DOI:10.19476/j.ysxb.1004.0609.2018.02.23
异极矿成矿规律及浮选药剂作用机理研究现状
章晓林1, 2,王其宏1, 2,景 满2,饶 霏3,武鲁庆2,李康康2,曹世明4,刘殿文1, 2
(1. 昆明理工大学 省部共建复杂有色金属资源清洁利用国家重点实验室,昆明 650093;
2. 昆明理工大学 国土资源工程学院,昆明 650000;
3. 西北大学 外国语学院,西安 710069;
4. 中国矿业大学 化工学院,徐州 221000)
摘 要:
本文主要考察了异极矿的成矿规律、结构特征以及异极矿与浮选药剂之间的作用规律,详细分析了影响异极矿可浮性的重要因素,总结了国内外与异极矿相关的浮选药剂与异极矿之间的作用规律,研究结果表明:在成矿的前期阶段,异极矿易与粘土矿物混杂,采用浮选法进行回收时含锌矿物易损失于矿泥中,从而使得异极矿的浮选回收率较低;胺类捕收剂的极性基团—NH2中N、H原子与异极矿表面的Zn、Si、O、H等原子之间并未形成有效的化学键,捕收剂与异极矿之间的作用方式仅为静电吸附;由于异极矿中SiO4-的体积比Zn2+更大,在与浮选药剂发生作用时,矿物表面的Zn2+往往被SiO42-屏蔽,使得矿物溶解度降低,从而使得S2-、HS-等起硫化作用的离子很难与Zn2+结合,硫化时间增长;螯合剂法有望成为异极矿浮选新的高效选别方法。
关键词:
文章编号:1004-0609(2018)-02-0406-10 中图分类号: 文献标志码:A
锌是一种重要的有色金属原材料,目前,锌在有色金属的消费中仅次于铜和铝。广泛应用于合金制造、电镀及建筑等领域,还可用作橡胶和油漆中的化学添加剂[1-2]。仅管含锌矿物仍以硫化矿为主,但含量高、可浮性好、目的矿物嵌布均匀的矿石已不能满足市场需求。随着人们对氧化锌矿资源的重视及研究的深入,就技术层面而言,将90%以上的含锌矿物与脉石分离是可行的[3]。在氧化锌矿中,具有经济价值的矿物主要包括异极矿、菱锌矿和硅锌矿等。由于异极矿和硅锌矿具有亲水性较强、含泥量大、易受铁质污染等特点,因此,与其他氧化锌矿相比更加难选。
由于成矿环境如温度、压力等不同,所形成的矿物晶型有所不别[4],而矿物晶体的结构特性对矿物的可浮性会产生极大地影响[5],某些硫化矿如辉钼矿、辉锑矿、雌黄等为层状、链状构造,在破裂过程中,完全解离面上会存在较弱分子键力,因而会表现出一定的天然可浮性[6];而异极矿、硅锌矿等含硅矿物其本身无天然可浮性,但可经硫化后用胺类捕收剂进行浮选[7]。因此,研究矿物的成矿过程和晶体结构,探索矿物晶体结构与可浮性之间的内在关联和规律[8],有助于寻求难选矿石的分离方法。
本文作者主要以异极矿为研究对象,从矿石的成矿规律、结构特征、异极矿与浮选药剂之间的作用机理等几个方面进行了全面的分析和总结,以期有助于对异极矿的可浮的理解性、异极矿与浮选药剂之间的作用规律。
1 异极矿成矿规律
常见的异极矿一般产于铅锌硫化物矿床所属氧化带中,并与Fe、Pb、Zn等金属的硫化物共生[9]。目前,异极矿的成矿规律主要存在以下两种看法:一是由于氧化、淋滤带往往存在二氧化硅凝胶体,可认为在氧化带进一步发育过程中,在先形成的菱锌矿变成Zn(HCO3)2和ZnSO4的情况下,Zn(HCO3)2或ZnSO4与二氧化硅凝胶体相结合形成异极矿[10];二是异极矿集合体在表生作用过程中,由富含SiO2的水溶液与闪锌矿的次生氧化产物作用而形成的[11]。云南金顶异极矿所属铅锌矿床氧化带处于非常发育的表生环境中,刘琰等[12]探究了该矿床与其他锌氧化矿物及次生氧化物的特征,采用湿法化学全分析对5组异极矿样品进行了分析,结果表明异极矿是在不同时期表生形成的。斯米尔诺夫著作中提到[13],通过大量人工合成异极矿实验材料和对氧化带的观察,指出异极矿的形成是含SiO2的水溶液与锌的重碳酸盐溶液,而不是与锌的硫酸盐溶液相互作用的结果。在地质结构的演变过程中,岩石中的元素Si如砂岩等与反应的过程产物硫酸作用生成原硅酸或偏硅酸,硅酸再与锌的重碳酸盐溶液反应生成异极矿。按照硫化物矿床氧化带的氧化顺序及成矿分带情况,其反应式如下:
ZnS+2O2=ZnSO4 (1)
ZnSO4+CaCO3=CaSO4+ZnCO3 (2)
2H2SiO3+4Zn(HCO3)2=Zn4(Si2O7)(OH)2↓+5H2O+8CO2↑ (3)
由于岩石中大量粘土的存在,使得异极矿表面在早期的成矿阶段易受石英及粘土矿物如白云石、蒙脱石等浸染,后期形成的异极矿则会沿风化裂隙壁呈脉状充填,其表面受污染程度较小[14]。由于异极矿的形成过程通常伴有大量的褐铁矿,因而地表存在的异极矿一般均伴有褐铁矿,为使受污染的异极矿与脉石矿石有效分离,浮选前须用水玻璃与稀硫酸组成的混合溶液进行充分搅拌[15],以消除铁质对异极矿浮选过程的影响。
2 异极矿结构构造
异极矿结构构造复杂,就常见异极矿矿物组成2ZnO·SiO2·H2O而言[16],其晶格中Zn原子与硅氧四面体所属的三个氧原子及一个羟基组成新的四面体,并且Zn在晶格中的活性较低[17]。HILL[18]在早些时候已对其晶体结构进行测定,刘琰等[19]研究过加热过程对异极矿的影响,发现经800~1300 热处理后,异极矿结构会转变为硅锌矿。异极矿的晶胞模型见图1。
异极矿在破碎及磨碎过程中,由于Si—O键间的结合强度比Zn—O键间结合强度大,因而一般断裂的是Zn—O键。加之矿物内部结构的复杂性,导致异极矿表面结构呈现出不均匀性,在Zn—O断裂时,矿物表面往往存在SiO42-和Zn2+化学键[16, 20]。与Zn2+相比,SiO42-的体积更大,活动空间较宽,因而在与药剂发生作用时,矿物表面的Zn2+往往被SiO42-屏蔽,多数情况下表现出与石英相似的表面结构,这种结构性质使得矿物溶解度降低,表面Zn原子的活性减小,Zn2+迁移能力降低[21],从而使得S2-、HS-等起硫化作用的离子很难与Zn结合。相对于菱锌矿等碳酸盐类矿物而言,异极矿所需硫化时间较长,硫化速度减慢[22],浮选时一般采用预先硫化或延长硫化时间。
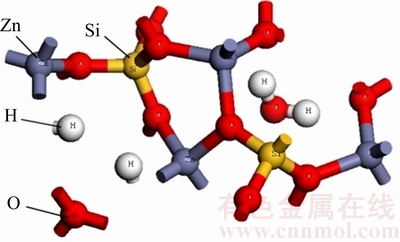
图1 异极矿的晶胞模型
Fig .1 Cell model of hemimorphite
3 浮选药剂与异极矿之间的作用规律
了解浮选药剂与异极矿之间的作用机理,不仅对选矿试验方案的确定和新药剂的合成具有十分重要的意义,而且对如何排除杂质离子的干扰也具有重要的参考价值。
浮选过程中,浮选药剂研究的基础为捕收剂和调整剂,这也是浮选理论研究的重点与难点,本文将对浮选药剂与矿物之间的作用机理进行详细讨论。
异极矿的浮选工艺主要包括硫化浮选法(硫化胺法和硫化黄药法)、脂肪酸浮选法和螯合剂浮选法。捕收剂类型主要包括阴离子捕收剂、阳离子捕收剂和螯合剂等。
3.1 硫化-黄药法
硫化-黄药法是指先对氧化锌矿物进行硫化,然后用硫酸铜活化,再以黄药为捕收剂进行浮选。石道民等[23]曾系统研究过硫化-黄药法,发现随着Na2S浓度的增大,硫在矿物表面的吸附量也随之增大,但过大浓度的Na2S容易阻止矿物表面黄药的吸附,从而对浮选不利。JANUSZ等[24]研究了使用硫氢离子(HS-)和铜离子(Cu2+)活化碳酸锌后,以黄药为捕收剂进行浮选。研究结果表明该法可用于浮选碳酸锌,但所需的药剂浓度较大,Cu2+和HS-在矿物表面吸附40个分子层以上时才能成功浮选。意大利戈尔诺选厂[23]原矿为菱锌矿和异极矿,原矿锌品位6.3%,浮选在45~50 ℃的矿浆中进行,先将其硫化,再用硫酸铜对氧化锌矿物进行活化,最后用戊基黄药捕收,最终精矿品位为38%,回收率为76.4%。会泽铅锌矿采用加温-硫化-黄药法能得到品位为40%,回收率为73.2%的锌精矿指标,但若以丁基黄药为捕收剂,药剂消耗量大[25]。STACHURSKI等[26]在623~773 K温度条件下,采用硫蒸汽对波兰某混合锌矿进行硫化处理,比较了不同硫酸铜用量及不同碳链长度的黄药对氧化锌矿石浮选的影响,经高温硫化处理后的氧化锌矿表现出较好的可浮性。针对某高铁泥化氧化铅锌矿的综合利用,陈锦全等[27]以硫化-胺法浮选氧化铅矿,以硫化-黄药法浮选氧化锌矿,将铅、锌品位从3.54%、5.86%分别提升至45.23%和40.56%,回收率达73%以上。
虽然硫化-黄药法在实际应用中取得一定效果,但仍有诸多缺点[28]:① 对高含泥的氧化锌矿,采用泥沙分选会造成锌金属的损失,也会对工艺过程的稳定产生一定的影响;② 处理含铁量高的氧化锌矿或硅锌矿(如异极矿)时,其浮选指标较低;③ 用该法浮选硅酸锌类矿物难以获得较高的回收;④ 一旦硫化钠用量过大就会使氧化锌矿物受到明显的抑制作用。⑤ 浮选时的硫化过程通常需要加温,致使工艺更加复杂和选矿成本的增大。
3.2 脂肪酸法
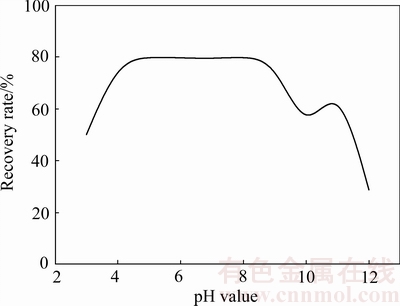
图2 油酸钠用量为3×10-4 mol/L时pH值对异极矿回收率的影响[31]
Fig. 2 Flotation recovery of hemimorphite as function of pH with 3×10-4 mol/L sodium oleate (Replotted from LIU et al[31])
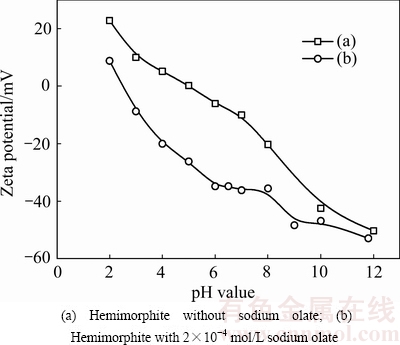
图3 不同pH值下异极矿表面Zeta电位[31]
Fig. 3 Zeta potential of hemimorphite as function of pH value[31]
脂肪酸类捕收剂可直接用于浮选氧化锌,也可在反浮选时使用以提高金属锌的回收率[29,30]。具有代表性的脂肪酸类捕收剂包括:油酸、油酸钠、合成脂肪酸等。而油酸钠作为捕收剂与矿物间的作用机理如化学吸附、静电吸附、离子-分子二聚复合吸附等报道甚多[31-33]。HOSSEINI等[34]对以油酸为捕收剂浮选菱锌矿进行了研究,发现油酸与矿物表面的锌反应生成油酸锌,致使矿物表面疏水性增强,电负性增大,从而使矿物的表面疏水上浮。刘诚等[31, 35]通过纯矿物试验对油酸钠与异极矿浮选体系进行了研究。不同条件下,油酸钠对异极矿浮选回收率的影响见图2。由图2可知,当捕收剂用量为3×10-4 mol/L时,异极矿在pH值为4~8、11的范围内均表现出较好可浮性,其回收率接近80%。Zeta电位测试表明(见图3),在相同的pH值条件下,油酸钠的加入使矿物Zeta电位发生负移,尤其在pH值等于4~8的范围内,负移距离最大。与异极矿表面带同种电荷的油酸根离子能很好地吸附在矿物表面,说明油酸根与矿物之间的作用为化学吸附,主要作用在矿物亥姆霍兹面[36]。根据金属离子水解平衡原理,异极矿发生水解作用主要发生以下羟基络合反应[37-38]:
Zn(OH)2(s)  Zn2++2OH-,Ksp=1×10-15.76 (4)
Zn2++2OH-,Ksp=1×10-15.76 (4)
Zn2++OH-  Zn(OH)+,β1=1×105.0 (5)
Zn(OH)+,β1=1×105.0 (5)
Zn2+2OH-  Zn(OH)2(aq),β2=1×1011.1 (6)
Zn(OH)2(aq),β2=1×1011.1 (6)
Zn2+3OH-  Zn(OH)3-, β3=1×1013.6 (7)
Zn(OH)3-, β3=1×1013.6 (7)
Zn2+4OH-  Zn(OH)42-, β4=1×1014.8 (8)
Zn(OH)42-, β4=1×1014.8 (8)
在矿浆环境pH值为4~8的范围内,异极矿可浮性较好。通过对Zn2+在溶液中的水解组分含量分析发现[31],此时溶液中锌离子的优势组分主要是Zn2+和少量Zn(OH)+,说明油酸根与上述离子发生了键合作用;而在pH值为9~11的范围内,溶液中存在大量的Zn(OH)2,但异极矿的回收率与Zn(OH)2含量成负相关关系,分析原因可能是由于此时溶液中油酸根离子与矿物表面的Zn(OH)2发生了离子交换的作用,其关系可用方程(9)[39]表示,这表明过多Zn(OH)2的存在不利于异极矿的回收,但可添加活化剂如Pb(NO3)2进行活化[40],原因在于Pb2+能在矿物表面生成Pb(OH)+羟基络合物及Pb(OH)2沉淀,从而增加矿物表面的活性质点,促进捕收剂吸附;当矿浆pH值大于11时,异极矿回收率也较低,此时锌离子的优势组分为Zn(OH)3-,表明OH-很可能与油酸根在矿物表面发生了竞争吸附。LIU等[40]和王淀佐等[41],当矿浆溶液pH值大于3.2时,离子 -分子缔合物RCOOH·RCOO-的含量增加(见图4),由于其具有较大的表面活性,因此RCOOH·RCOO-在一定程度上也促进了异极矿的回收。
Zn(OH)2(surf)+RCOO-  Zn+RCOO-+OH- (9)
Zn+RCOO-+OH- (9)
前人的研究成果表明[23],脂肪酸已广泛地应用于氧化矿脉石矿物的反浮选,对提高锌精矿指标效果明显。但对含铁量高、脉石矿物组成复杂的硅酸盐矿物如异极矿的分选效果较差,因此,对硅酸盐类矿石的实际应用仍相对较少。
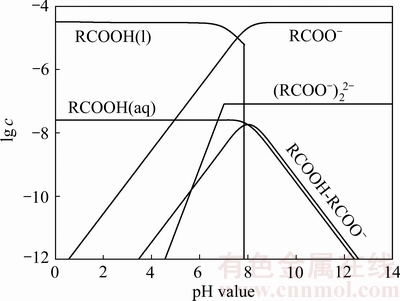
图4 油酸钠溶液组分图[42]
Fig. 4 Species distribution diagram of oleic acid with cT=3.0×10-5 mol/L[42] (Replotted from SOMASUNDARAN et al[42])
3.3 硫化-胺法
硫化-胺法也称为雷(Rey)法,是当前用于回收氧化锌矿物的主要方法,浮选时先将氧化锌矿物硫化,然后采用胺类作为氧化锌矿的捕收剂。该法首先由Paul Raffinot发现,并证明了伯胺类捕收剂最为有效[43],浮选过程可在常温条件下进行。由于胺类捕收剂对矿浆pH值敏感,一般须在弱酸性条件下进行浮选[44],这是氧化锌矿浮选工艺的一大突破,并为世界各国广泛采用。
EJTEMAEI等[22]研究发现,硅酸锌与异极矿同属硅酸盐矿物,但硅酸锌可浮性比异极矿好(尤其在弱碱性条件下),这一现象可通过矿物的化学性质及其结构进行解释:硅酸锌是一种典型硅氧四面体(SiO44-)与锌离子(Zn2+)连接而成的岛状硅酸盐矿物[45],而异极矿中锌原子与硅氧四面体中的3个氧原子及一个羟基组成新的四面体结构[16],与硅酸锌相比,异极矿中Zn2+的活性低[17, 19]。为了降低矿泥对浮选作业的干扰可将脂肪胺与硫化钠预先混匀,或将其溶解在煤油中制成乳浊液再进行浮选[14-15]。
将椰油胺40%、燃料油5%、松油5.5%以及EthomeenC25(1 g分子椰油脂肪胺与2.5 g分子环氧乙烷反应产物)的乳化液作捕收剂,在pH=12的高碱度浮选条件下选别氧化锌矿石,所得精矿氧化锌品位37%~40%,回收率60%以上[23]。

图5 pH值对异极矿可浮性影响[16]
Fig. 5 Influence of pH value on floatability of hemimorphite[16]
表1 氧化锌矿可能的定位离子[23]
Table 1 Possible locating ions for zinc oxide[23]

陈晔[16]以硫化钠为活化剂,考察了十八伯胺、十六伯胺、十四伯胺、十二伯胺四种胺类捕收剂对异极矿可浮性的影响。试验结果表明,在同一浮选环境下,脂肪胺碳原子数越多,其选择性也越好,捕收能力也越强。以十二胺伯胺浮选异极矿为例,矿浆pH值对异极矿可浮性影响见图5(Na2S用量8×10-4 mol/L,十二胺用量40 mg/L,pH调整剂为NaOH和H2SO4)。如图中所示,异极矿在pH值=6.0时,回收率仅为43.61%,根据对十二胺溶液组分分析[46],此时溶液中胺主要以RNH3+、(RNH3+)22+两种阳离子形式存在,而异极矿表面定位离子[23]在此pH值范围内应既有阳离子Zn2+、H+,也有阴离子Si2O76-,根据试验结果,推测此时定位离子应以阳离子为主;在pH值属9.0~10.5范围内,异极矿回收率基本在75%以上,此时矿物表面的定位离子以阴离子为主,有利于胺类捕收剂的吸附;但随着pH值的进一步增大,胺主要以分子形式存在,即使矿物表面存在大量阴离子也无较强作用力,故回收率下降较快。
为了考查十二胺分子中的极性基团—NH2与异极矿表面的作用规律,论文基于密度泛函理论第一性原理的计算对十二胺分子与异极矿之间的吸附规律进行了模拟和计算,结果表明,极性基团—NH2中N、H原子与异极矿表面Zn、Si、O、H等原子间的实际距离均远大于原子间共价半径[47-48](结果见表2),说明上述原子之间并没有化学键的形成,综合上述理论认为,十二胺与异极矿之间没有形成化学键,胺类捕收剂在异极矿表面以物理吸附为主,还可能存在一定的氢键作用[49]。
佩雷拉等[50]利用BET对异极矿、硅锌矿比表面积,孔隙平均直径等进行了测定(结果见表3),发现异极矿比表面积大,因而与Na2S作用时间要比硅酸锌矿长,其表面达到相同Zn2+浓度所需硫化时间大约是硅锌矿所需时间的2倍[21]。以Na2S作硫化剂对氧化锌矿进行浮选已在选矿实践中应用并取得较好效果(见表4),相比于NaHS而言,Na2S价格便宜,作为pH值调整剂时能调节pH值在较高(10.5~12.0)范围 内[51-52],而这一pH值范围也适合氧化锌矿的浮选[53]。硫化-胺法中,过量Na2S的使用并不会对硅酸锌、异极矿的可浮性造成影响[21],但过量S2-会对菱锌矿的浮选造成抑制[3, 54-55]。
表2 —NH2基团中N、H原子与异极矿表面原子间距及成键情况[47-48]
Table 2 Atomic spacing and bonding situation between N and H atoms of —NH2 groups and surface atom of hemimorphite[47-48]
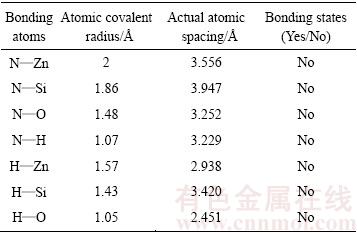
表3 利用BET测定的硅酸锌、异极矿相关物理性质[50]
Table 3 Physical properties of willemite and hemimorphite measured by BET[50]

表4 硫化-胺法在不同研究项目中的应用比较
Table 4 Comparison between different research projects by sulfidizing–amine flotation method

3.4 螯合剂法
螯合捕收剂因选择性高而受到人们的重视。它一般由多个有机大分子构成,并能通过两个或多个官能团与金属离子形成一个或多个圆环[22]。该类药剂具有较高的稳定性和选择性,这主要取决于其电子分布及空间结构。每一螯合剂至少有一官能团能将电子对贡献给与之成键的金属离子,这就使得两者除共价键外,还有配位键的作用。螯合剂中的活性原子被称为给予体或配位体原子,如O、N、P、S原子等[46]。
在浮选异极矿工艺中,胺类捕收剂相比于硫醇类捕收剂而言回收率高,但也仅在50%~60%[59],且药剂消耗量大,在生产实际中还存在泡沫难消等问题。为此,人们研究过将有机螯合剂用于异极矿的浮选,如二甲酚橙、水杨醛肟、邻氨基苯甲酸、α-安息香肟等系列活化剂。螯合剂与异极矿的活化机理(以二甲酚橙为例),是由于二甲酚橙与异极矿表面锌离子螯合后,锌离子微溶解发生相转移,矿物表面活性锌离子浓度增加(以ZnH2L2-形式存在),进而促进了胺离子在矿物表面的疏水性多层吸附[60]。不同活化剂条件下,异极矿回收率与pH值间变化关系的研究结果(见图6)[61]表明,有机螯合剂在pH值=8~9的范围内就能使异极矿的回收率达80%以上,水杨醛肟、邻氨基苯甲酸、α-安息香肟等在异极矿表面的初步疏水性活化作用,既有对矿物表面的活化,也有对捕收剂吸附性能的活化[62],与Na2S作活化剂相比,不仅所需矿浆pH值低,且回收率指标高。冷娥[63]通过混合矿试验考察了异极矿在上述活化剂条件下与石英、方解石等的分离状况。结果表明,在脱除石英后,以水杨醛肟活化异极矿、木素磺酸钙抑制方解石,能使异极矿品位从12.45%上升至29.45%,回收率达72.34%,远高于无螯合剂活化的指标,说明螯合剂浮选异极矿是可行有效的。但是,目前螯合捕收剂售价较高,且单独用作捕收剂时难以矿物表面造成足够的疏水性,所需用量较大,因此目前广泛应用于生产实践中的难度较大。
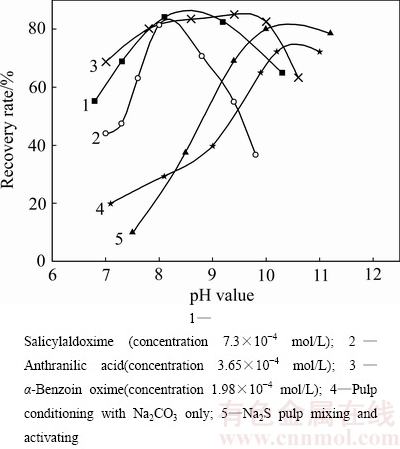
图6 不同活化剂条件下,异极矿回收率与pH值的关系[61]
Fig. 6 Relationships between recovery rate of hemimorphite and pH value under different activators condition[61]
4 结论
1) 异极矿的形成规律主要是含二氧化硅的水溶液与锌的重碳酸盐溶液作用的结果,而不是与锌的硫酸盐溶液相互作用的结果。
2) 早期形成的异极矿表面易受粘土矿物的浸染,后期形成的异极矿会沿着风化裂隙壁呈脉状充填,其表面受污染程度较小。
3) 由于异极矿的形成过程通常伴有大量的褐铁矿,为使受污染的异极矿与脉石矿石有效分离,浮选前须用水玻璃与稀硫酸消除铁质对异极矿浮选过程的影响。
4) 晶体结构中,异极矿一般断裂的是Zn—O键,与Zn2+相比,SiO42-的体积更大,在与药剂发生作用时,矿物表面的Zn2+往往被SiO42-屏蔽,起硫化作用的离子很难与Zn2-结合,故硫化时间较长。
5) 异极矿浮选工艺及与药剂作用机理表明:硫化-黄药法、脂肪酸法、硫化-胺法虽在生产实际中有一定应用,但均有不足之处,而采用螯合剂活化-胺类捕收剂浮选无论在捕收能力还是在活化效果方面均优于前者,螯合剂法有望为异极矿的浮选提供新的方向,但由于药剂成本过高,其使用受到一定程度的限制。
REFERENCES
[1] 孙 毅. 氧化锌矿高附加值绿色化综合利用的研究[D]. 东北大学, 2014: 1-5.
SUN Yi. Study on high value-added and green utilization of zinc oxide ore[D]. Northeastern University, 2014: 1-5.
[2] SUZETTE M. Mineral commodity summaries[M]. Reston, Virginia. Geological Survey, 2016: 192-193.
[3] IRANNAJAD M, EJTEMAEI M, GHARABAGHI M. The effect of reagents on selective flotation of smithsonite- calcite-quartz[J]. Minerals Engineering, 2009, 22(9/10): 766-771.
[4] 马广鹏, 韩建儒, 仪修杰, 等. 氧化锌单晶的水热生长与结晶习性[J]. 人工晶体学报, 2005, 34(5): 772-777.
MA Guang-peng, HAN Jian-ru, YI Xiu-jie, YANG Chang-hong, JIANG Fu-yi, GU Sheng-li, YANG Dong-mei, CHEN Huan-chu. Hydrothermal growth and crystalline morphology of bulk ZnO single crystal[J]. Journal of Synthetic Crystals, 2005, 34(5): 772-777.
[5] 幸伟中. 矿物的可浮性及其分类[J]. 金属学报, 1965(2): 259-269.
XING Wei-zhong. The floatability of minerals and its classification[J]. Acta Metallurgica Sinica, 1965(2): 259-269.
[6] 赵翠华, 陈建华, 吴伯增, 等. 硫化矿物表面天然疏水性的密度泛函理论研究(英文)[J]. 中国有色金属学报(英文版), 2014(2): 491-498.
ZHAO Cui-hua, CHEN Jian-hua, WU Bo-zeng, LONG Xian-hao. Density functional theory study on natural hydrophobicity of sulfide surfaces[J]. Transactions of Nonferrous Metals Society of China, 2014(2): 491-498.
[7] 龚明光. 矿物可浮性分类与软硬酸碱原则[J]. 有色金属(选矿部分), 1980(2): 51-57.
GONG Ming-guang. Floatability clasification and soft-hard base-acid principle of minerals[J]. Nonferrous Metals (Mineral Processing Section), 1980(2): 51-57.
[8] 印万忠, 孙传尧. 矿物晶体结构与表面特性和可浮性关系的研究[J]. 国外金属矿选矿, 1998(4): 8-11.
YIN Wan-zhong, SUN Chuan-yao. Research on the relationships between crystal structure and surface properties and floatability of minerals[J]. Metallic Ore Dressing Abroad, 1998(4): 8-11.
[9] 王 濮, 潘兆橹, 翁玲宝. 系统矿物学(中册)[M]. 北京: 地质出版社, 1984: 243-244.
WANG Pu, PAN Zhao-lu, WENG Ling-bao. Systematic mineralogy(Vol.2)[M]. Beijing: Geological Publishing House, 1984: 243-244.
[10] 黄典豪. 云南乐红铅锌矿床氧化带中异极矿的矿物学特征及其意义[J]. 岩石矿物学杂志, 2000, 19(4): 349-354.
HUANG Dian-hao. Mineralogical character of hemimorphite from the oxidized zone of the lehong Pb-Zn deposit in Yunnan Province and its significance[J]. Acta Petrologica et Mineralogica, 2000, 19(4): 349-354.
[11] 周国平. 宝石学[M]. 武汉: 中国地质大学出版社, 1986: 390-398.
ZHOU Guo-ping. Gemmology[M]. Wuhan: China University of Geosciences Press, 1986: 390-398.
[12] 刘 琰, 邓 军, 杨立强, 王庆飞,周应华, 高帮飞. 表生异极矿成因研究及其找矿意义[J]. 矿物岩石, 2005, 25(2): 1-6.
LIU Yan, DENG Jun, WANG Li-qiang, WANG Qing-fei, ZHOU Ying-hua, GAO Bang-fei. Crystal chemistry and color genesis of the hemimorphite from Jinding Pb-Zn Deposit, Yunnan Province[J]. Geological Journal of China Universities, 2005, 25(2): 1-6.
[13] 谢尔盖·谢尔盖耶维奇·斯米尔诺夫. 硫化物矿床氧化带的研究[M]. 陈 良, 译. 北京: 地质出版社, 1956: 32.
SERGEI S S. Research on oxidation zone of sulfide deposits[M]. CHEN Liang, transl. Beijing: Geological Publishing House, 1956: 32.
[14] 张祥峰. 异极矿浮选理论与工艺研究[D]. 中南大学, 2012: 3-8.
ZHANG Xiang-feng. Study on theory and process of floatation of hemimorphite[D]. Central South University, 2012: 3-8.
[15] 温蔚龙, 张 正, 焦宝忠. 被褐铁矿严重污染的异极矿浮选富集[J]. 金属材料与冶金工程, 1982(4): 22-26.
WEN Wei-long, ZHANG Zheng, JIAO Bao-zhong. Flotation and enrichment of hemimorphite seriously polluted by limonite[J]. Metal Materials and Metallurgy Engineering, 1982(4): 22-26.
[16] 陈 晔. 阳离子胺类捕收剂浮选异极矿氧化锌及其作用机理研究[D]. 广西大学, 2006: 10-12.
CHEN Ye. The study of flotation of hemimorphite zinc oxide by using cation amine collector and it’smechanism[D]. Guangxi University, 2006: 10-12.
[17] EVANS R C. An introduction to crystal chemistry[M]. Cambridge: Cambridge University Press, 1964: 338-350.
[18] HILL R J, GIBIIS G V, CRAIG J R, ROSS F K, WILLIAMS J M. A neutron-diffraction study of hemimorphite[J]. Zeitschrift Fur Kristallographie, 1977, 146(4/6): 241-259.
[19] 刘 琰, 邓 军, 杨立强, 王庆飞. 异极矿加热过程的研究[J]. 岩石学报, 2005, 21(3): 993-998.
LIU Yan, DENG Jun, YANG Li-qiang, WANG Qing-fei. The dehydration of hemimorphite[J]. Acta Petrologica Sinica, 2005, 21(3): 993-998.
[20] 文书明, 张文彬, 刘邦瑞. 无机硫代类活化剂对异极矿胺法浮选的影响[J]. 昆明理工大学学报(自然科学版), 1994(5): 112-114.
WEN Shu-ming, ZHANG Wen-bin, LIU Bang-rui. The effects of inorganic thiol activators on amine flotation of hemimorphite[J]. Journal of Kunming University of Science and Technology (Natural Science Edition), 1994(5): 112-114.
[21] SALUM M J G, ARAUJO A C D, PERES A E C. The role of sodium sulphide in amine flotation of silicate zinc minerals[J]. Minerals Engineering, 1992, 5(3/5): 411-419.
[22] EJTEMAEI M, GHARABAGHI M, IRANNAJAD M. A review of zinc oxide mineral beneficiation using flotation method[J]. Advances in Colloid & Interface Science, 2014, 206(2): 68-78.
[23] 石道民, 杨 敖. 氧化铅锌矿的浮选[M]. 昆明: 云南科技出版社, 1996: 28-35, 53.
SHI Dao-min, YANG Ao. Flotation of lead-zinc oxide ore[M]. Kunming: Yunnan Science & Technology Press, 1996: 28-35, 53.
[24] JANUSZ W. Flotation of synthetic zinc carbonate using potassium ethylxanthate[J]. Int J Miner Process, 1983, 2(11): 79-88.
[25] 李端人. 会泽铅锌矿脉矿选矿工艺研究[J]. 云南冶金, 1984(1): 35-39.
LI Duan-ren. Study on mineral processing technologies of lead-zinc ore deposit in Huize[J]. Yunnan Metallurgy, 1984(1): 35-39.
[26] STACHURSKI J, SANAK S. Use of xanthates for flotation separation of oxidized sulfide ores of zinc and tin subjected to prior thermochemical treatment[J]. Archiwum Gornictwa, 1985, 30(1): 107-117.
[27] 陈锦全, 周德炎, 魏宗武, 陈建华. 高铁泥化氧化铅锌矿的浮选试验研究[J]. 矿业研究与开发, 2007, 27(5): 50-51.
CHEN Jin-quan, ZHOU De-yan, WEI Zong-wu, CHEN Jian-hua. Experimental study on flotation of a mudded lead-zinc oxide ore with high iron content[J]. Mining Research and Development, 2007, 27(5): 50-51.
[28] 王 资. 氧化锌矿浮选研究现状[J]. 昆明冶金高等专科学校学报, 1997(3): 20-26.
WANG Zi. Existing state of flotation study of zinc oxide[J]. Journal of Kunming Technical College of Metallurgy, 1997(3): 20-26.
[29] FAN X, ROWSON N A. The effect of Pb(NO3)2 on ilmenite flotation[J]. Minerals Engineering, 2000, 13(2): 205-215.
[30] FUERSTENAU D W, PRADIP. Zeta potentials in the flotation of oxide and silicate minerals[J]. Advances in Colloid & Interface Science, 2005, 114/115(114/115): 9-6.
[31] 刘 诚, 冯其明, 张国范. 油酸钠作用下异极矿的浮选行为及作用机理[J]. 中国有色金属学报, 2016, 26(4): 878-883.
LIU Cheng, FENG Qi-ming, ZHANG Guo-fan. Flotation behaviors and mechanism of hemimorphite using sodium oleate as collector[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 878-883.
[32] YIN Xi-hui, GUPTA V, DU Hao, WANG Xu-ming, MILLER J D. Surface charge and wetting characteristics of layered silicate minerals[J]. Advances in Colloid & Interface Science, 2012, 179/182(13): 43-50.
[33] RAO K H, ANTTI B M, FORSSBERG E. Mechanism of oleate interaction on salt-type minerals, Part Ⅱ. Adsorption and electrokinetic studies of apatite in the presence of sodium oleate and sodium metasilicate[J]. International Journal of Mineral Processing, 1990, 28(1/2): 59-79.
[34] HOSSEINI S H, FORSSBERG E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid[J]. Minerals & Metallurgical Processing, 2006, 23(2): 87-96.
[35] LIU Cheng, FENG Qi-ming, ZHANG Guo-fan. Electrokinetic and flotation behaviors of hemimorphite in the presence of sodium oleate[J]. Minerals Engineering, 2015, 84: 74-76.
[36] MEHDILO A, IRANNAJAD M, REZAI B. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 2013, 428(3): 761-767.
[37] 王云燕, 柴立元, 王庆伟, 舒余德. 重金属离子(Zn2+,Cu2+,Cd2+,Pb2+)-水系羟合配离子配位平衡研究[J]. 中国有色金属学报, 2008, 18(s1): 183-191.
WANG Yun-yan, CHAI Li-yuan, WANG Qing-wei, SHU Yu-de. The rmodynamic equilibrium of hydroxyl complex ions in heavy metals (Zn2+,Cu2+,Cd2+,Pb2+)·H2O system[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 183-191.
[38] POKROVSKY O S, SCHOTT J. Surface chemistry and dissolution kinetics of divalent metal carbonates[J]. Environmental Science & Technology, 2002, 36(3): 426-32.
[39] HOSSEINI S H, FORSSBERG E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid[J]. Minerals & Metallurgical Processing, 2006, 23(2): 87-96.
[40] LIU Cheng, FENG Qi-ming, ZHANG Guo-fan, MA Wan-kun, MENG Qing-you, CHEN Yan-fei. Effects of lead ions on the flotation of hemimorphite using sodium oleate[J]. Minerals Engineering, 2016, 89: 163-167.
[41] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 32-41.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Hunan Science and Technology Press, 1988: 32-41.
[42] SOMASUNDARAN P, NAGARAJ D R. Chemistry and applications of chelating agents in flotation and flocculation[J]. , 1984: 209-219.
[43] 李明晓, 刘殿文, 张文彬. 氧化锌矿处理方法的研究现状[J]. 矿山机械, 2010(22): 7-13.
LI Ming-xiao, LIU Dian-wen, ZHANG Wen-bin. Research on methods of processing zinc oxide ore[J]. Mining & Processing Equipment, 2010(22): 7-13.
[44] MCKENNA W J, VIRGIL L, PETERSON E C. Froth flotation of oxidized zinc ores: US2482859[P]. 1949-09-27: 209-166.
[45] WILLS B A. Mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery[M]. Pergamon Press, 1988: 30(4): 651-659.
[46] SOMASUNDARAN P, WANG Dian-zuo. Solution chemistry: Minerals and reagents[M]. Elsevier, 2006: 32-36.
[47] 杨进元. 键长与极性的关系[J]. 科学通报, 1979, 24(9): 395-399.
YANG Jin-yuan. Relationships between bond length and polarity[J]. Chinese Science Bulletin, 1979, 24(9): 395-399.
[48] 徐永群. 计算共价键长的经验公式[J]. 黄冈师范学院学报, 1997(1): 65-67.
XU Yong-qun. Empirical formulas for calculating covalent bond length[J]. Journal of Huanggang Teachers College (Natural Science Edition), 1997(1): 65-67.
[49] LI Kang-kang, ZHANG Xiao-lin, CAO Shi-ming, LIU Dian-wen. The mechanism research of collector and hemimorphite[J]. Advanced Materials Research, 2014, 1073/1076: 2163-2167.
[50] C·A·佩雷拉, 杨辉亚, 林 森. 浮选含异极矿的锌矿石的药 剂[J]. 国外金属矿选矿, 2005, 42(5): 18-19.
PARREIRA A C, YANG Hui-ya, LIN Sen. Reagents for flotation of zinc ore with hemimorphite[J]. Metallic Ore Dressing Abroad, 2005, 42(5): 18-19.
[51] FUERSTENAU M C, MILLER J D, KUHN M C. Chemistry of flotation[M]. New York: Metallurgical, 1985: 105-115.
[52] RAUSCH R D, MARIACHER B C. Mining and concentrating of lead and zinc[R]. American Institute of Mining, Metallurgical, and Petroleum Engineers, 1970: 721-731.
[53] KEQING F A, MILLER J D, TAO Jiang, LI Guang-hui. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores[J]. Transactions of Nonferrous Metals Society of China, 2005, 15(5): 1138-1144.
[54]  G., BULUT G., GüL A., KANGAL O, PEREK K T, ARSLAN F. Flotation of aladag oxide lead-zinc ores[J]. Minerals Engineering, 2005, 18(2): 279-282.
G., BULUT G., GüL A., KANGAL O, PEREK K T, ARSLAN F. Flotation of aladag oxide lead-zinc ores[J]. Minerals Engineering, 2005, 18(2): 279-282.
[55] PEREIRA C A, PERES A E C. Reagents in calamine zinc ores flotation[J]. Minerals Engineering, 2005, 18(2): 275-277.
[56] HOSSEINI H S, TAJI M. Flotation behavior of Iranian oxidized zinc ore using different types of collectors (cationic, anionic and mixed (cationic/anionic)[J]. International Journal of Mining Engineering and Mineral Processing, 2015, 4(1): 18-27.
[57] WET J R D, SINGLETON J D. Development of a viable process for the recovery of zinc from oxide ores[J]. Journal of the Southern African Institute of Mining & Metallurgy, 2008, 108(5): 253-259.
[58] KASHANI A H N, RASHCHI F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents [J]. Minerals Engineering, 2008, 21(12/14): 967-972.
[59] BULATOVIC S M. Handbook of flotation reagents[M]. Amsterdam: Elsevier Science & Technology Books, 2007: 130-149.
[60] 羊依金, 刘邦瑞, 冷 娥. 用二甲酚橙活化异极矿浮选的研究[J]. 云南冶金, 1992(2): 35-38.
YANG Yi-jin, LIU Bang-rui, LENG E. Study on activation of hemimorphite with xylenol orange[J]. Yunnan Metallurgy, 1992(2): 35-38.
[61] 徐晓军. 氧化矿有机螯合剂活化浮选理论[M]. 昆明: 云南科技出版社, 2000: 126-140.
XU Xiao-jun. The theory of oxidized mineral floatation using organic chelating agents as activators[M]. Kunming: Yunnan Science & Technology Press, 2000: 126-140.
[62] 徐晓军, 刘邦瑞. 黄药浮选氧化铜矿物时螯合剂的协同活化作用[J]. 中国有色金属学报, 1995, 5(2): 61-64.
XU Xiao-jun, LIU Bang-rui. Synergistic activation of chelating agents when the xanthate flotation of copper oxide minerals[J]. The Chinese Journal of Nonferrous Metals, 1995, 5(2): 61-64.
[63] 冷 娥, 汪 伦, 刘邦瑞. 应用有机螯合剂活化异极矿的研究[J]. 有色金属工程, 1991(1): 44-49.
LENG E, WANG Lun, LIU Bang-rui. Research on the application of organic chelating agents in the activation of hemimorphite[J]. Nonferrous Metals, 1991(1): 44-49.
Research status of metallogenic regularity of hemimorphite and mechanism of flotation reagents
ZHANG Xiao-lin1, 2, WANG Qi-hong1, 2, JING Man2, RAO Fei3, WU Lu-qing2, LI Kang-kang2, CAO Shi-ming4, LIU Dian-wen1, 2
(1. National Key Laboratory for Clean Application of Complex Non-ferrous Metal Resources, Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Land Resource Engineering, Kunming University of Science and Technology, Kunming 650000, China;
3. School of Foreign Languages, Northwest University, Xi’an, Shaanxi 710069, China;
4. School of Chemical Engineering & Technology, China University of Mining and Technology, Xuzhou 221000, China)
Abstract: Metallogenic regularity and structural characteristics of hemimorphite and action regularity between hemimorphite and flotation reagents were studied. The important factors affecting the floatability of hemimorphite were analyzed in details and interaction rules between hemimorphite and reagents were summarized. The results show that the hemimorphite mix easily with clay minerals in the early stage of mineralization, which leads to the fact that zinc containing mineral losts in the slime when the flotation method is used and the recovery rate of hemimorphite is low. No chemical bonds are formed between N and H atoms in polar group -NH2 of amine collectors and Zn, Si, O, H on the surface of hemimorphite, and the acting way between hemimorphite and collectors is electrostatic adsorption only. The mineral surface of Zn2+ is often shielded by SiO42- because the volume of SiO42- is larger than that of Zn2+. When they act with flotation reagents, the solubility of minerals is reduced and the sulfurication ion such as S2- and HS- are difficult to combine with Zn2+ and more sulfurization time is needed. Chelating agent method is expected to become the high efficient separation method of the flotation of hemimorphite.
Key words: hemimorphite; metallogenic regularity; floatability; mechanism
Foundation item: Project(51264019) supported by the National Natural Science Foundation of China; Project (2016T20070151) supported by 昆明理工大学分析测试基金项目
Received date: 2016-11-29; Accepted date: 2017-04-27
Corresponding author: ZHANG Xiao-lin; Tel: +86-871-5187068; E-mail: xiaolin6001@sina.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51264019);昆明理工大学分析测试基金项目(2016T20070151)
收稿日期:2016-11-29;修订日期:2017-04-27
通信作者:章晓林,副教授,博士;电话:0871-5187068;E-mail: xiaolin6001@sina.com
摘 要:本文主要考察了异极矿的成矿规律、结构特征以及异极矿与浮选药剂之间的作用规律,详细分析了影响异极矿可浮性的重要因素,总结了国内外与异极矿相关的浮选药剂与异极矿之间的作用规律,研究结果表明:在成矿的前期阶段,异极矿易与粘土矿物混杂,采用浮选法进行回收时含锌矿物易损失于矿泥中,从而使得异极矿的浮选回收率较低;胺类捕收剂的极性基团—NH2中N、H原子与异极矿表面的Zn、Si、O、H等原子之间并未形成有效的化学键,捕收剂与异极矿之间的作用方式仅为静电吸附;由于异极矿中SiO4-的体积比Zn2+更大,在与浮选药剂发生作用时,矿物表面的Zn2+往往被SiO42-屏蔽,使得矿物溶解度降低,从而使得S2-、HS-等起硫化作用的离子很难与Zn2+结合,硫化时间增长;螯合剂法有望成为异极矿浮选新的高效选别方法。
[1] 孙 毅. 氧化锌矿高附加值绿色化综合利用的研究[D]. 东北大学, 2014: 1-5.
[2] SUZETTE M. Mineral commodity summaries[M]. Reston, Virginia. Geological Survey, 2016: 192-193.
[4] 马广鹏, 韩建儒, 仪修杰, 等. 氧化锌单晶的水热生长与结晶习性[J]. 人工晶体学报, 2005, 34(5): 772-777.
[5] 幸伟中. 矿物的可浮性及其分类[J]. 金属学报, 1965(2): 259-269.
[6] 赵翠华, 陈建华, 吴伯增, 等. 硫化矿物表面天然疏水性的密度泛函理论研究(英文)[J]. 中国有色金属学报(英文版), 2014(2): 491-498.
[7] 龚明光. 矿物可浮性分类与软硬酸碱原则[J]. 有色金属(选矿部分), 1980(2): 51-57.
[8] 印万忠, 孙传尧. 矿物晶体结构与表面特性和可浮性关系的研究[J]. 国外金属矿选矿, 1998(4): 8-11.
[9] 王 濮, 潘兆橹, 翁玲宝. 系统矿物学(中册)[M]. 北京: 地质出版社, 1984: 243-244.
[10] 黄典豪. 云南乐红铅锌矿床氧化带中异极矿的矿物学特征及其意义[J]. 岩石矿物学杂志, 2000, 19(4): 349-354.
[11] 周国平. 宝石学[M]. 武汉: 中国地质大学出版社, 1986: 390-398.
ZHOU Guo-ping. Gemmology[M]. Wuhan: China University of Geosciences Press, 1986: 390-398.
[12] 刘 琰, 邓 军, 杨立强, 王庆飞,周应华, 高帮飞. 表生异极矿成因研究及其找矿意义[J]. 矿物岩石, 2005, 25(2): 1-6.
[13] 谢尔盖·谢尔盖耶维奇·斯米尔诺夫. 硫化物矿床氧化带的研究[M]. 陈 良, 译. 北京: 地质出版社, 1956: 32.
[14] 张祥峰. 异极矿浮选理论与工艺研究[D]. 中南大学, 2012: 3-8.
[15] 温蔚龙, 张 正, 焦宝忠. 被褐铁矿严重污染的异极矿浮选富集[J]. 金属材料与冶金工程, 1982(4): 22-26.
[16] 陈 晔. 阳离子胺类捕收剂浮选异极矿氧化锌及其作用机理研究[D]. 广西大学, 2006: 10-12.
[19] 刘 琰, 邓 军, 杨立强, 王庆飞. 异极矿加热过程的研究[J]. 岩石学报, 2005, 21(3): 993-998.
[20] 文书明, 张文彬, 刘邦瑞. 无机硫代类活化剂对异极矿胺法浮选的影响[J]. 昆明理工大学学报(自然科学版), 1994(5): 112-114.
[23] 石道民, 杨 敖. 氧化铅锌矿的浮选[M]. 昆明: 云南科技出版社, 1996: 28-35, 53.
[25] 李端人. 会泽铅锌矿脉矿选矿工艺研究[J]. 云南冶金, 1984(1): 35-39.
[27] 陈锦全, 周德炎, 魏宗武, 陈建华. 高铁泥化氧化铅锌矿的浮选试验研究[J]. 矿业研究与开发, 2007, 27(5): 50-51.
[28] 王 资. 氧化锌矿浮选研究现状[J]. 昆明冶金高等专科学校学报, 1997(3): 20-26.
[31] 刘 诚, 冯其明, 张国范. 油酸钠作用下异极矿的浮选行为及作用机理[J]. 中国有色金属学报, 2016, 26(4): 878-883.
[41] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 32-41.
[43] 李明晓, 刘殿文, 张文彬. 氧化锌矿处理方法的研究现状[J]. 矿山机械, 2010(22): 7-13.
[47] 杨进元. 键长与极性的关系[J]. 科学通报, 1979, 24(9): 395-399.
[48] 徐永群. 计算共价键长的经验公式[J]. 黄冈师范学院学报, 1997(1): 65-67.
[50] C·A·佩雷拉, 杨辉亚, 林 森. 浮选含异极矿的锌矿石的药 剂[J]. 国外金属矿选矿, 2005, 42(5): 18-19.
[60] 羊依金, 刘邦瑞, 冷 娥. 用二甲酚橙活化异极矿浮选的研究[J]. 云南冶金, 1992(2): 35-38.
[61] 徐晓军. 氧化矿有机螯合剂活化浮选理论[M]. 昆明: 云南科技出版社, 2000: 126-140.
[62] 徐晓军, 刘邦瑞. 黄药浮选氧化铜矿物时螯合剂的协同活化作用[J]. 中国有色金属学报, 1995, 5(2): 61-64.
[63] 冷 娥, 汪 伦, 刘邦瑞. 应用有机螯合剂活化异极矿的研究[J]. 有色金属工程, 1991(1): 44-49.


