Trans. Nonferrous Met. Soc. China 22(2012) 3126-3130
Solvent impregnated resin prepared using ionic liquid Cyphos IL 104 for Cr(VI) removal
YANG Xiu-yun1, ZHANG Jian-ping1,2, GUO Lin2,3, ZHAO He1,2, ZHANG Yang2,3, CHEN Ji2
1. School of Chemistry and Environmental Engineering, Changchun University of Science and Technology, Changchun 130022, China;
2. State Key Laboratory of Rare Earth Resources Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China;
3. University of Chinese Academy of Sciences, Beijing 100039, China
Received 12 December 2011; accepted 21 May 2012
Abstract:
Ionic liquid (IL) trihexyl (tetradecyl) phosphonium bis 2,4,4-trimethylpentylphosphinate (Cyphos IL 104) was impregnated on XAD-7 resin. The solvent impreganated resin (SIR) was prepared and applied in Cr(VI) removal. The morphology and the thermal stability of the resins were explored. The effects of equilibrium time and initial pH value on Cr(VI) adsorption were investigated. Adsorption isotherm, separation and desorption of the SIR, and selectivity of SIR were also explored. The results show that Cyphos IL 104 exists in the inner XAD-7 resin, and the optimum pH value range of the SIR for Cr(VI) extraction is 0 to 2. When NaOH used as desorption solution, the Cr(VI) can be effectively desorbed from the SIR.
Key words:
solid-liquid extraction; Cyphos IL 104; Cr(VI); resin; adsorption isotherm; separation; desorption;
1 Introduction
The heavy metal chromium especially hexavalent Cr(VI) form is recognized to be one of the most toxic elements because of its hazard for living organisms. These chromium compounds are widely applied in many industries such as pigments, metallurgy, electroplating, leather tanning, stainless steel production, textiles, and photography. In order to control and release the emission of chromium compounds into environment, some methods, i.e., liquid-liquid extraction [1], adsorption [2-4] and membrane separation [5] have been used for Cr(VI) removal. Aliquat 336 [6] and tertiary amines [5,7] are the traditional extractants for the removal of Cr(VI).
In recent years, ionic liquids (ILs) have gained a considerable attention and have been widely used in extraction, chemical catalysis and electrochemistry, due to their high-thermal stability, non-inflammability and non-volatility [8-10]. Environment-friendly advantages of ILs give a choice to replace the volatile organic compounds (VOCs) in solvent extraction. Imidazolium ILs are frequently used as diluent in liquid-liquid extraction systems [11,12]. DIETZ et al [13] reported that the strontium-crown ether complex (Sr·CE2+) was exchanged for [Cnmim]+ with the cation exchange mechanism. A series of imidazolium ILs were used as extractants to separate Hg2+ and Cd2+ [14]. Ion exchange process in extraction may lose the IL components into the aqueous phase, then the cost of extractants would be increased. Phosphonium ILs have been used as effective extractant for the removal of metal ions and organic acids [15-17]. Trihexyl (tetradecyl) phosphonium bis 2,4,4-trimethylpentylphosphinate (Cyphos IL 104) has been used in lactic acid separation [18] and metal ions separation [19,20]. Compared with liquid-liquid extraction, solid-liquid extraction provides an effective method for ILs application in separation. When ILs are immersed into the macroporous resins, the lattice of resins can afford more chelating sites [21]. Solid-liquid extraction could reduce the consumption of ILs and decrease the cost. In this work, Cyphos IL 104 was chosen as the extractant for removal of Cr(VI), and it was impregnated on intermediate polarized XAD-7 resin.
Then the solvent impregnated resin (SIR) was studied for Cr(VI) separation.
2 Experimental
2.1 Materials and instruments
Amberlite XAD-7 was purchased from Aldrich whose surface area was 450 m2/g and pore volume was 1.14 mL/g. Cyphos IL 104 (>95.0%) was kindly supplied by Cytec Canada Inc (Fig. 1). The stock solution of Cr(VI) was prepared by dissolving K2Cr2O7 (primary standard grade, Tianjin Benchmark Chemical Reagent Co., Ltd., China) in deionized water. All other chemicals were of analytical grade (Beijing Beihua Fine Chemicals Co., Ltd., China).

Fig. 1 Chemical structure of Cyphos IL 104
The morphology of the samples was observed using field-emission scanning electron microscope (FESEM, XL30, Philips). Thermogravimetric analysis (TGA) was determined by a thermal analysis instrument (SDT 2960, TA Instruments, New Castle, DE, USA) from room temperature to 800 °C at a heating rate of 10 °C/min under N2 atmosphere. The pH values of the aqueous phase were measured by a model PHS-3C pH meter (Leici, Shanghai, China). The concentration of Cr(VI) was measured at 540 nm based on the reaction of diphenylcarbazide and Cr(VI) [3] using a UV–vis spectrophotometer. The FTIR measurements were performed with a Bruker Vertex 70 FTIR spectrometer (Bruker,  , Switzerland). The mixture metal ions (i.e. Fe(III), Co(II), Cu(II), Zn(II) and Cr(VI)) concentrations were determined using inductively coupled plasma optical emission spectrometers (ICP-OES, Thermo iCAP 6000).
, Switzerland). The mixture metal ions (i.e. Fe(III), Co(II), Cu(II), Zn(II) and Cr(VI)) concentrations were determined using inductively coupled plasma optical emission spectrometers (ICP-OES, Thermo iCAP 6000).
2.2 Experimental methods
XAD-7 resins were washed with ethanol three times to remove impurities. Then filtering and drying overnight at 50 °C to remove excess ethanol in the resins. 1 g of XAD-7 resin was immersed into 0.25 g/mL ethanol containing Cyphos IL 104 for 12 h with slow stirring. The resins were separated through a porous filter using a vacuum pump, washed with deionized water and dried at 50 °C.
0.04 g of solvent impregnated resin and 10 mL Cr(VI) solution were added in 50 mL erlenmeyer flask. The flask was shaken for 60 min with the help of a desktop constant temperature oscillator (TH2-318, Equipment Co., Ltd. Shanghai Jing Hong) at 25 °C. Solution sample was then centrifuged at 2000 r/min for 5 min. The amount of Cr(VI) adsorbed at equilibrium, q (mg/g) was calculated according to the following equation:
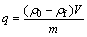 (1)
(1)
where ρ0 and ρf are the initial and final concentrations (mg/L) of Cr(VI) in aqueous phase, respectively; V is the volume of metal solution (L) and m is the mass of sorbents (g). The desorption of Cr(VI) was explored by different concentrations of NaOH solutions. The desorption ratio (S, %) of Cr(VI) from the sorbent was calculated by
 (2)
(2)
where m(Crd) is the amount of desorbed Cr(VI) in NaOH, and m(Cra) is the amount of adsorbed Cr(VI) by SIR.
3 Results and discussion
3.1 Morphology of resins
The morphology of Cyphos IL 104 impregnated XAD-7 resin is shown in Fig. 2. When Cyphos IL 104 was added in XAD-7 resin, the surface of resin was smooth and ILs were not observed on the surface of resin. The phenomenon indicated that Cyphos IL 104 did not coat on the surface of resin, while existed in the inner XAD-7 resin. This avoided the loss of extractant from the surface of resin.

Fig. 2 SEM image of surface of Cyphos IL 104 impregnated XAD-7 resin
3.2 Thermal stability of resins
As shown in Fig. 3, the thermal stability of XAD-7 resin and Cyphos IL 104 impregnated XAD-7 resin was investigated by thermogravimetric analysis (TGA). In Fig. 3 curve (a) showed the degradation process of XAD-7 resin and curve (b) showed the degradation process of Cyphos IL 104 impregnated XAD-7 resin. The degradation temperature of XAD-7 resin was 306 °C. When Cyphos IL 104 was added in XAD-7 resin, the degradation temperature decreased to 279 °C. Therefore, the thermal stability of resins was decreased with adding Cyphos IL 104.

Fig. 3 TG curves of XAD-7 resin and SIR
3.3 Effect of equilibrium time on extraction efficiency
To get the enough shaking time for the extraction equilibrium, the amount of Cr(VI) adsorbed (q) versus extraction time is shown in Fig. 4. With the increase of time from 10 min to 90 min, q increased. Then the plot reached a plateau and the amount of Cr(VI) adsorption came to the maximum. Therefore, extraction equilibrium time of this experiment was determined to be 90 min.

Fig. 4 Cr(VI) uptake kinetics by SIR (100 mg/L Cr(VI) in 1.0 mol/L HCl, 0.04 g SIR, 10 mL Cr(VI) solution )
3.4 Effect of pH on adsorption of Cr(VI)
The influence of initial pH value from 0 to 12 on the extraction of Cr(VI) with Cyphos IL 104 SIR was studied. As shown in Fig. 5, with the increase of pH value, q decreased. The q decreased greatly with the increase of pH. And q decreased almost to 0 when pH value was larger than 7. The optimum pH value range for Cr(VI) extraction was 0 to 2, since Cyphos IL 104 was an effective extractant for the removal of H2CrO4 [22]. When the pH value changed from 0 to 2, H2CrO4 was the dominant species in the aqueous solution [23,24]. With the increase of pH value, the amount of H2CrO4 decreased.  is dominant, when the pH value was higher than 8.
is dominant, when the pH value was higher than 8.
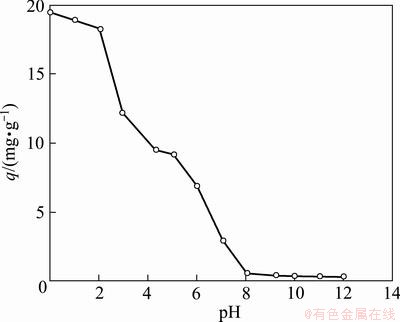
Fig. 5 Effect of initial pH value on adsorption of Cr(VI) (100 mg/L Cr(VI), 0.04 g SIR, 10 mL Cr(VI) solution)
3.5 Adsorption isotherm of Cr(VI)
The Langmuir adsorption isotherm model was applied to the adsorption data of the present work and it described monolayer coverage of adsorbate [25]. The Langmuir model was calculated by
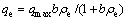 (3)
(3)
where ρe is the equilibrium concentration (mg/L), qe is the amount of Cr(VI) removed at equilibrium (mg/g); qmax (mg/g) and b (L/mg) are the Langmuir constants related to the maximum adsorption capacity and energy of sorption, respectively. The Freundlich isotherm is an equation based on the heterogeneous surfaces. The binding sites are not equivalent and/or independent. The Freundlich model is described by [26]
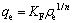 (4)
(4)
where KF and 1/n are the indicators of the adsorption capacity and adsorption intensity, respectively. The sorption parameters of Langmuir and Freundlich isotherms models were obtained by non-linear regression analysis. As shown in Fig. 6, the correlation coefficient (R2) of Freundlich model was 0.997. The value was higher than that of Langmuir model (R2=0.971), then the Freundlich model fitted better than Langmuir model. The Freundlich equation was more applicable to describe sorption data of the SIR in Cr(VI) adsorption. The Langmuir model served to estimate the maximum adsorption capacities (qmax). With the non-linear regression analysis, the qmaxb value was 2.377 and b was 0.053, then qmax of Cr(VI) was 44.85 mg/g.
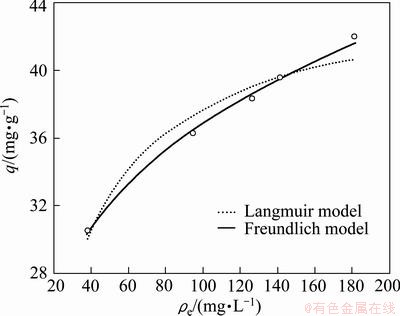
Fig. 6 Langmuir adsorption isotherm and Freundlich adsorption isotherm of SIR for Cr(VI) (0.04 g SIR, 10 mL Cr(VI) solution)
3.6 Analysis of IR spectra
The IR spectra of XAD-7 resin, SIR and SIR loaded Cr(VI) are shown in Figs. 7(a)-(c), respectively. As shown in Fig. 7(b) and Fig. 7(c), the IR peaks around 2930 cm-1 2859 cm-1 and 2957 cm-1 were the stretching vibrations of CH3 and CH2 in Cyphos IL 104. XAD-7 resin was a highly crosslinked macroreticular acrylic resin [27] and the IR peak around 1735 cm-1 was the vibration of C=O in XAD-7 resin. When Cyphos IL 104 impregnated XAD-7 resin combined with Cr(VI), the IR peak of resin functional group shifted from 983 cm-1 to 947 cm-1. It was attributed to the stretching vibration of the bond Cr—O.
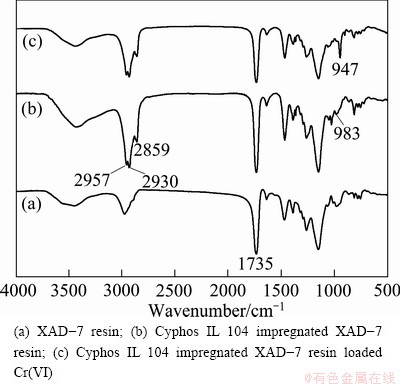
Fig. 7 IR transmittance spectra of SIR and SIR loaded Cr(VI)
3.7 Desorption property
The desorption properties of SIR are shown in Fig 8. The desorption ratio increased with increasing the concentration of NaOH. Adsorption ratio of Cr(VI) was almost 0, when pH value was lager than 8. Then in base environment, Cr(VI) and Cyphos IL 104 in resins were separated and Cr(VI) went into desorption solution. When the concentration of NaOH was 0.05 mol/L, the desorption ratio reached a plateau. The Cr(VI) could be effectively desorbed by NaOH at 0.05 mol/L.

Fig. 8 Desorption of Cr(VI) by NaOH
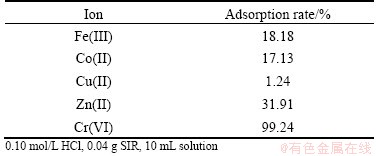
3.8 Adsorption behavior of other metal ions
To investigate the selectivity of SIR for Cr(VI) and other metal ions, the effects rate of Fe(III), Co(II), Cu(II) and Zn(II) on Cr(VI) adsorption were explored. As shown in Table 1, the adsorption rate of Cr(VI) was 99.24% with 18.18% Fe(III), 17.13% Co(II), 1.24% Cu(II) and 31.91% Zn(II) adsorbed in SIR. Therefore, the adsorption selectivity of Cr(VI) was higher than those of the other four metal ions under this experimental condition. The concentrations of five metal ions are all 0.001 mol/L.
Table 1 Effect of other metal ions on adsorption of Cr(VI)
4 Conclusions
The SIR with Cyphos IL 104 was prepared and applied in Cr(VI) adsorption. The adsorption efficiency of Cr(VI) decreases with the increase of pH value. The adsorption capacity of the SIR and Cr(VI) is fitted to Freundlich isotherm rather than Langmuir isotherm. The selectivity of SIR was investigated. Cr(VI) can be removed from other metal ions existing in the solution.
References
[1] AKAMA Y, SALI A. Extraction mechanism of Cr(VI) on the aqueous two-phase system of tetrabutylammonium bromide and (NH4)2SO4 mixture [J]. Talanta, 2002, 57: 681-686.
[2] GUPTA S, BABU B V. Removal of toxic metal Cr(VI) from aqueous solutions using sawdust as adsorbent: equilibrium, kinetics and regeneration studies [J]. Chem Eng J, 2009, 150: 352-365.
[3] LIU Ying-hui, GUO Lin, ZHU Li-li, SUN Xiao-qi, CHEN Ji. Removal of Cr(III, VI) by quaternary ammonium and quaternary phosphonium ionic liquids functionalized silica materials [J]. Chem Eng J, 2010, 158: 108-114.
[4] ZHU Li-li, LIU Ying-hui, CHEN Ji. Synthesis of N-methylimidazolium functionalized strongly basic anion exchange resins for adsorption of Cr(VI) [J]. Ind Eng Chem Res, 2009, 48: 3261-3267.
[5] KOZLOWSKI C A, Kinetics of Chromium(VI) transport from mineral acids across cellulose triacetate (CTA) plasticized membranes immobilized by tri-n-octylamine [J]. Ind. Eng Chem Res, 2007, 46: 5420-5428.
[6] SOMEDA H H, EL-SHAZLY E A, SHEHA R R. The role of some compounds on extraction of chromium(VI) by amine extractants [J]. J Hazard Mater, 2005, 117: 213-219.
[7] KOZLOWSKI C A, WALKOWIAK W. Applicability of liquid membranes in chromium (VI) transport with amines as ion carriers [J]. J Membr Sci, 2005, 266: 143-150.
[8] DUPONT J, de SOUZA R F, SUAREZ P A Z. Ionic liquid (molten salt) phase organometallic catalysis [J]. Chemical Reviews, 2002, 102: 3667-3691.
[9] CAPELLO C, FISCHER U, HUNGERBUEHLER K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents [J]. Green Chem, 2007, 9: 927-934.
[10] CHUN S, DZYUBA S V, BARTSCH R A. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether [J] Anal Chem, 2001, 73: 3737-3741.
[11] SUN Xiao-qi, PENG Bo, CHEN Ji, LI De-qian, LUO Fang. An effective method for enhancing metal-ions' selectivity of ionic liquid-based extraction system: Adding water-soluble complexing agent [J]. Talanta, 2008, 74: 1071-1074.
[12] SUN Xiao-qi, PENG Bo, JI Yang, CHEN Ji, LI De-qian. Chitosan (Chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption [J]. AIChE J, 2009, 55: 2062-2069.
[13] DIETZ M L, DZIELAWA J A. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: implications for the ‘greenness’ of ionic liquids as diluents in liquid-liquid extraction [J]. Chem Commun, 2001: 2124-2125.
[14] VISSER A E, SWATLOKI R P, REICHERT W M, MAYTON R, SHEFF S, WIERZBICKI A, DAVIS J H, RODERS R D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions [J]. Chem Commun, 2001: 135-136.
[15] VINCENT T, PARODI A, GUIBAL E. Pt recovery using Cyphos IL-101 immobilized in biopolymer capsules [J]. Sep Purif Technol, 2008, 62: 470-479.
[16] GALLARDO V, NAVARRO R, SAUCEDO I, AVILA M, GUIBAL E. Zinc(II) extraction from hydrochloric acid solutions using amberlite XAD-7 impregnated with Cyphos IL 101 (tetradecyl(trihexyl) phosphonium chloride) [J]. Sep Sci Technol, 2008, 43: 2434-2459.
[17] MARTAK J, SCHLOSSER S. Liquid-liquid equilibria of butyric acid for solvents containing a phosphonium ionic liquid [J]. Chem Pap, 2008, 62: 42-50.
[18] MARTAK J, SCHLOSSER S. Extraction of lactic acid by phosphonium ionic liquids [J]. Sep Purif Technol, 2007, 57: 483-494.
[19] LIU Ying-hui, ZHU Li-li, SUN Xiao-qi, CHEN Ji. Toward greener separations of rare earths: bifunctional ionic liquid extractants in biodiesel [J]. AIChE J, 2010, 56: 2338-2346.
[20] LIU Ying-hui, ZHU Li-li, SUN Xiao-qi, CHEN Ji, LUO Fang. Silica materials doped with bifunctional ionic liquid extractant for yttrium extraction [J]. Ind Eng Chem Res, 2009, 48: 7308-7313.
[21] SUN Xiao-qi, JI Yang, CHEN Ji, MA Jiu-tong. Solvent impregnated resin prepared using task-specific ionic liquids for rare earth separation [J]. Journal of Rare Earths, 2009, 27: 932-936.
[22] GUO Lin, LIU Ying-hui, ZHANG Chao, CHEN Ji. Preparation of PVDF-based polymer inclusion membrane using ionic liquid plasticizer and Cyphos IL 104 carrier for Cr(VI) transport [J]. J Membr Sci, 2011, 372: 314-321.
[23] SENGUPTA A K, CLIFFORD D. Important process variables in chromate ion-exchange [J]. Environ Sci Technol, 1986, 20: 149-155.
[24] PARK H J, TAVLARIDES L L. Adsorption of chromium(VI) from aqueous solutions using an imidazole functionalized adsorbent [J]. Ind Eng Chem Res, 2008, 47: 3401-3409.
[25] LANGMUIR I. The constitution and fundamental properties of solids and liquids Part I Solids [J]. J Am Chem Soc, 1916, 38: 2221-2295.
[26] FREUNDLICH H. Over the adsorption in solution [J] Z Phys Chem, 1906, 57: 385-470.
[27] SAHA B, GILL R J, BAILEY D G, KABAY N, ARDA M. Sorption of Cr(VI) from aqueous solution by Amberlite XAD-7 resin impregnated with Aliquat 336 [J]. React Funct Polym, 2004, 60: 223-244.
离子液体Cyphos IL 104的溶剂浸渍树脂的制备及其在铬(VI)回收中的应用
杨秀云1,张建苹1,2, 郭 琳2,3, 赵 贺1,2, 张 阳2,3, 陈 继2
1. 长春理工大学 化学与环境工程学院,长春 130022;
2. 中国科学院 长春应用化学研究所,稀土资源利用国家重点实验室,长春 130022;
3. 中国科学院大学,北京 100039
摘 要:采用XAD-7 树脂浸渍离子液体二(2,4,4 三甲基戊基)亚膦酸三己基十四烷基烷基鏻(Cyphos IL 104)。研究该种溶剂浸渍树脂 (SIR) 的制备过程及其在Cr(VI) 分离中的应用,并对树脂的表面形貌和热稳定性进行表征。研究平衡时间和初始pH值对Cr(VI) 吸附的影响,讨论溶剂浸渍树脂的吸附等温线、吸附和解吸附过程以及浸渍树脂的选择性。结果表明,萃取剂Cyphos IL 104浸入了树脂的内部孔道中,溶剂浸渍树脂吸附Cr(VI)的最佳pH范围为0-2。当Cyphos IL 104作为解吸附溶液时,Cr(VI)可以从树脂上有效地脱附。
关键词:固-液萃取;Cyphos IL 104;Cr(VI);树脂;吸附等温线;吸附;解吸附
(Edited by LI Xiang-qun)
Foundation item: Project (51174184) sponsored by the National Natural Science Foundation of China; Project (2012CBA01202) supported by the National Basic Research Program of China; Project (KGZD-EW-201-1) supported by the Key Research Program of the Chinese Academy of Sciences
Corresponding author: CHEN Ji; Tel: +86-431-85262646; E-mail: jchen@ciac.jl.cn
DOI: 10.1016/S1003-6326(12)61764-6
Abstract: Ionic liquid (IL) trihexyl (tetradecyl) phosphonium bis 2,4,4-trimethylpentylphosphinate (Cyphos IL 104) was impregnated on XAD-7 resin. The solvent impreganated resin (SIR) was prepared and applied in Cr(VI) removal. The morphology and the thermal stability of the resins were explored. The effects of equilibrium time and initial pH value on Cr(VI) adsorption were investigated. Adsorption isotherm, separation and desorption of the SIR, and selectivity of SIR were also explored. The results show that Cyphos IL 104 exists in the inner XAD-7 resin, and the optimum pH value range of the SIR for Cr(VI) extraction is 0 to 2. When NaOH used as desorption solution, the Cr(VI) can be effectively desorbed from the SIR.


