J. Cent. South Univ. Technol. (2007)03-0296-05
DOI: 10.1007/s11771-007-0058-4
![]()
Curing mechanism of TDE-85/MeTHPA epoxy resin modified by polyurethane
LI Zhi-hua(李芝华)1, ZHENG Zi-qiao(郑子樵)1, REN Dong-yan(任冬燕)2, HUANG Yao-peng(黄耀鹏)1
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2.Department of Materials Engineering, Mianyang Vocational and Technical College, Mianyang 621000, China)
Abstract:
Diglycidyl 4,5-epoxy tetrahydro phthalate/methyl tetrahydrophthalic anhydride (TDE-85/MeTHPA) epoxy resin modified by polyurethane (PU) was prepared with 1,4-butanediol (1,4-BDO), trimethylol propane (TMP) and polyurethane prepolymer synthesized by polypropylene glycol and toluene diisocynate. Chemical reaction and curing mechanism of this system were discussed by incorporating the results of infra spectrum analysis. The results indicate that the epoxy polymeric networkⅠis obtained by the curing reaction between TDE-85 and MeTHPA, while the PU polymeric network Ⅱ is obtained by the chain-extended and crosslinking reaction between 1,4-BDO, TMP and polyurethane prepolymer(PUP). The graft chemical bonds are formed between polymer networksⅠ and Ⅱ that therefore increase the degree of blend and compatibility between epoxy polymer and PU.
Key words:
polyurethane; epoxy resin; modification; curing mechanism;
1 Introduction
Epoxy resins can offer good properties, such as high mechanical strength, strong adhesion, low shrinkage, nice stability, excellent processability, therefore are widely used in coating, adhesive, electronic product, civil construction, composite material, etc[1-3]. But the brittle and bad impact resistance limit its further application, so the toughness of epoxy resin has to be improved[4-5]. Inter-penetration polymer network (IPN) is formed by more than two kinds of cross-linkable polymers that inter-penetrate or tangle with each other randomly, so it has the action of “compelled mutual solution” and “synergism effect”. At present, IPN is a new unit of polymer family[6-8]. Compared with the properties of the polymer modified by the ordinary methods, the inter-penetration polymer network might further enhance the impact strength, tensile strength and thermal stability[9]. Nowadays, the important method of modification of diglycidyl 4,5-epoxy tetrahydro phthalate /methyl tetrahydrophthalic anhydride (TDE-85/MeTHPA) epoxy resin by polyurethane (PU) is usually designed to form the IPN between PU and TDE-85/MeTHPA epoxy resin in certain conditions, which can improve the tensile strength, thermal stability, and impact strength of this epoxy resin at the same time[10-11].
However, the compositions of TDE-85/MeTHPA epoxy resin modified by PU are various, furthermore, the functional groups of the components may react and affect with each other at certain levels, which makes the mechanism of curing process even more complex. In the published researches, only the reactions between those functional groups, the structural analysis of reactants or final products were roughly reported, no further study about the mechanism of the reactions of TDE-85/MeTHPA epoxy resin modified by PU was made. In this paper, a series of experiments were designed to study the curing mechanism of TDE-85/MeTHPA epoxy resin modified by PU.
2 Experimental
2.1 Materials
Diglycidyl 4,5-epoxy tetrahydro phthalate (TDE-85) was provided by Tianjin Jingdong Chemical Plant. Polypropylene glycol (PPG) was industrical product supplied by Tianjin No.3 Petroleum Chemical Plant. Toluene diisocynate (TDI) was of chemical pure grade and from Shanghai No.1 Reagent Plant. Dibutyltin dilaurate, trimethylol propane (TMP), and 1,4-butanediol (1,4-BDO) with chemical pure grade were provided by Shanghai Reagent Plant. The industrical product of methyl tetrahydrophthalic anhydride (MeTHPA) was supplied by Wenzhou Qingming Chemical Plant. 2,4,6-tris(dimethyl-aminomethyl) phenol (DMP30) was provided by Changsha Chemical Industry Research Institute. All other chemicals used in this study were of chemical pure grade.
2.2 Synthesis of polyurethane prepolymer (PUP)
The PPG was added to a four-neck glass flask, and warmed to 80 ℃ under vacuum for dehydration. A stoichiometric amount of TDI was added slowly at 80 ℃ under stirring, and the reaction was stopped after 2 h. The isocynate capped PUP was obtained and cooled to room temperature.
2.3 Synthesis of TDE-85/MeTHPA resin modified by
PU
Five samples were designed to study the reactions of all the components and curing mechanism of TDE-85/MeTHPA resin modified by PU in the experiments. The formulation of the samples designed is shown in Table 1. The TDE-85 was put into a vacuum oven for drying at 80 ℃ for 1 h, then cooled down to 60 ℃, and mixed with PUP synthesized, 1,4-BDO, TMP, MeTHPA and DMP30 according to the mass shown in Table 1. The mixture was put into the vacuum oven at 80 ℃ for 30 min, then poured to a Teflon board and annealed with a heating procedure (80 ℃, 1 h→100 ℃, 2 h→140 ℃, 10 h).
Table 1 Formulation of samples designed (mass/g)

2.4 FT-IR measurement
FT-IR spectra were recorded on a Nicolet AVATAR360 spectrophotometer. Solid samples were tested with potassium bromide pressed disc method, and liquid sample was tested directly or after diluted with tetrachloromethane.
3 Results and discussion
3.1 Curing reaction between TDE-85 and MeTHPA
The FT-IR spectra of TDE-85 and sample R1 are shown in Fig.1. From the IR spectra of TDE-85, saturated C-H bond stretching vibration peak at about 3 000 cm-1 and carbonyl stretching vibration peak at 1 736 cm-1 can be found. Two peaks of ether bonds C-O-C absorption appear at 1 180 cm-1 nearby, and the one in high-frequency is asymmetric peak, however, the other in low-frequency is symmetric peak. The characteristic peak of epoxy group appears at 908 cm-1. Through the comparison of the IR spectra of TDE-85 and sample R1, the epoxy peak at 908 cm-1 disappears in sample R1, and a new absorption peak appears at 3 629 cm-1, which should be double-frequency of the C==O double bond. During the curing process of sample R1, epoxy polymeric network Ⅰis formed through reaction between TDE-85 and MeTHPA. Combined with the results in Refs.[12-13], a mechanism of the curing reaction of TDE-85 and MeTHPA is proposed as a multi-step reaction shown in Fig.2.
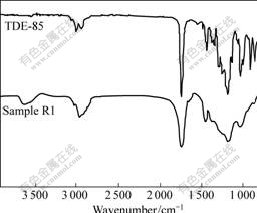
Fig.1 FT-IR spectra of TDE-85 and sample R1
Firstly, carboxylic acid salt anion is formed from the reaction of DMP30 with the anhydride (MeTHPA). Then, a carboxylic acid salt anion attacks the epoxy group in TDE-85, an oxide anion is formed in the step II. Finally, a new carboxylic acid salt anion is produced by the reaction of oxide anion with another molecule of MeTHPA. The polymerization occurs through the repeating of steps II and III alternately.
The FT-IR spectra of TDE-85 and sample R2 are shown in Fig.3. It can be found that the curves of TDE-85 and sample R2 are extremely similar except that sample R2 has an extra O-H stretching vibration peak at 3 535 cm-1. At the sequential curing temperature sample R2 remains in liquid state, which indicates that there is no reaction between TDE-85 and 1,4-BDO. The extra O-H stretching vibration peak is introduced by 1,4-BDO.
3.2 Chain-extended reaction between 1,4-BDO and PUP
The FT-IR spectra of PPG, PUP and sample R3 are shown in Fig.4. The PPG has its characteristic stretching vibration peak of O-H group at 3 474 cm-1. But in the FT-IR spectrum of PUP, the peak of O-H bond disappears, the characteristic peak of isocyanate (-NCO) appears at 2 270 cm-1, and the characteristic peak of
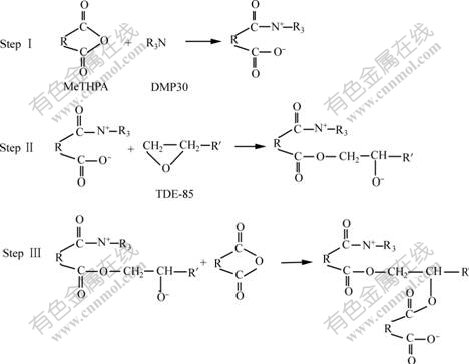
Fig.2 Proposed mechanism of curing reaction
carbonyl in urethane at 1 730 cm-1 and the stretching vibration of N-H at 3 300 cm-1 emerge. This indicates that only one -NCO group in TDI reacts with O-H group in the PPG to form polyurethane prepolymer capped with isocyanate on both ends[14-15].
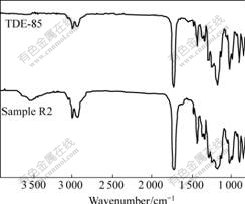
Fig.3 FT-IR spectra of TDE-85 and sample R2
Sample R3 is obtained by mixing the PUP with 1,4-BDO, a chain-extended reagent. The peak of isocyanate at 2 270 cm-1 in the FT-IR spectrum of
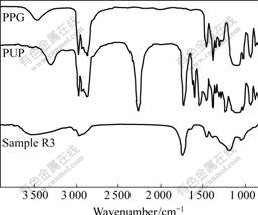
Fig.4 FT-IR spectra of PPG, PUP and sample R3
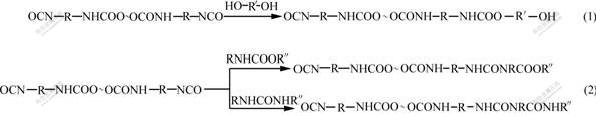
sample R3 disappears, which indicates that the -NCO group reacts with 1,4-BDO. During the curing process of sample R3, PU polymeric network Ⅱis generated by the chain-extended and cross-linked reactions between 1,4-BDO, TMP and PUP. According to Refs.[16-17], the reaction is shown in reaction 1. Meanwhile, a further reaction (reaction 2) might take place, and allophanate and biuret are formed in reaction 2.
3.3 Graft polymerization reaction between TDE-85 and PUP
The FT-IR spectrum of sample R4 is shown in Fig.5. The sample R4 only contains TDE-85 and PUP, but both the characteristic peak of isocyanate in the PUP at 2 270 cm-1 and the characteristic peak of epoxy group at 908 cm-1 in TDE-85 disappear in the FT-IR spectrum of sample R4. The graft chemical bonds are formed between polymer networksⅠ and Ⅱ. Combined with the previous reports[4-5,18], it can be inferred that the TDE-85 reacts with the isocyanate group in the PUP as follows:
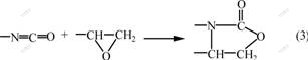
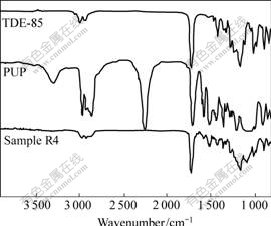
Fig.5 FT-IR spectra of TDE-85, PUP and sample R4
3.4 Curing mechanism of TDE-85/MeTHPA resin modified by PU
The FT-IR spectrum of sample R5(Fig.6) shows that the peak of -NCO group at 2 270 cm-1 and the peak of epoxy group at 908 cm-1 disappear completely. According to the analysis of samples R1, R2, R3 and R4, the following reactions take place during the curing process of TDE-85/MeTHPA resin modified by PU(sample R5): curing reaction between TDE-85 and MeTHPA, chain-extension reaction between 1,4-BDO and PUP, and graft polymerization reaction between TDE-85 and PUP. Epoxy polymeric network Ⅰand PU polymeric network Ⅱare all formed during the curing process of PU-modified TDE-85/MeTHPA epoxy resin. The epoxy polymeric network Ⅰis formed through reaction between TDE-85 and MeTHPA, and PU polymeric network Ⅱ is generated by the chain-extended and cross-linked reactions between 1,4-BDO, TMP and PUP. The graft chemical bonds are formed between polymer networksⅠ and Ⅱ, which can increase the degree of blend and compatibility between epoxy polymer and PU.
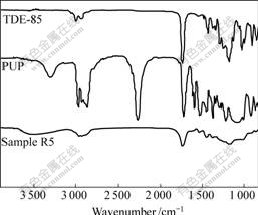
Fig.6 FT-IR spectra of TDE-85, PUP and sample R5
4 Conclusions
1) The polyurethane prepolymer capped with isocyanate group on both ends is obtained through the reaction between PPG and TDI. The epoxy polymeric networkⅠ is obtained by the curing reaction between TDE-85 and MeTHPA, while the PU polymeric network Ⅱ is obtained by the chain-extended and crosslinking reaction of 1,4-BDO, TMP and PUP.
2) The graft chemical bonds are formed between polymer networks Ⅰ and Ⅱ, which can increase the degree of blend and compatibility between epoxy polymer and PU.
References
[1] LI Zhi-hua, XIE Ke-yu, ZHENG Zi-qiao. Morphology and mechanical properties of MG/PU/EP encapsulating composite[J]. Journal of Central South University: Natural and Technology, 2007, 38(1): 51-55. (in Chinese)
[2] ZHANG Chun-hua, HAN Bin, HUANG Yu-ding. Study on properties of TDE-85/aromatic diamine matrix and carbon fiber composites[J]. Journal of Harbin University of Science and Technology, 2000, 5(5): 60-63.
[3] LI Zhi-hua, REN Dong-yan, ZHENG Zi-qiao, et al. Curing reaction of TDE-85/MeTHPA resin modified by polyurethane[J]. Journal of Central South University: Natural and Science, 2007, 38(2): 213-217. (in Chinese)
[4] HSIEH K H, HAN J L, YU C T,et al. Graft interpenetrating polymer networks of urethane-modified bismale-imide and epoxy (I): Mechanical behavior and morphology[J]. Polymer, 2001, 42(6): 2491-2500.
[5] HUA F J, HU C P. Interpenetrating polymer networks of epoxy resin and urethane acrylate resin(1): Kinetics of network formation[J]. European Polymer Journal, 1999, 35(1): 103-112.
[6] YEGANEH H, LAKOURAJ M M, JAMSHIDI S, et al. Synthesis and properties of biodegradable elastomeric epoxy modified polyurethanes based on poly(ε-caprolactone) and poly(ethylene glycol)[J]. European Polymer Journal, 2005, 41(10): 2370-2379.
[7] CHEN Li,CHEN Su. Latex interpenetrating networks based on polyurethane polyacrylate and epoxy resin[J]. Progress in Organic Coating, 2004, 49(3): 252-258.
[8] ANAND PRABU A, ALAGAR M. Mechanical and thermal studies of intercross-linked networks based on siliconized polyurethane-epoxy/unsaturated polyester coatings[J]. Progress in Organic Coating, 2004, 49(3): 236-243.
[9] SHI Lin-yi, ZHANG Jian-ping. Preparation and tensile property of PU/EP graft-IPNs[J]. Journal of Shanghai University: Natural Science, 1998, 4(6): 36-47. (in Chinese)
[10] XIE Hong-quan, GUO Jun-shi. Room temperature synthesis and mechanical properties of two kinds of elastomeric interpenetrating polymer networks based on castor oil[J]. European Polymer Journal, 2002, 38(11): 2270-2277.
[11] WIDMAIER J M, NILLY A, CHEMAL J M, et al. Dependence of the phase separation process on the relative onset of network formation in simultaneous interpenetrating polymer networks[J]. Polymer, 2005, 46(10): 3318-3322.
[12] HORICHKO E Y, KUKSIN A M, HORICHKO V V, et al. Kinetics of the step-growth polymerization of epoxide in the presence of the linear polyurethane: Effect of the phase separation of components[J]. Reactive and Functional Polymers, 1997, 33(2/3): 351-357.
[13] HE Shang-jin, SHI Ke-yu, BAI Jie, et al. Studies on the properties of epoxy resins modified with chain -extended ureas[J]. Polymer, 2001, 42(23): 9641-9647.
[14] ROSU L, CASCAYAL C N, CIOBANU C. Effect of UV radiation on the semi-interpenetrating polymer networks based on polyurethane and epoxy maleate of bisphenol A[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2005, 169(2): 177-185.
[15] YANG Ya-hui, FU Wang-li. Study on epoxy resin toughened and reinforced by polyurethane[J]. Thermosetting Resin, 2001,16(1): 5-8. (in Chinese)
[16] RAGOSTA G, MUSTO P, SCARINZI G, et al. Effects of perfluoroether concentration and curing protocol on morphology and mechanical properties of toughened TGDDM/MNA resin systems[J]. Polymer, 2003, 44(7): 2081-2090.
[17] WEN Qing-zhen. The influence of chain extenders on the reaction rate of polyether-urethane prepolymers[J]. Journal of Naval University of Engineering, 2003, 15(1): 23-26. (in Chinese)
[18] LIN Mu-shi, WANG Ming-wei, CHENG Long-an. Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-A diacrylate[J]. Polymer Degradation and Stability, 1999, 66(3): 343-347.
(Edited by CHEN Wei-ping)
Foundation item: Project(2003AA84ts04) supported by the National High-Tech Research and Development Program of China
Received date: 2006-08-24; Accepted date: 2006-10-27
Corresponding author: LI Zhi-hua, PhD; Tel: +86-731-8830838; E-mail: ligfz@mail.csu.edu.cn
- Curing mechanism of TDE-85/MeTHPA epoxy resin modified by polyurethane


