adsorption kinetics were taken under the temperatures of 288, 298, 308 K, respectively, measuring europium ion concentration at intervals until adsorption equilibrium. The results are shown in Fig. 2, which reveal that the rate of adsorption is fast at the beginning, but the rate gradually decreases along with adsorbing until reaching adsorption equilibrium.
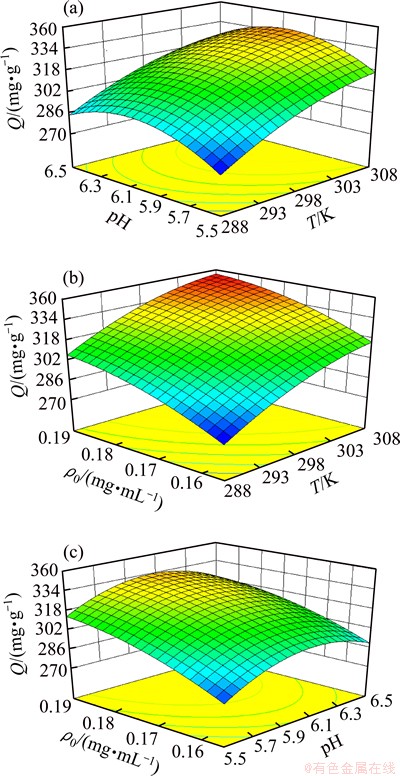
Fig. 1 Three-dimensional plots showing effects of pH and temperature (a), temperature and initial Eu(III) concentration (b), and pH with initial Eu(III) concentration interactions (c) on adsorption capacity of 110-H resin for Eu(III)

Fig. 2 Adsorption kinetics and capacity Q at different time and different temperatures
The Lagergren-first-order model can be described by
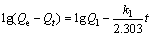 (4)
(4)
At the same time, the pseudo-second-order model can be explained by
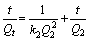 (5)
(5)
where Qe and Qt are the adsorption amounts at equilibrium time and at certain time, respectively, k1 and k2 are the adsorption rate constants of the first-order kinetics and second-order, respectively, Q1 and Q2 are saturated adsorption amounts in theory which were respectively calculated by the first-order kinetic equation and secondary dynamics equation. The effectivenesses of these models were based on the linear relations, which were drawn by lg(Qe-Qt) vs t and t/Qt vs t, fitting first order kinetics equation and secondary dynamics equation, respectively [22].
According to correlation coefficient R2 in Tables 4 and 5, the first order kinetics model is more effective than the secondary dynamic model; and from the formulas, the saturated adsorption amount in theory Q1 is close to the actual adsorption amount. Therefore, the adsorption is suitable for the Lagergren-first-order model.
Table 4 Linear relations of first-order kinetics for Eu(III) on 110-H resin under various temperatures
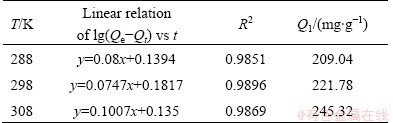
Table 5 Linear relations of second-order kinetics for Eu(III) on 110-H resin under various temperatures

3.4 Adsorption isotherm curve
Adsorption isotherm is a curve which refers to the relationship between equilibrium adsorption amount and metal ion concentration at a constant temperature. It reflects the surface properties of the resin, pore distribution and the interrelation between resin and metal ions. It is used to describe the adsorption behavior, evaluate the preferential adsorption process, determine adsorbent dosage, and can be used as the screening basis for the best absorber. In addition, the isothermal curve is always used for the analysis and design of adsorption system, which has also played a great role in the prediction simulation. Langmuir equation, deduced or summarized in practice, is mathematical model that can explain and describe the adsorption isotherm properly.
Isothermal adsorption type of Langmuir [23] is
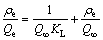 (6)
(6)
where Q∞ is the monolayer capacity of the 110-H resin while KL is the Langmuir constant that reflects quantitatively the affinity between Eu(III) and the 110-H resin. The Langmuir curve obtained by plotting ρe/Qe vs ρe(Langmuir) for the adsorption of Eu(III) onto the 110-H gives a straight line.
It is clear from Fig. 3 that the adsorption of Eu(III) onto 110-H resin fits well with the Langmuir isotherm model, as indicated by the R2 values (R2288K=0.985, R2298K=0.9944, R2308K=0.9991). In other words, there is no interaction between the adsorbed molecules located in monolayer adsorption [24].
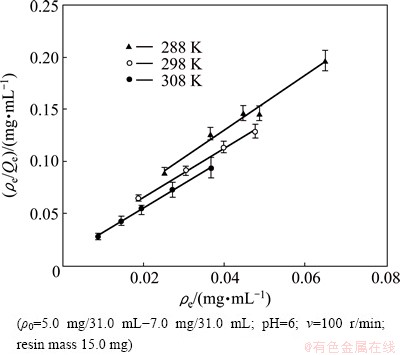
Fig. 3 Langmuir isotherm curves at different temperatures
3.5 Thermodynamic parameter
Thermodynamic parameters of adsorption were determined by using classical thermodynamic equations under the conditions of 15.0 mg resin and ρ0=6.0 mg/ 31 mL at pH 6.0 and 100 r/min. By plotting lgD vs 1/T as shown in Fig. 4 (R2=0.9986), three important thermo- dynamic parameters, i.e., enthalpy ΔH, entropy ΔS and Gibbs free energy ΔG can be calculated respectively. The slope and intercept of lg D vs 1/T curve were used for ΔH and ΔS.
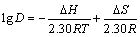 (7)
(7)
where D is the distribution coefficient of adsorption of Eu(III) onto 110-H resin (D=ρ/ρe), R is the mole gas constant, and T is the reaction temperature. The results are listed in Table 6 along with the Gibbs free energy calculated by
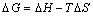 (8)
(8)
Table 6 shows that enthalpy ΔH=36.1 kJ/mol, which indicates that the adsorption process is endothermic and the rise of temperature is conducive to the adsorption. The negative ΔG shows that the adsorption process was spontaneous. All these thermodynamic data show that the decrease of free energy and the increase of entropy are the impetus for the resin to adsorb europium ions.
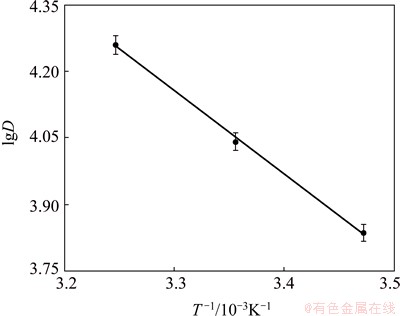
Fig. 4 Influence of temperature on distribution coefficient
Table 6 Thermodynamic parameter

3.6 Desorption and recycling
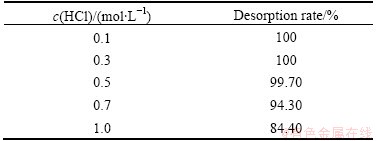
The resin containing Eu(III) was eluted with HCl solution after it was washed thrice by HAc-NaAc buffer solution (pH 6.0) at constant temperature. As shown in Table 7, desorption rates of europium are different at different concentrations of hydrochloric acid. When the concentration of hydrochloric acid solution ranges from 0.5 to 1.0 mol/L, the desorption rate is undesirable. Considering the hydrochloric acid pollution, the less hydrochloric acid dosage is better for the eluent. Namely, 0.1 mol/L HCl is the best eluent in this experiment, whose one-time desorption rate reaches 100%.
Table 7 Desorption rate of europium under different concentrations of hydrochloric acid
3.7 Dynamic adsorption curve
The test of dynamic adsorption was conducted by a glass column which was mentioned above, packed with 150 mg newly pretreated 110-H resin at the temperature of 298 K. After the column was packed completely, a 0.150 mg/mL Eu(III) standard solution was fed into the column continuously at a constant flow rate of 0.167 mL/min until ρe=ρo. The adsorption amount of europium in the glass column could be calculated through the following equation [25]:
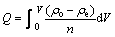 (9)
(9)
where ρo is the initial concentration of europium, namely 0.150 mg/mL; ρe is the concentration of europium after the solution flows past the glass column completely, n is the amount of absorbent. According to Fig. 5, the adsorption amount of europium is 348.75 mg/g.

Fig. 5 Breakthrough curve for adsorption of Eu(III)
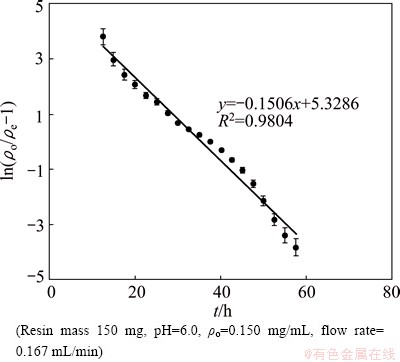
Fig. 6 Thomas model for continuous adsorption of Eu(III)
Thomas model can predict that the concentration change follows time, and the adsorption capacity of resin can thus be obtained. The model has the following form:
 (10)
(10)
Usually, we transform it into a linear model:
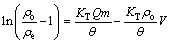 (11)
(11)
where KT is Thomas rate constant, θ is the rate of liquid flow. The value of KT is 1.67×10-2 mL/(min·mg) and Q is 355.24 mg/g, given from Fig. 6 which is made by plotting ln[(ρo/ρe)-1] vs t. On the basis of the theoretical predictions, model parameters are compared with those of the observed data (Fig. 5). Because the Thomas curve has a good correlation coefficient (R2=0.9804) and the theory adsorption is very close to experimental data, we can conclude that the experimental data fit well with the Thomas model.
3.8 Dynamic desorption curve
In order to make sure that 110-H resin can absorb and be desorbed repeatedly, efficient elution adsorbed solute is essential. In the dynamic desorption, 0.1 ml/L HCL was used as an eluent to desorb 110-H resin. The experimental results accord with the curve obtained by plotting ρe vs V. As shown in Fig. 7, the total elution volume is 40 mL, and desorption process lasts for 4.0 h. Therefore, 0.1 mol/L hydrochloric acid eluent can easily handle and remove europium ion from the resin.
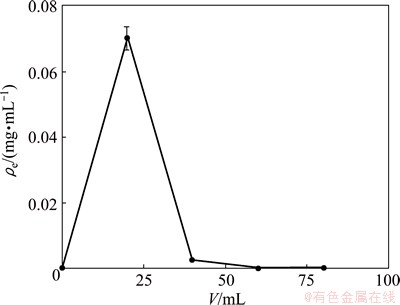
Fig. 7 Dynamic desorption curve
3.9 Influence of organic matter
Wastewater normally contains many organic matters mainly including phenolic and surface-active substances. In this work, phenol and sodium dodecyl benzene sulfonate (SDBS) [26], types of phenolic and surface- active substances were used to evaluate the effect of organic matter on the adsorption of Eu(III) by 110-H resin, respectively. The experiments were conducted at 298 K in the conical flasks containing 30.4 mL adsorption solutions (25 mL HAc-NaAc buffer solution at pH 6.0, 5 mL europium solution (1.0 mg/mL) and 0.4 mL phenol/SDBS solution (5.0 mg/mL) and 15.0 mg of dried 110-H resin until adsorption equilibrium was reached. The adsorption capacities of Eu(III) were 317.70 mg/g and 314.17 mg/g under the interference of phenol and SDBS, respectively, both of which were close to adsorption capacity without organic matter (Q= 324.91 mg/g) . Therefore, organic matters could slightly but not significantly decrease the adsorption capacity of 110-H resin for Eu(III).
3.10 IR spectra
The 110-H resin and Eu(III)-loaded 110-H resin were characterized by infrared technology, and the infrared spectra are shown in Fig. 8. It is deduced that the adsorption of Eu(III) by 110-H resin (ΔH>0) is a chemical process. Therefore, the functional groups of 110-H, C—OH, C=O and Eu(III) are supposed to form chemical bonds. It is found from Fig. 8 that the characteristic peak of the bond C—OH shifts from 3438.51 cm-1 to 3421.86 cm-1. The characteristic adsorption peak of the bond C=O(1719.01 cm-1) weakens, and a new peak at (1560.36 cm-1) occurs. All these results indicate that there are coordination bonds between oxygen atoms and Eu(III) ions.
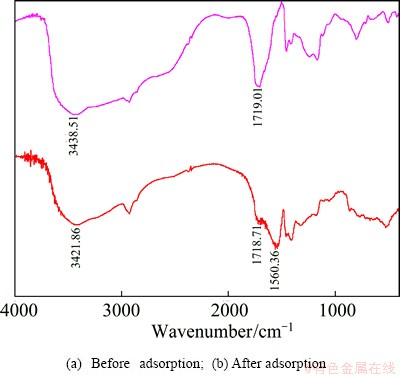
Fig. 8 IR spectra of 110-H resin
3.11 SEM analysis
The surface compositions of 110-H resin and Eu(III)-loaded 110-H resin are shown in Fig. 9, which clearly demonstrate that the 110-H resin has no pores in-house in the dry condition. Compared the surface of 110-H resin with that of Eu(III)-loaded 110-H resin, the smooth surface of 110-H resin turns thicker and coarser with granular flake material. Hence, the results reveal that Eu(III) ions are loaded onto the surface of 110-H resin.
3.12 Application in actual water
In this experiment, the removal of Eu(III) from actual water was carried out by using 110-H resin. Adsorption experiment was conducted in the conical flasks under the conditions of 15.0 mg 110-H resin, ρ0=5.0 mg/31 mL, a little buffer solution and reacted at 100 r/min and 298 K for 48 h in the shaker. The adsorption capacity of Eu(III) approached to 254.56 mg/g. Therefore, the 110-H resin can be considered as a potential candidate for Eu(III) removal from actual water in large scale operations.
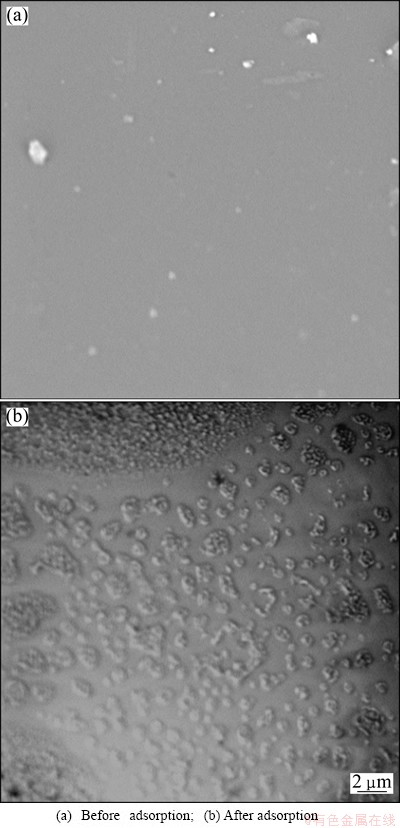
Fig. 9 SEM images for 110-H resin
4 Conclusions
1) RSM views that the optimal adsorption conditions of 110-H resin for absorbing Eu(III) from aqueous solutions are 308 K, pH=6.04 and ρ0=0.19 mg/mL.
2) Adsorption kinetics studies show that the adsorption process conforms to the Lagergren-first-order model, and isothermal studies demonstrate that it obeys the Langmuir model. Thermodynamic parameters ΔH0, ΔS0 and ΔG0 indicate that the adsorption is a spontaneous and endothermic reaction in nature. The Eu(III) adsorbed on 110-H resin can be eluted by 0.1 mol/L HCl quantitatively. Dynamic adsorption study shows that 110-H resin has a high sorption capacity of Eu(III) ion (348.75 mg/g), and can be recyclable from dynamic desorption. And organic matters do not much affect the adsorption performance of 110-H resin for Eu(III).
3) Infrared spectra show that oxygen atoms and Eu(III) ions form coordination bonds, and SEM images show that the surface of 110-H resin becomes thicker and coarser after Eu(III) is loaded.
4) The 110-H resin is successfully applied in the removal of Eu(III) in actual water.
References
[1] WEI Ping, TANG Hui-quan, CHEN Yao, CHEN Xiao-hong. Measuring technical efficiency of Chinese nonferrous metals enterprises on a background of industry consolidation [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(9): 2797-2806.
[2] KIM C J, YOON H S, CHUNG K W, LEE J Y, KIM S D, SHIN S M, LEE S J, JOE A R, LEE S I, YOO S J, KIM S H. Leaching kinetics of lanthanum in sulfuric acid from rare earth element (REE) slag [J]. Hydrometallurgy, 2014, 146: 133-137.
[3] YANG Hua-ling, CHEN Ji, ZHANG Dong-li, WANG Wei, CUI Hong-min, LIU Yu. Kinetics of cerium(IV) and fluoride extraction from sulfuric solutions using bifunctional ionic liquid extractant (Bif-ILE) [A336][P204] [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1937-1945.
[4] GENNARI F C. Mechanochemical synthesis of erbium borohydride: Polymorphism, thermal decomposition and hydrogen storage [J]. Journal of Alloys and Compounds, 2013, 581: 192-195.
[5] BINNEMANS K, JONES P T, BLANPAIN B, GERVEN T V, YANG Y X, WALTON A, MATTHIAS B. Recycling of rare earths: A critical review [J]. Journal of Cleaner Production, 2013, 51: 1-22.
[6] LIN Mei-juan, WANG Xiao-ping, TANG Qiang, LING Qi-dan. Luminescence properties of polymers containing europium complexes with 4-tert-butylbenzoic acid [J]. Journal of Rare Earths, 2013, 31(10): 950-956.
[7] LI Yin-yan, TIAN Ying, HUA You-jie, XU Shi-qing. Europium(III)- doped ionogels with improved luminescent properties [J]. Journal of Non-Crystalline Solids, 2013, 376: 38-42.
[8] LI Han-yu, YANG Tian-lin, DING Ling, WANG Wen-hua. Synthesis, characterization, fluorescence and DNA-binding studies of europium (III) pirates complexes with amide-based 2,3-dihydroxynaphthalene derivatives [J]. Journal of Rare Earths, 2012, 30(4): 297-303.
[9] KIBOMBO H S, WEBER A S, WU C M, RAGHUPATHI K R, KOODALI R T. Effectively dispersed europium oxide dopants in TiO2 aerogel supports for enhanced photocatalytic pollutant degradation [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2013, 269: 49-58.
[10] MATLOCK M M, HOWERTON B S, ATWOOD D A. Chemical precipitation of heavy metals from acid mine drainage [J]. Water Research, 2002, 36: 4757-4764.
[11] KHAN M A, BUSHRA R, AHMAD A, NABI S A, KHAN D A, AKHTAR A. Ion exchangers as adsorbents for removing metals from aquatic media [J]. Archives of Environmental Contamination and Toxicology, 2014, 66(2): 259-269.
[12] KONDO K, KAMIO E. Separation of rare earth metals with a polymeric microcapsule membrane [J]. Desalination, 2002, 144: 249-254.
[13] SHARMA I G, ALEX P, BIDAYE A C, SURI A K. Electrowinning of cobalt from sulphate solutions [J]. Hydrometallurgy, 2005, 80: 132-138.
[14] MINOWA H, EBIHARA M. Separation of rare earth elements from scandium by extraction chromatography: Application to radiochemical neutron activation analysis for trace rare earth elements in geological samples [J]. Analytica Chimica Acta, 2003, 498: 25-37.
[15] LU Xiao-ying, HUO Guang-sheng, LIAO Chun-hua. Separation of macro amounts of tungsten and molybdenum by ion exchange with D309 resin [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 3008-3013.
[16] XIONG Chun-hua, YAO Cai-ping, WANG Li, KE Jia-jun. Adsorption behavior of Cd(II) from aqueous solutions onto gel-type weak acid resin [J]. Hydrometallurgy, 2009, 98(3-4): 318-324.
[17] JIA Qian, XIONG Chun-hua, CHEN Xin-yi, ZHOU Su-guo, YAO Cai-ping, MA Chu-nan. Optimization of conditions for Cu(II) adsorption on 110 resin from aqueous solutions using response surface methodology and its mechanism study [J]. Desalination and Water Treatment, 2013, 51(22-24): 4613-4621.
[18] XIONG Chun-hua, ZHENG Zhan-wang. Evaluation of D113 cation exchange resin for the removal of Eu(III) from aqueous solution [J]. Journal of Rare Earths, 2010, 28(6): 862-867.
[19] XIONG Chun-hua, YAO Cai-ping. Study on the adsorption of cadmium(II) from aqueous solution by D152 resin [J]. Journal of Hazardous Materials, 2009, 166(2-3): 815-820.
[20] XIONG Chun-hua, CHEN Xin-yi, YAO Cai-ping. Enhanced adsorption behavior of Nd(III) onto D113-III resin from aqueous solution [J]. Journal of Rare Earths, 2011, 29(10): 979-985.
[21] XIONG Chun-hua, ZHENG Yu-qiang, FENG Yu-jie, YAO Cai-ping, MA Chun-nan, ZHENG Xu-ming, JIANG Jian-xiong. Preparation of a novel chloromethylated polystyrene-2-amino-1,3,4-thiadiazole chelating resin and its adsorption properties and mechanism for separation and recovery of Pt(IV) from aqueous solutions [J]. Journal of Materials Chemistry A, 2014, 2(15): 5379-5386.
[22] XIONG Chun-hua, WU Xiang-mei. Adsorption of copper using macroporous phosphonic acid resin [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(6): 1446-1450.
[23] XIONG Chun-hua, LI Yan-li, WANG Guo-tao, FANG Lei, ZHOU Su-guo, YAO Cai-ping, CHEN Qing, ZHENG Xu-ming, QI Dong-ming, FU Ya-qin, ZHU Yao-feng. Selective removal of Hg(II) with polyacrylonitrile-2-amino-1,3,4-thiadiazole cleating resin: Batch and column study [J]. Chemical Engineering Journal, 2015, 259: 257-265.
[24] XIONG Chun-hua, PI Lei-lei, CHEN Xin-yi, YANG Li-qun, MA Chun-nan, ZHENG Xu-ming. Adsorption behavior of Hg2+ in aqueous solutions on a novel chelating cross-linked chitosan microsphere [J]. Carbohydrate Polymers, 2013, 98(1): 1222-1228.
[25] AKSU Z,  F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curves [J]. Process Biochemistry, 2004, 39(5): 599-613.
F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curves [J]. Process Biochemistry, 2004, 39(5): 599-613.
[26] PAPADAKIA M, EMERY R J, ABU-HASSAN M A,  A, METCALFE I S, MANTZAVINOS D. Sonocatalytic oxidation processes for the removal of contaminants containing aromatic rings from aqueous effluents [J]. Separation and Purification Technology, 2004, 34: 35-42.
A, METCALFE I S, MANTZAVINOS D. Sonocatalytic oxidation processes for the removal of contaminants containing aromatic rings from aqueous effluents [J]. Separation and Purification Technology, 2004, 34: 35-42.
响应面法优化凝胶型弱酸性树脂对铕(III)的吸附性能
陈 婷1,李 斌1,方 磊2,陈德睢1,许文斌1,熊春华1
1. 浙江工商大学 化学应用系,杭州 310018;
2. Department of Food Science and Human Nutrition, University of Florida, Bldg 475 Newell Drive, Gainesville, FL 32611, USA
摘 要:通过响应面法,对增强凝胶型弱酸性树脂(110-H)在水溶液中对稀土元素铕的吸附作用进行优化。实验表明在最佳条件下110-H对铕的吸附量(346.85 mg/g)令人满意,是目前对铕离子吸附量最高的材料之一。吸附过程拟合一级反应动力学和朗缪尔等温线模型,吸附热力学参数分别为ΔH=36.1 kJ/mol,ΔS=200 J/(mol·K)。解吸实验表明110-H可以用浓度为0.1 mol/L的HCl溶液洗脱以实现再生。用红外光谱和扫描电镜技术对比树脂吸附金属离子前、后特征以分析其吸附机理。
关键词:铕;响应面法;吸附;凝胶型弱酸性树脂
(Edited by Xiang-qun LI)
Foundation item: Project (20133326110006) supported by PhD Programs Foundation of Ministry of Education of China; Project (2014R408073) supported by College Student Innovation Projects of Zhejiang Province, China; Project supported by Zhejiang Provincial Key Laboratory of Industrial Textile Materials & Manufacturing Tech, China
Corresponding author: Chun-hua XIONG; Tel: +86-15168262591; E-mail: xiongch@163.com
DOI: 10.1016/S1003-6326(15)64071-7
Abstract: The conditions relating to enhanced adsorption procedure of earth element europium (Eu) onto gel-type weak acid resin (110-H) in aqueous solution were optimized by means of the response surface methodology (RSM), which proved that 110-H owned satisfactory adsorption capacity (346.85 mg/g) in optimum conditions, belonging to one of the high adsorption capacity materials. Then, the adsorption and desorption behaviors were investigated by batch studies. The adsorption performance showed high agreement with the Lagergren-first-order model and Langmuir isotherm with thermodynamic adsorption parameters of ΔH=36.1 kJ/mol and ΔS=200 J/(mol·K). Desorption study revealed that 110-H could be effectively eluted by a low concentration of HCl solution (0.1 mol/L) to regenerate and reuse. Finally, the 110-H and Eu(III) loaded 110-H were characterized by IR spectroscopy and scanning electron microscope (SEM) to analyze the mechanism of adsorption, which proved to be chemisorbed.


