Article ID: 1003-6326(2005)02-0300-06
Effect of multi-walled carbon nanotubes on
tribological properties of lubricant
CHEN Chuan-sheng(陈传盛), CHEN Xiao-hua(陈小华), HU Jing(胡 静),
ZHANG Hua(张 华), LI Wen-hua(李文华), XU Long-shan(许龙山), YANG Zhi(杨 植)
(College of Materials Science and Engineering, Hunan University, Changsha 410082, China)
Abstract:
After purified by mixture of sulfuric acid and nitric acid, the multi-walled carbon nanotubes(MWNTs) were modified with stearic acid(SA). The modified carbon nanotubes as lubricant additive were utilized to prepare lubricant, and the effects of carbon nanotubes on the tribological properties were investigated by using a pin-on-plate wear tester. The surface structure of MWNTs was examined by transmission electron microscopy, Raman spectroscopy and infrared spectroscopy. The results show that the surfaces of MWNTs are coated with a modified layer of SA. Furthermore, the modified MWNTs as lubricant additive can effectively improve the friction-reduction and anti-wear ability of lubricant. The friction coefficient of base lubricant decreases by about 10% and the wear loss of base lubricant decreases by 30%-40% when the concentration of modified MWNTs in lubricant is 0.45%. In addition, the mass ratio of SA to MWNTs influences the friction-reduction and anti-wear ability of the modified MWNTs as lubricant additive. The optimum mass ratio of MWNTs to stearic acid is about 3∶8-1∶2.
Key words:
carbon nanotube; modification; lubricant additive; tribological property CLC number: TG172.63;
Document code: A
1 INTRODUCTION
Since the discovery of carbon nanotubes(CNTs) in 1991[1], the CNTs have attracted a great deal of attention because of their unique structural, electronical, and mechanical properties. Many potential applications of CNTs, such as nanodevices, quantum wires, ultrahigh-strength engineering fibers, sensors, and catalyst supports[2-6], have been found. CNTs as reinforcing elements can improve the properties of metal base composite significantly[7-10], but it is well known that the CNTs are chemically inert, and the use of CNTs as reinforcing elements will suffer from poor dispersion capability and weak interfacial interactions. Therefore, activating and modifying CNTs are an essential prerequisite for improving the activity of CNTs and expanding their application areas. Currently, chemical modification of CNTs is an efficient means for modifying the chemical properties, physical properties and the wettability of CNTs. For example, surfactants and polymers have improved the dispersions of CNTs in solution[11-13]. Organic molecules like dyes, proteins or nucleic acids may be coupled with functionalized CNTs for sensor application[14]. Certain proteins and DNA have been demonstrated to modify the multi-walled carbon nanotubes(MWNTs)[15].
In addition, chemical modified CNTs provide an attractive method to improve the properties of CNTs composites. Hernadi et al[16] reported that the wettability of CNTs in composite material was improved by a proper inorganic coating. Gojng et al[17] used multi-functional amines to modify MWNTs, and then the functionalized nanotubes were embedded in the epoxy resin. Their results indicate that the functionalization leads to a reduced agglomeration and an improved interfacial interaction between the CNTs and the epoxy resin. In spite of various attempts of researchers, using CNTs as reinforcing additives, there are three major obstacles to overcome, such as, the poor solubility of CNTs in solvent, the wettability of CNTs surface and the load transfer from the matrix to CNTs. Hence, further development in the modification of CNTs is still required.
The stearic acid(SA), which is one of the most important lubricant and dispersant, was proposed as good candidate for practical applications. In this paper, a simple approach to modify MWNTs with SA through esterification was reported and the tribological properties of modified MWNTs as lubricant additive were investigated. Moreover, the variations of friction coefficient and wear loss with the mass ratio of SA to MWNTs were investigated.
2 EXPERIMENTAL
2.1 Preparation and purification of multi-walled carbon nanotube
The MWNTs used in this study were prepared by the chemical catalytic vapor deposition process(CCVD). The details of the MWNTs preparation have been discussed in Ref.[18]. MWNTs growth was obtained on the catalyst at 725℃ with a flow rate of acetylene 50mL/min and nitrogen 300mL/min for 30min. As-prepared products were purified by immersing in a mixture of concentrated HNO3 and HCl(V(HNO3)∶V(HCl)=1∶5) and refluxed for 2h. After filtered and washed with de-ionized water, the products were dried at 120℃. The purified MWNTs were mechanically milled for 20h with a planetary ball mill under nitrogen protection at a rotating speed of 257r/min. The mass ratio of steel ball to purified MWNTs is 50∶1. In order to remove the impurity in MWNTs and generate oxygenated functional groups, the ball-milled MWNTs were suspended in a mixture of concentrated H2SO4 and HNO3(V(H2SO4)∶V(HNO3)=3∶1) and refluxed for 0.5h, then filtered and washed with de-ionized water again, finally dried at 120℃. The MWNTs samples were characterized by transmission electron microscopy(H800, TEM), Raman spectroscopy(Jobin Yvon, Labran-010, maded in France) and infrared spectroscopy(300E Jasco spectrometer).
2.2 Modification of MWNTs
The preparation of modified MWNTs with stearic acid is as follows: firstly, the purified MWNTs were added into distilled water, and the mixture of MWNTs was ultrasoniced to disperse the MWNTs; secondly, the SA was added into the suspension of the MWNTs; thirdly, 10mL sulfuric acid(2mol/L) was dropped into the suspension of MWNTs, stirred and refluxed under 100℃ for 2h, the reaction mixture was cooled to ambient temperature and extracted with chloroform, then precipitated into hexane; finally, this suspension was filtered using a membrane with 0.1μm pore size and dried at 60℃. The resultant MWNTs was examined by TEM, Raman spectroscopy and infrared spectroscopy.
2.3 Preparation of composite lubricant
For the preparation of various lubricant, the modified MWNTs were dispersed in lubricant through sonication and stirring of magnetic force for 1h under 80℃(rotate speed is 500r/min). For comparison, the lubricants of graphite were prepared under the same condition. The mass fraction of graphite is 0.15% in lubricant.
2.4 Measurement of tribological properties
The friction and wear tests were conducted on a MPX-2000 model ring-on-plate wear-testing machine (made in Xinhua Hebei Tester Factory of China). A bearing steel plates used in the test were bearing steel of 34mm in diameter and 10mm in height with a hardness of HRC60, coupling with a bearing steel ring which inner diameter is 20mm, outer diameter is 32mm and hardness is HRC60. The steel plates were fixed in lubricant bath and the bearing steel ring rotated over the plate. All wear experiments were conducted at room temperature and rotating rate of 384r/min. The test duration was 100min. The wear tests were evaluated according to the mass loss of the bearing steel plate after wear testing by an analytical electron balance (precision 10-6kg). Before measuring, all plates were cleaned with alcohol in an ultrasonic bath for 10min, and then dried at room temperature in air. The friction coefficients were measured simultaneously during the process of wear test.
3 RESULTS AND DISCUSSION
3.1 Morphology of carbon nanotubes
Fig.1 shows the morphology of CNTs. A low magnification TEM image(Fig.1(a)) shows that the raw materials contain CNTs and non-nanotubes materials. After purified by acid, the metal particles, the oxide particles and nano-nanotubes carbon materials have been eliminated completely, and the high purity MWNTs have been obtained without damage. The MWNTs made by CVD have central hollow tubes; the outer diameter of most of the MWNTs ranges from 20nm to 40nm, as shown in Fig.1(b). In addition, it can also be seen form Fig.1(b) that the MWNTs are not only curved after purification, but also entangled into large mass. So, in order to obtain short and straight MWNTs, ball milling should be performed. TEM image of the ball-milled MWNTs for 20h is shown in Fig.1(c). It can be seen that the average length of the MWNTs after ball milling is reduced obviously. Moreover, all the MWNTs are distributed in the form of isolated tube. Fig.1(d) shows the TEM microstructure of MWNTs after modified. From this figure it can be seen that the SA appears as aggregates on the surfaces of the MWNTs. The result indicates that the MWNTs are coated by stearic acid.
3.2 Spectroscopy of MWNTS
The spectrum analyses of MWNTs are shown in Fig.2. The FTIR spectrum of the acid-treated

Fig.1 Microsturctures of CNTs
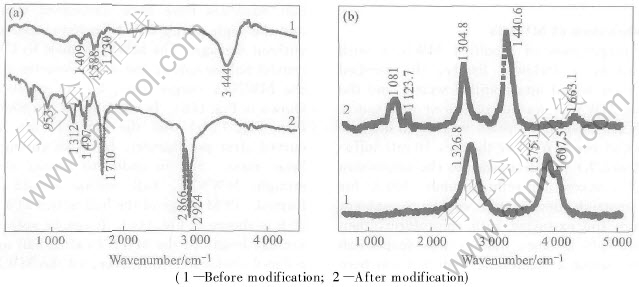
Fig.2 Infrared spectrums(a) and Raman spectrums(b) of CNTS
MWNTs shows the presence of many groups on the surface of the acid-treated MWNTs, such as carbonyl groups reveal at about 1730cm-1, oxygen-hydrogen bonds and C—C bonds at about 3444cm-1 and 1588cm-1, respectively(as shown in the curve 1 of Fig.2(a)). After modified with SA, the characteristic peaks of the SA are observed on the MWNTs at about 2924cm-1, 2866cm-1, 1312cm-1 and 953cm-1, respectively(see Fig.2(a)). The two new peaks at 2924cm-1 and 2866cm-1 are attributed to CH3 and CH2 stretching mode, respectively. In addition, the new peak observed at about 1497cm-1 should be attributed to —COO-, which is considered a shift from —COOH to —COO-. This confirms the effect of linkage between —OH on MWNTs and —COOH on SA by eaterification.
From curve 1 of Fig.2(b), it can be seen that the D and G modes of MWNTs are observed at about 1326.8cm-1and 1575.1cm-1, respectively. Moreover, a very strong peak is observed at 1607.5cm-1, designated as the D′ modes of MWNTs. The experimental measurements are very close to the earlier experimental result of Tan et al[19]. After MWNTs are modified, the G mode and D′ disappear, and two new peaks at 1440.6cm-1 and 1081cm-1 corresponding to CH3 and CH2 modes are observed(see Fig.2(b)). It is further confirmed that the MWNTs are coated by SA.
3.4 Tribological properties of MWNTs as lubricant additive
3.4.1 Effect of stearic acid content
Fig.3 depicts the variation of the friction coefficient and wear loss with content of SA for lubricant containing 0.2% MWNTs under 1000N. The results indicate that under a load of 1000N, the friction coefficient decreases with the increasing content of SA when the content of SA is below 0.4%. However, as the content of SA increases beyond 0.4%, the friction coefficient increases with the increasing content of SA(Fig.3(a)). Also, when the stearic acid content in the lubricant surpasses 0.4%, the wear loss increases with the increasing content of SA, as shown in Fig.3(b).

Fig.3 Variations of friction coefficient(a) and wear loss(b) with content of SA for
lubricant containing 0.2% MWNTs under 1000N
3.4.2 Effect of MWNTs content
Fig.4 shows variation of friction coefficient and wear loss with MWNTs content for lubricant containing 0.4% stearic acid under a load of 1000N. When the content of MWNTs in lubricant is below 0.15%, the friction coefficient and the wear loss show a steadily decreasing trend with the increasing content of MWNTs. However, with the increasing content of MWNTs higher than 0.15%, the friction coefficient and wear loss increase. The increasing friction coefficient and wear loss is likely to be attributing to that the MWNTs are easily conglomerated with the increasing concentration of MWNTs in lubricant. As discussed above, the presence of MWNTs can improve the friction-reduction and anti-wear ability of pure lubricant significantly. Furthermore, these results indicate that there is an optimum ratio between MWNTs and SA in improving the friction-reduction and antiwear ability of lubricant, and the optimum ratio between MWNTs and stearic acid is about 3∶8-1∶2.

Fig.4 Variations of friction coefficient(a) and wear loss(b) with MWNTs content for lubricant
containing 0.4% stearic acid under 1000N
3.4.3 Effect of different additives
The variations of friction coefficient(a) and wear loss(b) with load are depicted in Fig.5. The concentration of MWNTs additive in lubricant is 0.45%. From Fig.5, it is very evident that the modified MWNTs as lubricant additive have lower friction coefficient and wear loss than pure lubricant. In addition, the friction coefficient and wear loss of different additives under load of 700N are listed in Table 1. It can be seen that the modified MWNTs as lubricant additive have lower friction coefficient and wear loss than pure lubricant, the ball-milled MWNTs and modified graphite with stearic acid. The results imply that the modified MWNTs with SA can improve the friction-reduction and anti-wear ability of pure lubricant. Furthermore, it can be seen from Table 1 that the friction coefficient of ball-milled MWNTs is higher than that of the modified graphite, but the wear loss is lower than that of the modified graphite. The higher frictional coefficient is likely to be attributing to that the ball-milled MWNTs are easily conglomerated. However, the MWNTs have outstanding mechanical properties, resulting in decreasing the wear loss.
Table 1 Friction coefficient and wear loss of different additive under 700N
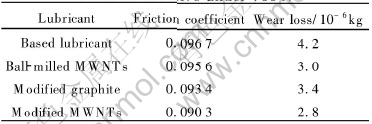
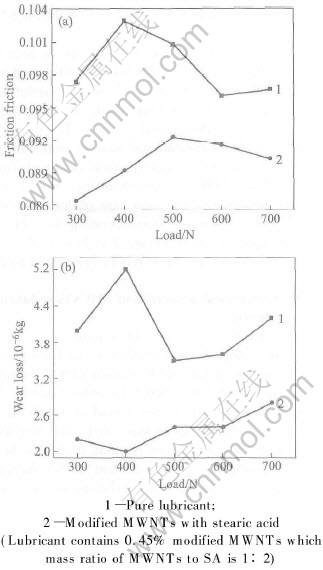
Fig.5 Variations of friction coefficient(a) and wear loss(b) with load
3.4.4 Discussion
Because the friction-reduction and anti-wear ability of nano-particle as lubricant additive depend on both the property of nano-particle and the dispersion of nano-particle in lubricant, the friction-reduction and anti-wear ability of the modified MWNTs with SA as lubricant additive can be explained by well dispersion in lubricant and superior property of MWNTs. Due to the enormous aspect ratio and the very small diameter of MWNTs, there are a very strong van der Waals attraction forces between MWNTs, resulting in forming the large MWNTs bundles. So, the ball-milled MWNTs without modification as lubricant additive possess higher frictional coefficient. However, the MWNTs have more superior mechanical property and better self-lubricant property than the graphite, leading to the decreasing wear loss.
Furthermore, as one of the good dispersant, SA could be efficient in preventing MWNTs from flocculating in base lubricant. It is well known that the SA molecules are long hydrocarbon chain molecules, which are a hydrocarbon segment(alkyl chain) and hydrophilic segment(carboxylic groups). Moreover, it can be seen from the infra-red spectrum(Fig.2(a)) that there were many hydroxy groups and carboxylic groups on the surface of MWNTs after oxidized by mixture acid. So, after the ball-milled MWNTs are modified with SA, many SA molecules are adsorbed on surface of MWNTs by the esterification reaction or physical attraction, i.e. the hydrophilic segment of SA molecules are anchored on the surface of MWNTs. When the modified MWNTs are dispersed in base lubricant, the long hydrocarbon segment(alkyl chain) stretched into base lubricant very easily, resulting in the formation of steric hindrance force. The steric hindrance force can conquer the van der Waals interaction between MWNTs, thereby help to separate them from each other. At the same time, the steric hindrance force can conquer the gravity and prevent the MWNTs from coagulating[20-22].
Because the modified MWNTs with SA can stability disperse in lubricant, two mating wear surfaces are easily filled with the dispersed MWNTs during the wear process, and then the MWNTs on wear surface can serve as spacers, preventing rough contact between the two mating wear surfaces, thereby improving the friction-reduction and anti-wear ability of pure lubricant considerably. Moreover, owing to MWNTs being shortened, the short and tube shape of the MWNTs will provide very easy shear and more easy slide or roll between the two mating wear surfaces, leading to decreasing the friction coefficient and wear loss.
In addition, it is well known that the SA is both good dispersant and good lubricant. With the addition of SA, the dispersion of MWNTs in lubricant is improved. Moreover, the SA on the surface of the MWNTs can form a layer of SA molecule in lubricant, resulting in forming the effective membrane of lubricant during the wear process, therefore the friction-reduction and anti-wear ability of lubricant are improved significantly. However, when the concentration of SA further increases in lubricant, the SA is easily decomposed and oxidized during the wear process due to instability of SA at high temperature, resulting in the friction coefficient and wear loss increase.
4 CONCLUSIONS
The SA direct-modified MWNTs can be produced by esterification reaction. The modified MWNTs with SA can effectively improve the friction-reduction and anti-wear ability of pure lubricant. When the concentration of modified MWNTs in lubricant is 0.45%, the friction coefficient of base lubricant decreases by about 10% and the wear loss decreases by 30%-40%. Moreover, the optimum ratio of MWNTs to SA is about 3∶8-1∶2.
REFERENCES
[1]Iijima S. Helical micro-tubules of graphitic carbon [J]. Nature, 1991, 345: 56-58.
[2]Collins P G, Zettle A, Bando H, et al. Nanotube nanodevice [J]. Science, 1997, 278: 100-103.
[3]Tans S J, Devoret M H, Dai H, et al. Individual single-wall carbon nanotubes as quantum wires [J]. Nature, 1997, 386: 474.
[4]Treacy M M J, Ebbesen T W, Gibson J M, et al. Exceptionally high Youngs modulus observed for individual carbon nanotubes [J]. Nature, 1996, 381: 678-680.
[5]Kong J, Franklin N R, Zhou C W, et al. Nanotube molecular wires as chemical sensors [J]. Science, 2000, 287:622-625.
[6]Che G L, Lakshmi B B, Martin C R, et al. Metal-nanocluster-filled carbon nanotubes: catalytic properties and possible applications in electrochemical energy store and production [J]. Langmuir, 1999, 15 (30): 750-758.
[7]DONG Shu-rong, ZHANG Xiao-bin. Mechanical properties of Cu based composites reinforced by carbon nanotubes [J]. Trans Nonferrous Met Soc China, 1999, 9(3): 457-461.
[8]WANG Lang-yun, TU Jiang-ping, YANG You-zhi, et al. Frication and wear behavior of multi-walled carbon nanotube/Cu matrix composites [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3):367-371.(in Chinese)
[9]ZHANG Gang, LI Shao-lu, CHEN Xiao-hua, et al. Corrosion behavior of carbon nanotubes/Ni composite coating [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 996-1000.(in Chinese)
[10]YI Guo-jun, CHEN Xiao-hua, JIANG Wen-zhong, et al. Surface modification and nickel coating of carbon nantubes [J]. The Chinese Journal of Nonferrous Metals, 2004,14(3): 479-483.(in Chinese)
[11]Chen J, Hamon M A, Hu H, et al. Solution properties of single-walled carbon nanotubes [J]. Science, 1998, 282: 95-98.
[12]Michael G C K, Banerjee S, Wong S S. Solubilization of oxidized single-walled carbon nanotubes in organic and aqueous solvents through organic derivatization [J]. Nano Lett, 2002, 2(11): 1215-1218.
[13]ZHAO Li-ping, GAO Lian. Stability of multi-walled carbon nanotubes dispersion with copolymer in ethanol [J]. Colloids and Surfaces A, 2003, 224: 127-134.
[14]Cattien V N, Delzeit L, Alan M C, et al. Preparation of nucleic acid functionalized carbon nanotube arrays [J]. Nano Lett, 2002, 2(10): 1079-1081.
[15]Williams K A, Veenhuizen P T M, Torre B G, et al. Carbon nanotubes with DNA recognition [J]. Nature, 2002, 420: 761.
[16]Hernadi K, Ljubovic E, Seo J W, et al. Synthesis of MWNTs-based composite materials with inorganic coating [J]. Acta Materialia, 2003, 51: 1447-1452.
[17]Gojny F H, Nastalczyk J, Roslaniec Z, et al. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites [J]. Chem Phys Lett, 2003, 370: 820-24.
[18]CHEN Xiao-hua, CHEN Chuan-sheng, CHEN Qing, et al. Non-destructive purification of multi-walled carbon nanotubes produced by catalyzed CVD [J]. Materials Letters, 2002, 57: 734-738.
[19]Tan P H, Zhang S L, Yue K T, et al. Comparative raman study of carbon nanotubes prepared by DC arc discharge and catalytic methods [J]. J Raman Spect, 1997, 28: 369-372.
[20]Gong X Y, Liu J, Baskaran S, et al. Surfactant-assisted processing of carbon nanotube/ polymer composites [J]. Chem Mater, 2000, 12: 1049-1052.
[21]Feng L, Li H J, Li F Y, et al. Functionalization of carbon nanotubes with amphiphilic molecules and their langmuir-blodgett films [J]. Carbon, 2003, 41: 2385-91.
[22]Zhao L P, Gao L. Stability of multi-walled carbon nanotubes dispersion with copolymer in ethanol [J]. Colloids and Surfaces A, 2003, 224: 127-34.
Foundation item: Projects(50372020; 59972031) supported by the National Natural Science Foundation of China; Project(01JJY2052) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2004-11-20; Accepted date: 2005-01-18
Correspondence: CHEN Xiao-hua, Professor, PhD; Tel: +86-731-8821610; Fax: +86-731-8821483; E-mail: hudacxh@sohu.com


