
Optimization of preparing V2O5 by calcination from ammonium metavanadate using response surface methodology
LIU Bing-guo1, 2, PENG Jin-hui1, 2, WAN Run-dong1, 2, ZHANG Li-bo1, 2, GUO Sheng-hui1, 2, ZHANG Shi-ming1, 2
1. Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology,
Kunming 650093, China;
2. Key Laboratory of Unconventional Metallurgy of Ministry of Education,
Kunming University of Science and Technology, Kunming 650093, China
Received 24 May 2010; accepted 23 July 2010
Abstract:
Parameters of technique to prepare vanadium pentoxide by calcination from ammonium metavanadate were optimized using central composite design of response surface methodology. A quadratic equation model for decomposition rate was built and effects of main factors and their corresponding relationships were obtained. The results of the statistical analysis show that the decomposition rate of ammonium metavanadate is significantly affected by calcination temperature and calcination time. The optimized calcination conditions are as follows: calcination temperature 669.71 K, calcination time 35.9 min and sample mass 4.25 g. The decomposition rate of ammonium metavanadate is 99.71%,which coincides well with experimental value of 99.27% under the optimized conditions, suggesting that regressive equation fits the decomposition rates perfectly. XRD reveals that it is feasible to prepare the V2O5 by calcination from ammonium metavanadate using response surface methodology.
Key words:
vanadium pentoxide; ammonium metavanadate; calcination; response surface methodology;
1 Introduction
Vanadium pentoxide crystallizes in an orthorhombic, weakly bonded layered structure, with vanadium surrounded by six oxygen atoms forming a strongly distorted octahedron unit[1]. It is the most stable compound in the V-O system, exhibiting highly anisotropic electrical and optical properties due to its orthorhombic structure[2]. Vanadium pentoxide is widely used in photocatalyst as photovoltaics, gas sensor, cathode for solid-state batteries, and window for solar cells and for electrochromic devices as well as for electronic and optical switches[3]. Besides, it is the most important metal used in metal oxide catalysis[4-6], due to the oxidation states of the vanadium atoms varying from two to five, and easy conversion between oxides of different stoichiometry. Recently, many different methods, such as vacuum evaporation[7-8], sol-gel process[9], sputtering[10], thermal decomposition, and chemical vapor deposition[11], have been used to fabricate vanadium pentoxide. Among these methods, the conventional thermal decomposition of ammonium metavanadate is being commercially applied at present, due to the process simplicity. However, the traditional approach “one variable at a time (OVAT)” is extremely laborious and time-consuming; moreover, it completely lacks representing the effect of interaction between different factors[12], from which the effect of each experimental factor is investigated by altering the level of one factor at a time while maintaining the level of the other factors constant. So as to solve the problem, it is necessary to find multivariate statistic techniques to optimize the preparation processes. Response surface methodology stands out as one appropriate method to solve this issue. Response surface methodology (RSM) may be summarized as a compilation statistical tool and technique for constructing and exploring an estimated functional relationship between a response variable and a set of design variables[12]. It is a collection of mathematical and statistical techniques that are useful for modeling and analysis of the problems having numerous variables influencing the response and the objective is to optimize the response[13-15]. This statistic method has by now been extensively applied in many areas of biotechnology and agricultural. However, as far as known to the authors of this work, there have been very few studies to optimize the preparation for vanadium pentoxide from ammonium metavanadate by the RSM approach.
Based on the above-mentioned facts, the main objective of this work is to determine the optimal process parameters using response surface methodology. The RSM technique attempts to optimize the responses of decomposition rate of ammonium metavanadate. A central composite design (CCD) was selected to study simultaneously the effects of three influencing variables i.e., calcination temperature, calcination time, and mass of sample on the response.
2 Experimental
2.1 Materials
Chemical pure ammonium metavanadate used in the present study was obtained from Tianjin Chemical Reagent Co., Ltd, China, which had particle size less than 100 μm.
2.2 Calcination experiments
The calcination experiments were carried out at different temperatures, calcination durations, and masses of sample. Initially, the conventional tubular electric oven was preheated at 30 K/min until the desired temperature was reached. Then the ammonium metavanadate was weighed and placed inside the ceramic reactor which was located in the centre of the conventional tubular electric oven. During the reaction, the temperature was monitored by a temperature controller system, the PID (proportional-integral- derivative) controller. Several cycles of experiment were repeated. For each cycle, the experiment was stopped after a fixed duration. The product was moved out from the tubular reactor and rapidly put into the drier. They were naturally cooled to room temperature. The final mass (m) of sample was weighed subsequently. The decomposition rate was calculated based on the following equation:
![]() (1)
(1)
where m and m0 are final mass and initial mass of ammonium metavanadate, respectively; wt is theory value of mass loss; γ is decomposition rate of ammonium metavanadate.
2.3 Designing experiment using response surface methodology
Based on the initial decomposition results and analysis of TG/DTG, a three-variable and two-level central composite design was employed to optimize the decomposition condition in order to obtain a high decomposition rate. This method helps to optimize the effective parameters with a minimum number of experiments and also analyze the interaction between the parameters and results[16]. The three independent variables set were χ1 (calcination temperature), χ2 (calcination time), χ3 (mass of sample), respectively, and each variable set had two levels. A total of 20 experiments were designed, and given in Table 1.
Table 1 Independent variables and their levels used for center composite rotatable design

To predict the optimal point, a second-order polynomial function was used to fit the correlation between independent variables and response on the basis of the above design. The recommended quadratic model was as follows:
![]() (2)
(2)
where γ is the predicated response; β0 is a constant; βi is the ith linear coefficient; βii is the ith quadratic coefficient; βij is the ijth interaction coefficient; and χij is the independent variable.
2.4 XRD analysis
X-ray diffraction analyses of the final solid products under optimization conditions were carried out using D/max-2200 diffractometer (Japan) with Ni-filtered Cu Kα radiation. The identification of the completeness of the vanadium pentoxide was made by comparing the diffraction peaks of each compound in the sample with those of the standard vanadium pentoxide. If the diffraction pattern of the final solid product satisfactorily matched with that of the standard vanadium pentoxide, the decomposition of ammonium metavanadate is complete.
3 Results and discussion
3.1 Thermal decomposition behavior of ammonium metavanadate
The thermal decomposition behavior of ammonium metavanadate was investigated at four heating rates, β=5, 10, 15, 20 K/min, using thermogravimetric analysis, respectively. TG and DTG curves for non-isothermal decomposition at various heating rates are presented in Figs.1 and 2, respectively. The results show that there are four mass loss stages in the TG curves, corresponding to four peaks in DTG curves. For the TG curves when β=10 K/min, stage I begins at 300 K and stops at 351.05 K, accompanied by 7.36% mass loss. The reason is the dehydration of the absorption water from outside air in ammonium metavanadate. The occurrence of stage II, stage III and stage IV was attributed to the decomposition of ammonium metavanadate. Beyond this temperature range, the mass change eases. On the basis of these obtained results, the thermal decomposition mechanism of ammonium metavanadate could be shown as[17]:
![]() (3)
(3)
![]()
![]() (4)
(4)
![]() (5)
(5)
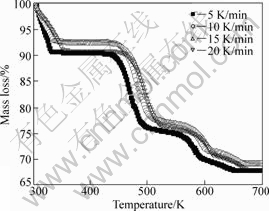
Fig.1 TG curves of ammonium metavanadate at different heating rates
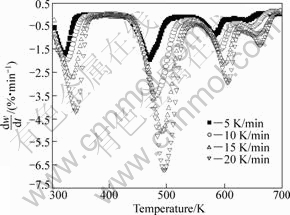
Fig.2 DTG curves of ammonium metavanadate at different heating rates
As can be seen, the mass loss is very significant for ammonium metavanadate in the range of 450-550 K, which indicates that decomposition reaction runs quickly.
3.2 Data analysis and evaluation of model by RSM
The experimental results concerning decomposition rate (γ) using three factor CCD experimental design with six replicates and six axial points are shown in Table 2. Decomposition rate of ammonium metavanadate obtained ranged from 39.12% to 99.46%. Runs 15-20 at the center point were used to determine the experimental error.
Table 2 Experimental design matrix and results for response surface of decomposition rate of ammonium metavanadate
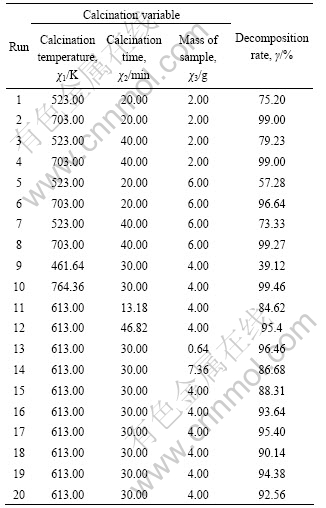
Data obtained from the experiments (Table 2) were analyzed by linear multiple regression software. The corresponding second-order response model for Eq.(2) founded after analysis for the regression is
γ=92.35+15.40χ1+2.99χ2-3.10χ3-2.18χ1χ2+2.72χ1χ3+ 1.83χ2χ3-7.80χ12-0.47χ22+0.080χ32 (6)
The experimental data were analyzed by analysis of variance (ANOVA, also a part of RSM) to assess the “goodness of fit,” which are listed in Table 3. Generally, the ANOVA and P-value are used to check the significance of each coefficient and indicate the interaction strength of each parameter. In the present experiment, the model with the P-value less than 0.0001 is statistically significant, which indicates that the model was suitable for this experiment. Meanwhile, the “lack of fit” of this model, with P-value of 0.189 2, was insignificant. The coeffocoent of determination (R2) and adjusted coefficient of determination (![]() ) are 0.974 and 0.951, respectively, implying that the accuracy and general availability of the polynomial model are adequate. The Model F-value of 41.58 implies that the model is signi?cant and there is only a 0.01% chance that a large “Model F-value” could occur due to noise.
) are 0.974 and 0.951, respectively, implying that the accuracy and general availability of the polynomial model are adequate. The Model F-value of 41.58 implies that the model is signi?cant and there is only a 0.01% chance that a large “Model F-value” could occur due to noise.
Table 3 Analysis of variance (ANOVA) for response surface quadratic model for decomposition rate
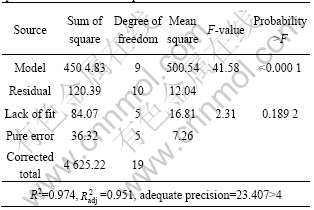
It would give poor or misleading results if the fit is inadequate. Therefore, checking the model adequacy is an important part of the data analysis procedure[18]. Multivariable linear regression was used to calculate the coefficients of the second-order polynomial equation and the obtained regression coefficients, whose significance was determined using the P-value, were summarized in Table 4. It can be seen that χ1, χ2, χ3, and the interaction terms (χ12) were signi?cant model terms whereas the interaction terms (χ1χ, χ2χ3, χ1χ3) were insigni?cant to the response.
3.3 Response surface analyses
The best way to visualize the influence of the independent variables on the response is to draw surface response plots of the model[19]. The three-dimensional response surface plot and its corresponding contour plot for the influence decmposition rate of ammonium mteavanadite by fitting quadratic polynomial model from regression analysis are given in Figs.3-4.
From Fig.3, it is observed that the decomposition rate significantly increases with increasing calcination temperature at the fixed mass of sample of 4 g. Increasing the calcination temperature up to 669.71 K gives an enhanced effect on the decomposition rate as the maximum predicated value at 99.71% is achieved because thermal decomposition of ammonium metavanadate is endothermic reaction and acceleration reaction of ammonium metavanadate would take place with increasing the temperature, calcination time, thus calcination reaction more complete. So, the decomposition
Table 4 Regression coefficient of polynomial function of response surface for decomposition rate of ammonium metavanadate
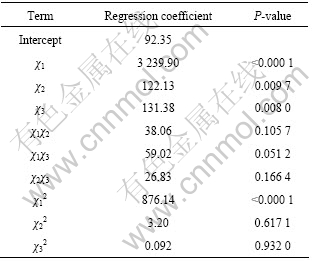
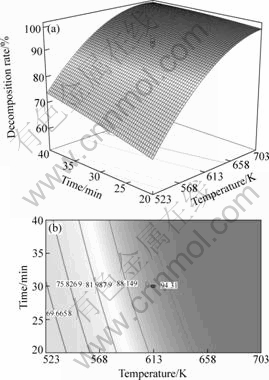
Fig.3 Response surface plot and respective contour plot of effect of decomposition rate (%) versus temperature and time
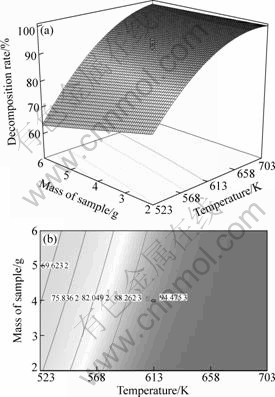
Fig.4 Response surface plot and respective contour plot of effect of decomposition rate (%) versus temperature and mass of sample
rates increase with increasing calcination temperature and calcination time. The figure reveals that the effect of the calcination temperature on the decomposition rate is more significant than calcination time.
Fig.4 shows the three-dimensional display of the response surface plot and their corresponding contour plot of the function of the decomposition rate versus the calcination temperature and mass of sample, with calcination time set at 30 min. The decomposition rate is seen to decrease with an increase in mass of sample within the experimental range. It is contributed to the fact that the diffusion of gas became difficult with increasing mass of the sample, resulting in decrease of decomposition rate.
3.4 Optimal conditions and verification of model
The optimized conditions of the influence decomposition rate of ammonium metavanadate, the calcination temperature, calcination time, and mass of sample, were obtained using RSM. The experimental value was compared with predicted one in order to determine the validity of the model.
From the model, optimized conditions were obtained and given in Table 5. The stationary point gives a maximum value as follows: calcination temperature 669.71 K, calcination time 35.9 min, and mass of sample 4.25 g. The predicted decomposition value was 99.71%, while the experimental value under these conditions was 99.27%, indicating that the experimental value is in agreement with the predicated one.
Table 5 Model validity of response surface for decomposition rate of ammonium metavanadate under optimal conditions

3.5 XRD analysis
Result of X-ray diffraction studies of the products under optimization conditions is shown in Fig.5. The results show that vanadium pentoxide is the only solid product identified whose diffraction pattern satisfactorily matches with the vanadium pentoxide pattern. Thus, it is feasible to prepare vanadium pentoxide by calcination from the ammonium metavanadate under optimum conditions.
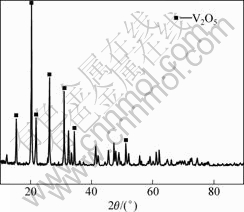
Fig.5 XRD pattern of final solid product under optimization conditions
4 Conclusions
1) The decomposition rate of ammonium metavanadate is significantly affected by calcination temperature and calcination time compared with the mass of sample.
2) The optimized calcination conditions are as follows: calcination temperature 669.71 K, calcination time 35.9 min and sample mass 4.25 g. The decomposition rate of ammonium metavanadate is 99.71%,which coincides well with experiments value of 99.27% under the optimized conditions, suggesting that regressive equation fits the decomposition rate perfectly.
3) It is feasible to prepare the V2O5 by calcination from ammonium metavanadate using response surface methodology.
References
[1] SU D S, ZANDBERGEN H W, TIEMEIJER P C, KOTHLEITNER G, H? VECKER M, H?BERT C, KNOP-GERICKE A, FREITAG B H, HOFER F, SCHL?GL R. High resolution EELS using monochromator and high performance spectrometer: Comparison of V2O5 ELNES with NEXAFS and band structure calculations [J]. Micron, 2003, 34: 235-238.
[2] LOSURDO M, BARRECA D, BRUNO G, TONDELLO E. Spectroscopic ellipsometry investigation of V2O5 nanocrystalline thin films [J]. Thin Sol Films, 2001, 384(1): 58-64.
[3] BAHGAT A A, IBRAHIM F A, EL-DESOKY M M. Electrical and optical properties of highly oriented nanocrystalline vanadium pentoxide [J]. Thin Sol Films, 2005, 489(1-2): 68-73.
[4] WECKHUYSEN B M, KELLER D E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis [J]. Catal Today, 2003, 78(1-4): 25-46.
[5] ERTL G, KNOZINGER H, WEITKAMP J. Handbook of hetero-geneous catalysis [M]. Weinheim: Wiley-VCH, 1997.
[6] HAGEN J. Industrial catalysis, a practical approach [M]. Weinheim: Wiley-VCH, 1999.
[7] GUAN Z S, YAO J N, YANG Y A, LOO B H. Electrochromism of the annealed vacuum-evaporated V2O5 films [J]. J Electroanal Chem, 1998, 443(2): 175-179.
[8] RAJENDRA KUMAR R T, KARUNAGARAN B, VENKATACHALAM S, MANGALARAJ D, NARAYANDASS S A K, KESAVAMOORTHY R. Influence of deposition temperature on the growth of vacuum evaporated V2O5 thin films [J]. Mater Lett, 2003, 57(24-25): 3820-3825.
[9] OZER N. Electrochemical properties of sol gel deposited vanadium pentoxide films [J]. Thin Sol Films, 1997, 305(1-2): 80-87.
[10] CAZZANELLI E, MARIOTTO G, PASSERINI S, SMYRL W H, GORENSTEIN A, ENERGYMATER S. Raman and XPS characterization of vanadium oxide thin films deposited by reactive RF sputtering [J]. Energy Mater Sol Cells, 1999, 56(3-4): 249-258.
[11] GHANASHYAM KRISHNA M, DEBAUGE Y, BHATTACHARYA A K. X-ray photoelectron spectroscopy and spectral transmittance study of stoichiometry in sputtered vanadium oxide films [J]. Thin Sol Films, 1998, 312(1-2): 116-122.
[12] VENTER G. Non-dimensional response surfaces for structural optimization with uncertainty [D]. Florida: University of Florida, 1998.
[13] SHARMA S, MALIK A, SATYA S. Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05 [J]. J Hazard Mater, 2009, 164(2-3): 1198-1204.
[14] MYERS R H, MONTGOMERY D C. Response surface methodology [M]. New York: John Wiley & Sons, Inc, 2002.
[15] CHEN M J, CHEN K , LIN C W. Optimization on response surface models for the optimal manufacturing conditions of dairy tofu [J]. J Food Eng, 2005, 68(4): 471-480.
[16] AZARGOHAR R, DAHAI A K. Production of activated carbon from luscar char: Experimental and modeling studies [J]. Micropor Mesopor Mater, 2005, 85(3): 219-227.
[17] ZHANG Mei, LIN Qin, XU Ai-ju. Application of thermal analysis in study of mechanism of thermal decomposition of NH4VO3 [J]. Modern Scientific Instruments, 2007(3): 98-100.
[18] KORBAHTI B K, RAUF M A. Determination of optimum operating conditions of carmine decoloration by UV/H2O2 using response surface methodology [J]. J Hazard Mater, 2009, 161(1): 281-286.
[19] XU X, GAO Y X, LIU G M, WANG Q, ZHAO J. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae thamnoides L.) oil using response surface methodology [J]. LWT-Food Sci Technol, 2008, 41(7): 1223-1231.
响应曲面法优化偏钒酸铵煅烧制备V2O5的工艺
刘秉国1, 2,彭金辉1, 2,万润东1, 2,张利波1, 2,郭胜惠1, 2,张世敏1, 2
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 昆明理工大学 非常规冶金省部共建教育部重点实验室,昆明 650093
摘 要:采用响应曲面法中的中心组合模式对偏钒酸铵煅烧制备V2O5工艺条件进行优化,建立偏钒酸铵煅烧制备V2O5的二次多项式数学模型,探讨主要因素的影响及其交互作用。方差分析结果表明:煅烧温度和煅烧时间对偏钒酸铵的分解率都有显著的影响。采用响应曲面法优化得出的最佳工艺条件为:煅烧温度669.71 K,煅烧时间35.9 min,物料量4.25 g。在最佳工艺条件下,偏钒酸铵的分解率预测值为99.71%,其与实验值99.27%相近,证实回归方程拟合度良好。XRD分析表明,采用响应曲面法所得的煅烧工艺参数是可行的。
关键词:V2O5;偏钒酸铵;煅烧;响应曲面法
(Edited by YANG Hua)
Foundation item: Project (50734007) supported by the National Natural Science Foundation of China; Project (2007GA002) supported by Science and Technology Planning of Yunnan Province, China; Project (2008-16) supported by Analysis and Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: PENG Jin-hui; Tel: +86-871-5192076; Fax: +86-871-5191046; E-mail: Jhpeng_ok@Yeah.net
DOI: 10.1016/S1003-6326(11)60764-4


