银/聚苯胺纳米复合材料的制备及其表征
李芝华,李珺杰,林伟
(中南大学 材料科学与工程学院,湖南 长沙,410083)
摘 要:
钠(SDS)为乳化剂,正己醇为助乳化剂,紫外-可见光辐照辅助,在反胶束体系中一步双原位合成银/聚苯胺(Ag/PANI)纳米复合材料。通过红外吸收光谱、X线衍射、透射电镜和四探针仪对产物的结构、形貌和性能进行表征分析。此外,还考察不同苯胺与硝酸银的物质的量比(n(An)/n(AgNO3))及水乳比W0对Ag/PANI纳米复合材料的结构、形貌及导电性能的影响。研究结果表明:在SDS反胶束体系中,紫外光可在还原银离子的同时引发苯胺聚合,形成聚苯胺包覆银纳米复合粒子;n(An)/n(AgNO3)及水乳比的增大对复合粒子的粒径有增大的影响;Ag/PANI纳米复合材料电导率较PANI有很大提高,并且随着n(An)/n(AgNO3)的减小而先增大后减小,当n(An)/n(AgNO3)=1/2时,电导率达到最大值50.24 S/cm;随着水乳比的增加而先增大后减小,当W0=22时,电导率达到最大值95.89 S/cm。
关键词:
中图分类号:TB333 文献标志码:A 文章编号:1672-7207(2014)06-1784-06
Preparation and characterization of Ag/polyaniline nanocomposite
LI Zhihua, LI Junjie, LIN Wei
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Silver/polyaniline(Ag/PANI) nanocomposite was successfully prepared within the micro-emulsion system of reverse micelle under ultraviolet irradiation. Sodium dodecyl sulfate (SDS) and n-hexyl alcohol were chosen as emulsifier and assisted surfactants of this micro-emulsion system, respectively. Fourier transform infrared spectrometer (FTIR), X-ray diffraction (XRD), Transmission electron microscope (TEM) and electrochemical tests were used to character the structure, morphology and, conductivity of Ag/PANI nanocomposite, respectively. The effect of the molar ratio of n(An)/n(AgNO3) and the water-oil ratio (W0) on structure, morphology and electrical properties of Ag/PANI nanocomposite was studied. The results show that UV triggers the polymerization of aniline at the same time as the reduction of silver ions, and eventually better forms nano-composite particles with the polyaniline as the shell and the silver nanoparticles as the core within the SDS reverse micelle system. With the increase of n(An)/n(AgNO3) and water/surfactant concentration ratio W0, the diameter of Ag/PANI nanocomposite takes on the tendency of increasing. Compared with pure polyaniline, the conductivity of Ag/PANI nanocomposite is improved significantly. The conductivity of Ag/PANI nanocomposite increases and then decreases, while the mole ratio of n(An)/n(AgNO3) decreases. The conductivity reaches maximum 50.24 S/cm when the ratio is 0.5. Similarly, with the increase of W0, the conductivity of Ag/PANI nanocomposite increases slightly and then decreases. The conductivity reaches maximum 95.89 S/cm when W0is 22.
Key words: PANI; Ag; reversed micelle; micro-emulsion; nanocomposite
廉价易得、合成工艺简单、性能稳定、电化学活性可逆、掺杂性能独特等优点使聚苯胺(PANI)成为最有应用价值的导电高分子之一,并被广泛应用于金属防腐、电磁屏蔽、抗静电、电极材料、生物检测传感器等领域[1-6]。金属纳米粒子具有尺寸效应、表面效应、小尺寸效应、宏观量子隧道效应等纳米效应,将其与聚苯胺复合,既能改善其原有性能,又能利用协同效应赋予材料新的性能,从而拓宽应用领域[7-8]。银纳米粒子作为金属纳米粒子的一种,具有优异的导电性能和热稳定性能、比表面积大、催化活性高等优点,可广泛应用于催化、抗菌、医药生物和电子元件等领域[9-10]:因此,在聚苯胺中引入银纳米粒子可使Ag/PANI复合材料呈现出不同于聚苯胺材料的光、电、磁、生物化学和催化等方面的新特性。目前,国内外有关Ag/PANI复合材料的制备和应用的研究较多。制备方法主要有化学氧化还原法和电化学方法。其中原位还原复合法[11]和紫外光、γ线等辅助还原原位聚合[12-13]方法分别是这2种方法的具体应用。本文作者充分利用反胶束体系的各向同性、热力学稳定性及分散均匀性,在不添加任何氧化剂和还原剂,并在紫外光辐照的条件下,采用一步双原位的制备方法,合成了粒径小、粒度分布均匀和形态可控的Ag/PANI纳米复合粒子,考察了不同苯胺与硝酸银的物质的量比n(An)/n(AgNO3)及水乳比W0对Ag/PANI纳米复合材料的结构与性能的影响。
1 实验
1.1 主要原料及试剂
苯胺,分析纯,上海凌峰化学试剂有限公司生产;硝酸银(AgNO3),分析纯,上海精细化工材料研究所生产;浓硝酸(HNO3),分析纯,株洲石英化玻有限公司生产;十二烷基硫酸钠(SDS),化学纯,广东汕头市西陇化工厂生产;环己烷,分析纯,天津市耀华化学试剂有限公司生产;正己醇,分析纯,国药集团化学试剂有限公司生产;无水乙醇,分析纯,天津市大茂化学试剂厂生产;丙酮,分析纯,湖南省株洲市化学工业研究所生产;甲醇,分析纯,湖南师范大学化学试剂厂生产。
1.2 银/聚苯胺纳米复合材料的制备
量取一定量的苯胺,加入0.5 mol/L硝酸溶液,配制成0.5 mol/L的苯胺/硝酸溶液,加入0.05 mol/L的SDS/正己醇/环己烷溶液(SDS与正己醇的浓度比为1/7),超声分散0.5 h使其形成反胶束溶液A。在0.05 mol/L的SDS/正己醇/环己烷溶液加入一定体积的0.5 mol/L硝酸银溶液,超声分散0.5 h使其形成反胶束溶液B。将反胶束A溶液和反胶束B溶液混合,开启紫光灯,于室温下磁力搅拌进行反应。经紫光灯辐照12 h后加入甲醇破乳,并通过离心分离机进行分离,所得沉淀依次用丙酮、无水乙醇、硝酸溶液和蒸馏水反复洗涤至溶液无色无沫,最后在 60 ℃下真空干燥24 h。
1.3 测试与表征
采用KBr压片法,用美国Nicolet公司AVATAR 360型傅立叶变换红外光谱仪进行红外光谱测试;采用Rigaku D/Max 2550型X线衍射仪进行物相分析;采用Tecnai G220ST型透射电子显微镜(TEM)进行形貌分析;取一定量试样在20 MPa压强下,采用SDJ-30型手动式液压机压成直径约为12 mm的薄圆片,采用SB2331直流数字电阻测试仪对压好的薄圆片进行电导率测试。
2 结果与讨论
2.1 Ag/PANI复合材料的红外光谱分析
图1所示为经HNO3掺杂的PANI和n(An)/n(AgNO3)=1/1时制备的Ag/PANI样品的FT-IR谱。由图1(a)可见:在3 450,1 570,1 480,1 370,1 298,1 129和798 cm-1等处出现了较强吸收峰,分别归属于聚苯胺链中N—H伸缩振动、醌环和苯环的C=C伸缩振动及C—N伸缩振动、苯环上C—H面内弯曲变形振动及C—N面外弯曲振动[14-15]。与图1(a)中PANI的FT-IR谱对比可见:图1(b)中Ag/PANI样品的特征吸收峰发生了蓝移,并且醌环和苯环的C=C键的伸缩振动吸收峰强度都明显增大,这些都表明复合材料中PANI与Ag之间不是简单的混合,而是发生了相互作用。
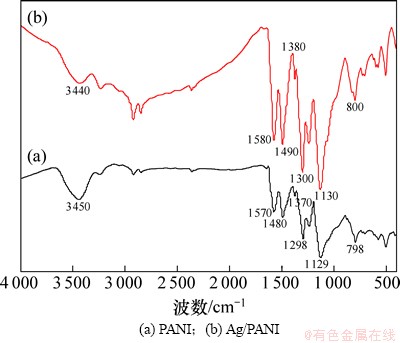
图1 PANI和Ag/PANI样品的FT-IR谱
Fig. 1 FT-IR spectra of PANI and Ag/PANI
图2所示为不同n(An)/n(AgNO3)下制备的Ag/PANI样品的FT-IR谱。由图2可知:不同银含量下制备的Ag/PANI样品,其吸收峰与掺杂态PANI的基本一致,说明复合材料中都有相似的主链结构,都存在掺杂态PANI,但是,其在1 580和1 490 cm-1处分别对应于醌环和苯环的C=C键的伸缩振动2个吸收峰的强度比(Q/B)差别较大;随着n(An)/n(AgNO3)的减小,醌环的C=C键的伸缩振动增强,说明所得复合材料中聚苯胺的氧化单元增加,这与其对Ag/PANI样品电导率的影响基本一致。
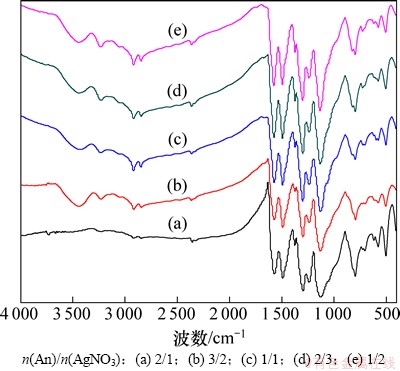
图2 不同n(An)/n(AgNO3)制备的Ag/PANI样品的FT-IR谱
Fig. 2 FT-IR spectra of Ag/PANI nanocomposites prepared at different n(An)/n(AgNO3)
2.2 Ag/PANI复合材料的XRD分析
图3所示为n(An)/n(AgNO3)=2/3,W0=44时,制备的Ag/PANI样品的XRD谱。图中2θ在15°,20°和25°附近出现了较明显的聚合物特征衍射峰,峰强度较弱且峰形较宽。这是因为经HNO3掺杂的聚苯胺分子链的高度取向,使得聚苯胺具有一定程度的结晶[16]。参照标准晶态银卡片(JCPDS NO.4-0783),图中2θ在38.04°,44.24°,64.4°和77.32°出现了4个明显的特征衍射峰,依次对应于银的(111),(200),(220)和(311)面,呈面心立方结构。尖锐的衍射峰的存在,表明制备的复合材料中存在银粒子。根据谢乐(Scherrer)公式估算出每一个面的晶粒尺寸分别为30.5,18.7,26.2和22.5 nm,银纳米粒子的平均粒径为24.4 nm。
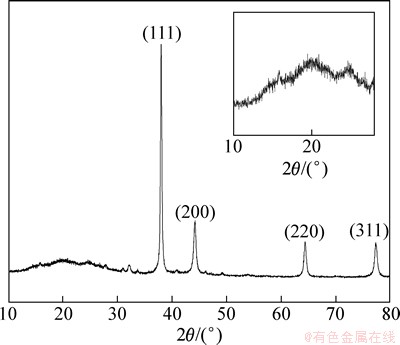
图3 Ag/PANI样品的XRD谱
Fig. 3 XRD pattern of Ag/PANI nanocomposite
2.3 Ag/PANI复合材料的形貌分析
图4所示为水乳比W0=66时,不同n(An)/n(AgNO3)物质的量的比下制备的Ag/PANI样品的TEM像。从图4可以看出:浅色阴影部分为PANI,黑色颗粒为银纳米粒子,银粒子基本嵌于PANI基体中,形成核-壳结构。复合粒子整体呈现颗粒状、类球形状。由于W0较大,制备的复合材料的粒径都较大。当n(An)/n(AgNO3)=2时(图4(a)),银粒子含量较少,且在Ag/PANI中分散不太均匀;当n(An)/n(AgNO3)=1时(图4(b)),银粒子在材料中分散较均匀,且被PANI完全包覆;当n(An)/n(AgNO3)=2/3时(图4(c)),制备的复合材料的粒径最小,且Ag在材料中分散均匀;当n(An)/n(AgNO3)=1/2时(图4(d)),Ag含量较多,出现了一些银纳米粒子的团聚现象。
图5所示为n(An)/n(AgNO3)=2/3时,不同水乳比下制备的Ag/PANI样品的TEM像。从图5可以看出:Ag/PANI样品基本上是核-壳结构,整体形貌为类球形状。随着W0的增大,制备的Ag/PANI样品的粒径增大,且聚苯胺对银纳米粒子的包覆效果、银纳米粒子在聚苯胺中的分散状态也受到一定的影响;当W0=11时,生成的Ag/PANI样品粒径为20 nm,银纳米粒子没有被聚苯胺完全包覆,且还有未被包覆的银粒子存在;当W0=22时,聚苯胺基本上将银粒子包覆其中,复合粒子粒径基本没变;当W0=66时,生成的Ag/PANI样品粒径增大,聚苯胺完全包覆纳米银粒子,纳米银粒子均匀的分散聚苯胺中;当W0=88时,生成的Ag/PANI样品粒径进一步增大,银纳米粒子发生明显的团聚现象。
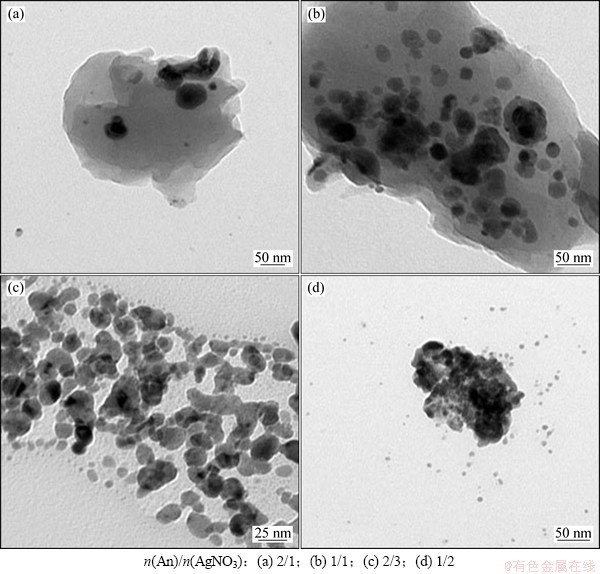
图4 不同n(An)/n(AgNO3)制备的Ag/PANI样品的TEM像
Fig. 4 TEM images of Ag/PANI nanocomposites prepared at different n(An)/n(AgNO3)
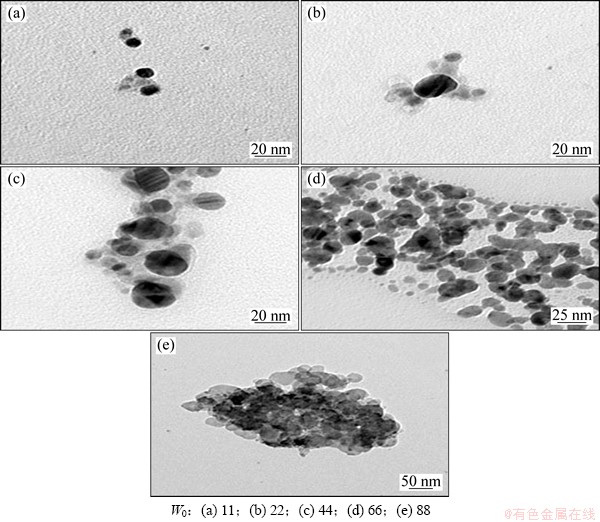
图5 不同水乳比W0制备的Ag/PANI样品的TEM像
Fig. 5 TEM images of Ag/PANI nanocomposites prepared at different water/surfactant concentration ratios
2.4 Ag/PANI复合材料的导电性分析
聚苯胺属于半导体范畴,而Ag为优良导体。因此,Ag/PANI的导电性将会随Ag含量的增加得到大幅度提高。图6所示为水乳比W0=66时,不同n(An)/n(AgNO3)下制备的Ag/PANI的电导率。从图6可以看出,其电导率随着n(An)/n(AgNO3)的减小而先增大后减小。因为复合材料的电导率还受到自身形貌及银粒子在聚苯胺基体中的分散情况的影响,当n(An)/n(AgNO3)较小时,Ag在复合材料中含量较高,银粒子容易出现团聚,且材料的包覆效果差,不利于形成导电通路,致使电导率下降;在n(An)/n(AgNO3)= 1/2时,制备的Ag/PANI样品的电导率达到最大,为50.24 S/cm,是纯聚苯胺(0.04 S/cm)的1 256倍。
利用反胶束为微反应器,胶束体系中的“水池”为反应场所制备Ag/PANI复合材料,可以通过调节水乳比W0,来控制Ag/PANI复合材料的粒径、形态、结构、性能[17-18]。图7所示为n(An)/n(AgNO3)=2/3时,不同水乳比下制备的Ag/PANI样品的电导率,从图7可以看出:随着W0的增大,制备的Ag/PANI样品的电导率先增大后减小,其增大的幅度远小于减小的幅度。因为水乳比的较小时,体系中反胶束尺寸增大而数目变小,胶束发生碰撞的几率下降,造成银纳米粒子没有被聚苯胺完全包覆或未包覆,复合材料中组成分布不均匀,导致电导率下降。W0=22时,所制备的复合材料的电导率最大,为95.89 S/cm,是纯聚苯胺(0.04 S/cm)的2 397倍。
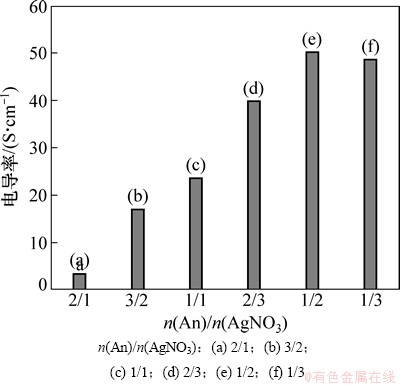
图6 不同n(An)/n(AgNO3)下制备的Ag/PANI样品的电导率
Fig. 6 Electrical conductivity of Ag/PANI nanocomposites prepared at different n(An)/n(AgNO3)
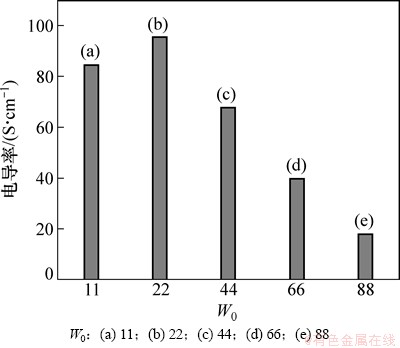
图7 不同水乳比制备的Ag/PANI样品的电导率
Fig. 7 Electrical conductivity of Ag/PANI nanocomposites prepared at different water/surfactant concentration ratios
3 结论
(1) 在反胶束体系中,不添加任何氧化剂和还原剂,利用紫外辅助一步双原位复合法制备了聚苯胺包覆银纳米粒子壳-核结构的Ag/PANI纳米复合材料,得到的纳米复合颗粒的粒径为20~100 nm。纳米复合材料中聚苯胺仍具有一定程度的结晶度,Ag为纳米粒子;PANI与Ag之间不是简单的混合,而是发生了相互作用。随着水乳比的增大,纳米复合颗粒粒径增大,当W0=11,颗粒平均粒径约为20 nm。
(2) 制备的Ag/PANI纳米复合材料电导率较PANI有了很大提高。纳米复合材料的电导率随着反应物n(An)/n(AgNO3)及水乳比的增大逐步增大而后减小。当W0=22和n(An)/n(AgNO3)=2/3时,电导率达到最大,为95.89 S/cm。
参考文献:
[1] Bhadra S, Khastgir D, Singha N K, et al. Progress in preparation, processing and applications of polyaniline[J]. Progress In Polymer Science, 2009, 34(8): 783-810.
[2] Pud A, Ogurtsov N, Korzhenko A, et al. Some aspects of preparation methods and properties of polyaniline blends and composites with organic polymers[J]. Progress In Polymer Science, 2003, 28(12): 1701-1753.
[3] Wessling B, Posdorfer J. Corrosion prevention with an organic metal (polyaniline): Corrosion test results[J]. Electrochimica Acta, 1999, 44(12): 2139-2147.
[4] Garjonyte R, Malinauskas A. Amperometric glucose biosensors based on Prussian Blue-and polyaniline-glucose oxidase modified electrodes[J]. Biosensors & Bioelectronics, 2000, 15(9/10): 445-451.
[5] WANG Hongzhi, ZHANG Peng, ZHANG Weiguo, et al. Electrodeposition and characterization of polyaniline film[J]. Chem Res Chinese Universities, 2012, 28(1): 133-136.
[6] Ma L, Zheng X, Gan, M Y, et al. Synthesis and characterize of polyaniline in magnetic field[J]. Journal of Wuhan University of Technology (Mater Sci Ed), 2008, 23(3): 316-318.
[7] 李新贵, 孙晋, 黄美荣. 聚苯胺/金属纳米粒子复合物的制备及性能[J]. 化学进展, 2007, 19(5): 787-795.
LI Xingui, SUN Jin, HUANG Meirong. Preparation and properties of nanocomposites of polyaniline and metal nanoparticles[J]. Progress In Chemistry, 2007, 19(5): 787-795.
[8] 李宇农, 何建军, 龙小兵. 金属纳米粒子的研究进展[J]. 稀有金属与硬质合金, 2003, 31(4): 47-50.
LI Yunong, HE Jianjun, LONG Xiaobing. The scientific development of metallic nanometer particles[J]. Rare Metals and Cemented Carbides, 2003, 31(4): 47-50.
[9] 张燕, 王强斌. 银纳米粒子的生物医学应用研究进展[J]. 生物物理学报, 2010, 26(8): 673-679.
ZHANG Yan, WANG Qiangbin. Progress of silver nanoparticles in biomedical application[J]. Acta Biophysica Sinica, 2010, 26(8): 673-679.
[10] 张万忠, 乔学亮, 陈建国. 银纳米材料的可控合成研究[J]. 稀有金属材料与工程, 2008, 37(11): 2059-2064.
ZHANG Wanzhong, QIAO Xueliang, CHEN Jianguo. Research progress on the controlled preparation of silver nanomaterials[J]. Rare Metal Materials and Engineering, 2008, 37(11): 2059-2064.
[11] Bober P, Stejskal J, Trchova M et al. Polyaniline-silver composites prepared by the oxidation of aniline with mixed oxidants, silver nitrate and ammonium peroxydisulfate: The control of silver content[J]. Polymer, 2011, 52(26): 5947-5952.
[12] Khanna P K, Singh N, Charan S, et al. Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism[J]. Materials Chemistry and Physics, 2005, 92(1): 214-219.
[13] Kang Y O, Choi S H, Gopalan A, et al. Tuning of morphology of Ag nanoparticle in the Ag/polyaniline nanocomposites prepared by γ-ray irradiation[J]. Journal of Non-Crystalline Solids, 2006, 352(6): 463-468.
[14] Arora M, Luthra V, Singh R, et al. Study of vibrational spectra of polyaniline doped with sulfuric acid and phosphoric acid[J]. Applied Biochemistry and Biotechnology, 2001, 96(1/3): 173-181.
[15] JING Shenyu, XING Shuangxi, YU Lianxiang, et al. Synthesis and characterization of Ag/polyaniline core-shell nanocomposites based on silver nanoparticles colloid[J]. Materials Letters, 2007, 61(13): 2794-2797.
[16] LI Xia, GAO Yu, GONG Jian, et al. Polyaniline/Ag composite nanotubes prepared through UV rays irradiation via fiber template approach and their NH3 gas sensitivity[J]. J Phys Chem C, 2009, 113(1): 69-73.
[17] Sato H, Asaji N, Komasawa I. A population balance approach for particle coagulation in reverse micelles[J]. I & EC Research, 2000, 39(2): 328-334.
[18] Bandyopadhyaya R, Kumar R, Gandhi K S. Simulation of precipitation reaction in reverse micelles[J]. Langmuir, 2000, 16(18): 7139-7149.
(编辑 陈爱华)
收稿日期:2013-07-17;修回日期:2013-09-23
基金项目:省部产学研项目(2009B090300327)
通信作者:李芝华(1963-),男,湖南邵东人,博士,教授,从事高分子材料研究;电话:0731-88830838;E-mail:ligfz@csu.edu.cn
摘要:以十二烷基硫酸钠(SDS)为乳化剂,正己醇为助乳化剂,紫外-可见光辐照辅助,在反胶束体系中一步双原位合成银/聚苯胺(Ag/PANI)纳米复合材料。通过红外吸收光谱、X线衍射、透射电镜和四探针仪对产物的结构、形貌和性能进行表征分析。此外,还考察不同苯胺与硝酸银的物质的量比(n(An)/n(AgNO3))及水乳比W0对Ag/PANI纳米复合材料的结构、形貌及导电性能的影响。研究结果表明:在SDS反胶束体系中,紫外光可在还原银离子的同时引发苯胺聚合,形成聚苯胺包覆银纳米复合粒子;n(An)/n(AgNO3)及水乳比的增大对复合粒子的粒径有增大的影响;Ag/PANI纳米复合材料电导率较PANI有很大提高,并且随着n(An)/n(AgNO3)的减小而先增大后减小,当n(An)/n(AgNO3)=1/2时,电导率达到最大值50.24 S/cm;随着水乳比的增加而先增大后减小,当W0=22时,电导率达到最大值95.89 S/cm。


