文章编号:1004-0609(2008)S1-0398-05
锂离子电池边角料中直接回收合成LiCoO2的性能
刘云建,胡启阳,李新海,王志兴,郭华军,彭文杰
(中南大学 冶金科学与工程学院,长沙410083)
摘 要:
研究了一种从锂离子蓄电池正极片的边角料中直接回收钴酸锂的新工艺。先用二甲基乙酰胺(DMAC)浸泡正极片,将LiCoO2从铝箔上剥离,再在高温下除去正极中的聚偏氟乙烯(PVDF)和碳粉等杂质。然后添加不同的锂盐(Li2CO3、LiOH·H2O和 LiAc·2H2O)调节回收粉末中的Li与Co的量比为1.00,再在850 ℃下焙烧12 h得到最终产品。用扫描电子显微镜(SEM)和X射线衍射(XRD)分析技术对得到的样品进行微观形貌与晶相结构的研究。研究结果表明,添加Li2CO3合成的LiCoO2层状结构发育最为完善,其首次放电容量和循环性能也最好;在3.0~4.3 V进行充放电,首次放电容量达到160 mA?h/g,经30次循环以后,仍有150 mA?h/g。
关键词:
中图分类号:TM 912.9 文献标识码:A
Synthesis and electrochemical performances of LiCoO2 recycled from incisors bound of Li-ion batteries
LIU Yun-jian, HU Qi-yang, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, PENG Wen-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: A new LiCoO2 recovery technology from Li-ion battery was studied. LiCoO2 was peeled off the Al foil with dimethyl acetamide(DMAC), and then the polyvinylidene fluoride(PVDF) and carbon powders in the active material were eliminated by high temperature calcining. And then the Li2CO3, LiOH·H2O and LiAc·2H2O were added into the recycled powders to adjust the molar ratio of Li to Co to 1.00. The new LiCoO2 was obtained by calcining the mixture at 850 ℃ for 12 h in the air. Structure and morphology of the recycled powders and resulted sample were observed by XRD and SEM technique, respectively. The layered structure of the LiCoO2 synthesized by adding Li2CO3 is best with the characteristics as cathode material in terms of charge–discharge capacity and cycling performance. The first discharge capacity is 160 mA?h/g from 3.0 V to 4.3 V. The discharge capacity after cycled 30 times is still 150 mA?h/g.
Key words: LiCoO2; recycle; synthesis; discharge performance; cycling performance
LiCoO2因为其良好的电化学性能,目前是锂离子电池中使用最广泛的正极材料。随着各种移动电子设备的需求越来越多,锂离子电池的需求量也在快速增长。据报道,截止2006年,锂离子电池的销售额已经突破100亿美元,这就需要大量的金属Co来满足锂离子电池市场的发展,而金属Co比较稀缺,并且价格昂贵[1]。所以,目前一方面人们正在积极开发低钴或是无钴的正极材料,如LiMn2O4[2-4]、LiFePO4[5-7]和 Li[NiCoMn]O2[8-9],另外一方面,许多研究工作者正在研究回收锂离子电池[10-14]。
JINSIK等[10]研究用硝酸浸泡溶解废旧锂离子电池中的LiCoO2,并以钛为电极,最终电解沉积得到Co3O4。ZHANG等[11]利用溶解萃取法从废旧锂离子电池中回收Co和Li。LAIN[12]采用萃取电解液、溶解正极和钴还原等方法,回收废旧电池中的每种元素。LEE和RHEE [13]、CONTESTAILE等[14]不仅回收废旧电池中的有价金属,并且利用它们来重新制备LiCoO2。上述研究都是针对废旧锂离子电池,且回收流程相对较长。随着锂离子电池需求量的增加,在锂离子电池制造过程中产生了大量未经充放电的边角废料。
为此,本文作者针对在锂离子电池制造过程中出现的边角料,首次报道处理该部分LiCoO2的方法。首先将正极材料中的PVDF,碳黑等杂质在高温下被除去,再在回收的粉末中添加Li2CO3、LiOH·H2O 和 LiAc·2H2O以调节粉末中的Li与Co的量比为1.00,并且对回收合成的LiCoO2的结构、形貌以及电化学性能进行研究。
1 实验先称取一定量的铝钴膜放入烧杯中,然后加入一定量的 N,N-二甲基乙酰胺(DMAC),浸泡3 h之后,取出铝片,将活性物质和溶剂静置2 h之后过滤。过滤后的样品先在120 ℃下烘烤12 h,取出研磨,然后将样品在450 ℃下加热2 h,再在600 ℃下加热5 h。最后将热处理样品用热水洗涤数遍。利用滴定法和原子吸收光谱测试粉末中的Co和Li的含量,利用碳硫分析和比色法分析C和F的含量。
加入一定量的Li2CO3、LiOH·H2O和 LiAc·2H2O调节粉末样品的Li与Co的量比为1.00。然后在850 ℃下焙烧12 h后随炉冷却至室温,得到LiCoO2样品粉末。
利用X射线衍射仪(Rigaku公司,日本)分别对回收粉末样品和合成的LiCoO2样品粉末进行物相分析。测试条件:以Cu Kα靶作为辐射源,电压为40 kV,电流为50 mA,步宽为0.02?,扫描速度为2(?)/min,扫描范围为10?~90?。用JSM-5600型扫描电子显微镜对再生LiCoO2的表面形貌进行表征。
通过2025型扣式电池测试材料的电化学性能。LiCoO2、乙炔黑(电池级,广东省化工进出口公司)和PVDF(聚偏二氟乙烯,电池级,法国阿托化学)按质量比8?1?1混合并研磨均匀后,加入适量有机溶剂N-甲基吡咯烷酮(NMP,纯度为99.9%,南京京龙化工厂生产) 研磨成均匀糊状物后涂于铝箔上,在120 ℃真空干燥12 h。在充满干燥氩气的手套箱中,以金属锂片作为负极,以Celgard2400微孔聚丙烯膜(Celgard Inc.USA)为隔膜,以1 mol/L LiPF6的EC(碳酸乙烯酯)/DMC(1,2-二甲基碳酸酯)/EMC(碳酸甲乙酯)(体积比为1?1?1)的溶液为电解液组装2025型扣式电池。在BTS-51型二次电池性能检测仪(深圳新威尔多电子设备有限公司生产)上测试电池的充放电性能。采用恒流恒压法对电池进行充、放电,充、放电电压为3.0~4.3 V,充电电流为0.2 C,温度维持在25 ℃左右。
2 实验结果 2.1 回收样品分析回收样品粉末的SEM图如图1所示。在图1中,有很多直径约为1 μm的小颗粒,这可能是在回收过程中烧结和洗涤造成的。
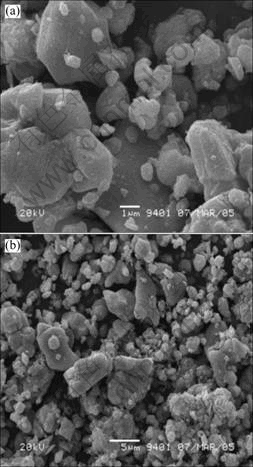
图1 回收样品的SEM图
Fig.1 SEM image of recycled powders
回收LiCoO2粉末样品的XRD谱如图2所示。在图2中,可以看到在衍射角为32.220?和36.821?处出现杂质Co3O4的衍射峰。将回收粉末用热水洗涤并过滤,再将CaCl2溶液加入到粉末的洗涤液中,溶液中出现白色沉淀。 这白色沉淀应该是CaF2。所以,可以推断,在加热过程中,PVDF分解放出HF,HF又和LiCoO2反应生成LiF。
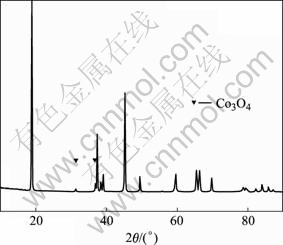
图2 回收样品的XRD谱
Fig. 2 XRD pattern of recycled powders
对回收样品中的元素含量进行分析,结果列于表1中。从表1可以换算出Li与Co的量比为0.914,这与正常的LiCoO2中Li与Co的量比有较大的差异。
表 1 回收样品中的各元素含量
Table 1 Content of elements in recycled powders (mass fraction, %)
![]()
在不同烧结温度下得到样品的SEM图如图3所示。与图1相比,经过烧结,回收样品中的小颗粒的粒径有所增大。这是因为熔融状态的锂盐促进了小颗粒的熔融长大。此外,从图3中还可以看出,添加LiAc·2H2O和LiOH·H2O合成的LiCoO2的粒径比添加Li2CO3合成的LiCoO2的粒径大。这可能是因为LiAc·2H2O和LiOH·H2O的熔点比Li2CO3的熔点低。
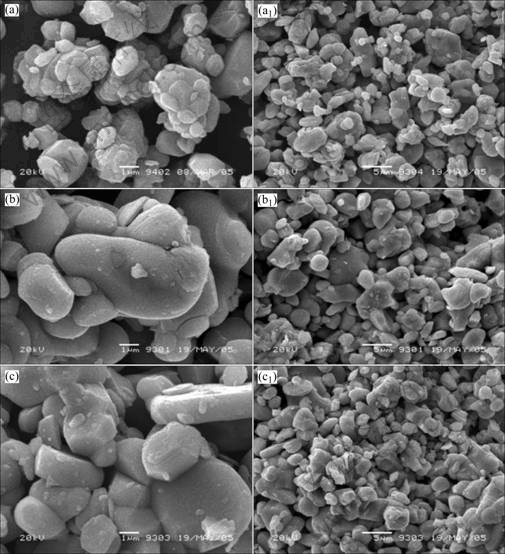
图 3 不同锂盐合成LiCoO2的形貌
Fig. 3 SEM images of LiCoO2 synthesized by adding different lithium salts: (a), (a1) Li2CO3; (b), (b1) LiAc·2H2O; (c), (c1) LiOH·H2O
添加不同锂盐合成LiCoO2的X射线衍射谱如图4所示。从图4中可以看出,没有出现Co3O4的衍射峰,并且其它杂质的衍射峰也没有出现。层状结构的LiCoO2的(003)晶面的衍射峰强度大,(006)/(012)和(018)/(110)晶面的衍射峰分裂明显。这表明,在高温下Co3O4和锂盐发生反应合成的LiCoO2呈现出明显的α-NaFeO2型层状结构。
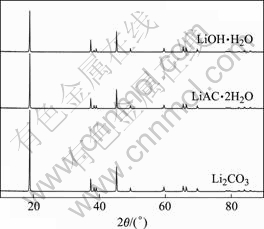
图 4 添加不同锂盐合成的LiCoO2的XRD谱
Fig. 4 XRD patterns of LiCoO2 synthesized by adding different lithium salts
添加不同锂盐合成的LiCoO2的I(003)/I(104)的值列于表2中。由表2可知,添加Li2CO3合成的LiCoO2的I(003)/I(104)最大,达到2.711。而添加LiAc·2H2O和LiOH·H2O合成LiCoO2的I(003)/I(104)分别为2.270和1.626。I(003)/I(104)越大,表明LiCoO2的层状结构发育越完善[15]。因此,利用LiAc·2H2O和LiOH·H2O合成LiCoO2的层状发育不理想,这可能是因为LiAc·2H2O和LiOH·H2O易于吸水,在称量过程中不易称量准确,造成合成的LiCoO2的Li与Co的量比与正常LiCoO2的粗比有差别,从而影响其层状结构的发育。
表 2 不同锂盐合成的LiCoO2的I(003)/I(104)值
Table 2 I(003)/I(104) of LiCoO2 synthesized by adding different lithium salts
![]()
将不同锂盐合成的LiCoO2做成扣式电池,对其进行电化学性能检测。充电方式为恒流恒压,充电电流约为0.2C,充电终止电压为4.3 V,放电终止电压为3.0 V。3个样品的首次放电曲线如图5所示。
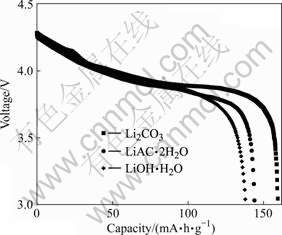
图 5 不同锂盐合成的LiCoO2电池材料的首次放电曲线
Fig. 5 Discharge curves of LiCoO2 synthesized by adding different lithium salts
3种样品的首次放电容量分别为160,144和138 mA?h/g。其中,利用Li2CO3合成的LiCoO2的首次放电容量最高,这是因为其I(003)/I(104)最大,层状结构发育最完善,Li+脱/嵌最容易。此外,从放电曲线中还可以看出,其首次放电的主平台为3.9~4.0 V,这和正常高温合成的LiCoO2的结构相一致。
用不同锂盐合成的LiCoO2的循环性能如图6所示。3个样品经30次充、放电后容量分别为150、133和126 mA?h/g,容量损失率分别为6.3%、7.6%和8.7%,表现出较好的循环性能,其中利用Li2CO3合成的LiCoO2的循环性能最佳。综合首次放电曲线和循环性能曲线可知,添加LiCoO2合成的LiCoO2的电化学性能最优,其I(003)/I(104)最大,层状结构发育最完善。
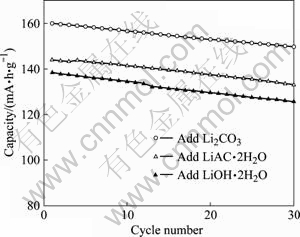
图 6 不同锂盐合成的LiCoO2的循环性能
Fig. 6 Cycling performance of LiCoO2 synthesized by adding different lithium salts
3 结论1) 利用二甲基乙酰胺浸泡锂离子电池正极边角料,将正极活性物质从铝箔上剥离,然后在高温下烧结除去PVDF和碳粉等杂质,得到的粉末中含有少量的Co3O4。
2) 在回收的粉末中添加一定量的Li2CO3、LiAc·2H2O和LiOH·H2O,然后在850 ℃下焙烧12 h,得到新的LiCoO2。其中添加Li2CO3合成的LiCoO2层状结构发育最为完善。
3) 电化学性能测试表明,利用Li2CO3合成的LiCoO2电化学性能最为优异,首次放电容量为160 mA?h/g,经30次循环以后,容量损失率为6.3%。
REFERENCES
[1] KIM J, BYOUG S K, LEE J G, CHO J, BYUNG PP W. Differential voltage analyses of high-power, lithium-ion cells(I): Technique and application[J]. J Power Sources, 2005, 139(1/2): 289-294.
[2] LI J G, HE X M, ZHAO R S. Electrochemical performance of SrF2-coated LiMn2O4 cathode material for Li-ion batteries[J]. Trans Nonferrous Met Soc China,2007, 17(6): 1324-1327.
[3] LI Yang, MICHIO T, WANG Bao-feng. A study on capacity fading of lithium-ion battery with manganese spinel positive electrode during cycling[J]. Electrochemical Acta, 2006, 51(9): 3228-3234.
[4] DENG B H, NAKAMURA H, YOSHIO M. Comparison and improvement of the high rate performance of different types of LiMn2O4 spinels[J]. J Power Sources, 2005, 141(1): 116-121.
[5] VERONICA P, AINTZANE G, IZASKUN G M, IRATXE M, MIGUEL B, OSCAR M, TEOFILO R. New freeze-drying method for LiFePO4 synthesis[J]. J Power Sources, 2007, 171(2): 879-885.
[6] WANG Yang-qiang, WANG Jiu-lin, YANG Jun, NULI A Y. High-rate LiFePO4 electrode material synthesis by a novel route from FePO4?4H2O[J]. Advanced Functional Materials, 2006, 16(2): 2135-2140.
[7] XIA Y G, MASAKI Y, HIDEYUKI N. Improved electrochemical performance of LiFePO4 by increasing its specific surface area[J]. Electrochimica Acta, 2006, 52(1): 240-245.
[8] ZHANG Shi-chao, QIU Xin-ping, HE Zhi-qi, WENG Dang-sheng, ZHU Wen-tao. Nanoparticle Li(Ni1/3Co1/3Mn1/3)O2 as cathode material for high-rate lithium-ion batteries[J]. J Power Sources, 2006, 153(1): 350-353.
[9] CHO T H, SHIOSAKI Y, NOGUCHI H. Preparation and characterization of layered LiMn1/3Ni1/3Co1/3O2 as a cathode material by an oxalate co-precipitation method[J]. J Power Sources, 2006, 159(4): 1322-1327.
[10] JINSIK M, JUNG Y W, LEE J Y, TAK Y S. Cobalt oxide preparation from waste LiCoO2 by electrochemical- hydrothermal method[J]. J Power Sources, 2002, 112(2): 639-642.
[11] ZHANG Ping-wei, YOKOYAMA T, ITABASHI O, SUZUKI T M, INOUE K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries[J]. Hydrometallurgy, 1998, 47(2/3): 259-271.
[12] LAIN M J. Recycling of lithium ion cells and batteries[J]. J Power Sources, 2001, 97/98(S1): 736-738.
[13] LEE C K, RHEE K I. Preparation of LiCoO2 from spent lithium-ion batteries[J]. J Power Sources, 2002, 109(1): 17-21.
[14] CESTAILE M, PANERO S, SCROSATI B. A laboratory-scale lithium-ion battery recycling process[J]. J Power Source, 2001, 92(1/2): 65-69.
[15] FANG T, DUH J, SHEEN S. LiCoO2 cathode material coated with nano-crystallized ZnO for Li-ion batters[J]. Thin Solid Films, 2004, 469/470(1): 361-365.
通讯作者:胡启阳,副教授;电话:0731-8836633;E-mail: hqyangbox@163.com


