Trans. Nonferrous Met. Soc. China 29(2019) 2534-2545
Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth
Jing-pu ZHENG1, Liang-jian CHEN1,2, Dai-yuan CHEN1, Chun-sheng SHAO1, Man-fei YI1, Bo ZHANG1
1. Department of Stomatology, Third Xiangya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 21 February 2019; accepted 11 November 2019
Abstract:
The effects of surface-modified porous titanium implants with different porosities and pore sizes on osseointegration were investigated in vivo. Three porous titanium implants (A30, A40 and A50 containing volume fractions of space-holder NaCl being 30%, 40% and 50%, respectively) were manufactured by metal injection moulding (MIM). The surface-modified implants were implanted into muscles and femurs of hybrid male dogs. Interface osteogenic activity and histological bone ingrowth of porous titanium implants were evaluated at 28, 56 and 84 d. The results showed that when additive space-holder amount of NaCl increased from 30% to 50% (volume fraction), the general porosity and mass fraction of macropores of porous titanium rose from 42.4% to 62.0% and from 8.3% to 69.3%, respectively. Histologic sections and fluorescent labeling showed that the A50 implant demonstrated a significantly higher osteogenic capacity at 28 d than other implants. Bone ingrowth into the A30 implant was lower than that into other implants at 84 d. Therefore, the pore structure of A50 implant was suitable for new bone tissue to grow into porous implant.
Key words:
porous titanium implant; porosity; aperture; interconnectivity; osseointegration;
1 Introduction
Titanium and its alloys have been extensively used as orthopedic and dental implants because of excellent biocompatibility and mechanical properties. However, they were generally bioinert materials. In order to improve the bioactivity of titanium implant surface and increase the initiative of osseointegration process, scholars often use plasma spraying, chemical deposition, grafting and coating surface modification technology. However, bonding strength between the coating and the matrix on the surface of the compact implant was low, and it was vulnerable to implant shear stress destroying and falling off, so its clinical application is limited. At the same time, researchers found that the elastic modulus of titanium and its alloys mismatched with that of the host bone. This mismatch could cause stress shielding effect under loading, and bone resorption may consequently lead to implant failure [1]. By adjusting its porosity, porous titanium could match its elastic modulus with the host bone [2,3]. Moreover, porous surface could enhance mechanical interlocking between the implant and the surrounding bone tissue, which resulted in long-term fixation and sufficient bone ingrowth in vivo [4,5]. The pore wall of porous layer could support the coating. If the coating is prepared on the porous inner wall of the porous surface, the failure of shear force could be effectively avoided. However, most researchers were limited to general description of the enhanced bone regeneration in certain porous titanium implants, rather than systematical analysis on the effects of structural factors in bone ingrowth, i.e., porosity, aperture and connectivity.
The architecture of a porous implant has been shown to substantially affect the bone ingrowth into pore space. The optimal parameters of porous titanium for bone ingrowth have been still unclear so far. Pore sizes between 400 and 700 μm and even up to 1.2 mm showed sufficient evidences for bone ingrowth [6-8]. Nevertheless, most researchers believed that optimal aperture for bone ingrowth ranged from
150 to 600 μm [9-11]. HOLY et al [12] found that porous implant with a pore aperture of 100-400 μm was more suitable for bone ingrowth, while ITALA et al [13] amended the pore size range to 50-125 μm. ST-PIERRE et al [14] declared that the pore size affected the cell proliferation, but had tiny effect on mineralization of bone matrix. Researches by OTSUKIA et al [15] indicated that the interconnectivity of pores was a critical factor for bone ingrowth and a proper aperture of communicating channels promoted bone ingrowth and differentiation. Higher porosity was expected to promote the bone growth, but it sacrificed the mechanical proprieties of the titanium implant. It is crucial to seek the balance among the pore size, porosity and mechanical properties of porous implants to optimize the functions of load-bearing and bone ingrowth. Therefore, the understanding and precise control of the structural parameters are necessary for the application of porous titanium implants in clinical practice.
Various methods have been developed to produce a porous titanium scaffold, including sintering with powders [16,17], solid-state foaming by expansion of argon-filled pores [18], compressing and sintering of titanium fibers [19], and polymeric sponge replication [20]. However, these methods cannot manufacture implants with precisely controlled porosity, pore shape, size, and interconnectivity suitable for inducing tissue ingrowth, in order to anchor the prosthesis to the surrounding bone and prevent implant loosening.
Metal injection moulding (MIM) is a powder metallurgy process currently used for the production of complicated and near-net shape parts of high performance materials. With NaCl as the space-holder, our group creatively regulated process parameters to prepare porous titanium with controllable porosity and aperture, which showed the superiority of MIM [21]. Surface modification of porous titanium was conducted by alkali heat treatment and biomimetic deposition of apatite coating. Moreover, the in vitro study indicated that the modified porous titanium implants were propitious to osteoblast cell’s adhesion, proliferation and differentiation with the increase of porosity [22]. In this work, we investigated the effects of structural parameters of porous titanium implant on the bone ingrowth in order to optimize porous structure for metal implants. Porous implants were fabricated by MIM with three additive amounts of NaCl (A30, A40, and A50 containing volume fractions of space-holder NaCl being 30%, 40% and 50%, respectively) . In vivo researches were conducted in cancellous bone in the dog femur to evaluate the biological performance, such as the bone ingrowth.
2 Experimental
2.1 Fabrication and characterization of porous titanium implants
Hydrogenation-dehydrogenation (HDH) titanium powder with particle sizes <77 μm was used in this study. NaCl powder with particle sizes <290 μm was added as a space-holder. The volume fractions of NaCl in mixed powders are 30%, 40% and 50%, respectively. In order to make them properly granulated, HDH titanium and NaCl powders, together with wax-based binders, were mixed in a XSM1/20-80 rubber mixer with a pair of roller rotor blades at 150 °C for 1 h. Green bodies were made by MIM method with a solid loading of 55 vol.%. After the binders and space-holders were removed, the green bodies were sintered at 1150 °C for 2 h under a vacuum of 1.33×10-3 Pa. The sintered parts were diced to sizes of approximately 4 mm × 3 mm × 6 mm for porous titanium implants, and sizes of 5 mm × 5 mm × 7 mm for mechanical properties test. According to the amount of NaCl added in the feeding, the three implants prepared by MIM were A30, A40 and A50, respectively. All implants were cleaned with acetone, anhydrous ethanol, and deionized water in the ultrasonic bath for 10 min successively.
The density, open porosity, and general porosity of implants were determined by Archmede method with the theoretical density of 4.5 g/cm3 for titanium. Pore size and the distribution of the interpore connections of samples were analyzed by the monolith mercury porosimeter (AutoPore II 9220 V3.04, Micromeritics Instrument Co., Atlanta, GA). The microstructures and morphologies of the implants were observed by scanning electron microscope (FE-SEM, JSM-6360LV, JEOL Techniques, Tokyo, Japan). The compress test was performed using a universal material testing machine (Instron 5565, Instron Corp., Canton, MA, USA) at a crosshead speed of 1 mm/min. The elastic modulus was calculated from the slope of the compressive stress- strain curve in the linear elastic region, and the compressive yield strength was determined from the stress-strain curve using the 0.2% offset method. Three samples of each group were tested to obtain the average values and the standard deviation.
2.2 Alkali-heat treatment and apatite deposition of porous titanium implants
All implants were immersed in NaOH solution (5 mol/L) in a shaking bath at 80 °C for 48 h. After being cleaned with deionized water repeatedly, implants were placed in a thermal treatment furnace where the temperature went up to 600 °C at a rate of 5 °C/min and maintained for 1 h. The implants were cooled down along with the furnace. Afterwards, the implants were dipped into the simulated body fluid (SBF) for 21 d. SBF was already supersaturated, its ion concentrations were as follows: 142.0 mmol/L Na+, 5.0 mmol/L K+, 1.5 mmol/L Mg2+, 2.5 mmol/L Ca2+, 147.8 mmol/L Cl-, 4.2 mmol/L 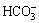 , 1.0 mmol/L
, 1.0 mmol/L 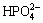 , 0.5 mmol/L
, 0.5 mmol/L 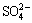 ; pH 7.4. The solution was replaced by fresh SBF every 3 d and the pH was adjusted to 7.25 by 1 mol/L HCl solution. Before the following implantation trial, all implants were sterilized in autoclave (103.4 kPa, 121.3 °C) for 2 h.
; pH 7.4. The solution was replaced by fresh SBF every 3 d and the pH was adjusted to 7.25 by 1 mol/L HCl solution. Before the following implantation trial, all implants were sterilized in autoclave (103.4 kPa, 121.3 °C) for 2 h.
2.3 Animal experiments
The animal study was approved by the Animal Research Committee of Third Xiangya Hospital, Central South University, Changsha, China. Three modified porous titanium samples (A30, A40, A50) were used as implants. Before implantation, porous implants were conventionally sterilized by ethylene oxide gas. The experimental animals of the study were 9 hybrid male dogs from Animal Experiment Center of Hunan Academy of Agricultural Sciences, which were about 2 years old and 9-12 kg. They were divided randomly into three groups and raised respectively for 28, 56 and 84 d after surgery. Surgery was performed under aseptic conditions. The experimental dogs were injected with ketamine (75 mg/kg) to induce general anaesthesia and supported by an inhalation mask of O2 and isoflurane (2.5 and 0.8-1.5 L/min, respectively). Analgesia was maintained by subcutaneous injection of buprenorfine (0.001-0.05 mg/kg), and antibiotic prophylaxis was by means of two injections of cefazoline (50 mg/kg). After a clearance of bone tissue contact, the porous titanium implants were inserted into the muscle (Fig. 1(a)). Using a motorized drill a bone defect (4 mm × 3 mm × 6 mm) was made in the lateral aspect of both femurs in all animals, using continuous irrigation with physiological saline to prevent bone necrosis. The porous titanium implant was embedded into the defect (Fig. 1(b)). For each group, the titanium implants with different porosities were implanted in bilateral femurs of 3 dogs (Fig. 1(c)). The distance between two implants was 2 cm. The same operation was conducted on the two groups. After the operation, all dogs received subcutaneous injection of antibiotics. Postoperatively, the dogs were allowed to move freely in their cages without external support.
The time courses of new bone formation and mineralization were assessed by sequential fluorescent labeling method. Xylenol orange (90 mg/kg) was administrated intravenously in three groups respectively at 7, 21 and 35 d after surgery. And sodium fluorescein (3 mg/kg) was injected intravenous labeling in three groups for 14, 35 and 56 d after surgery.
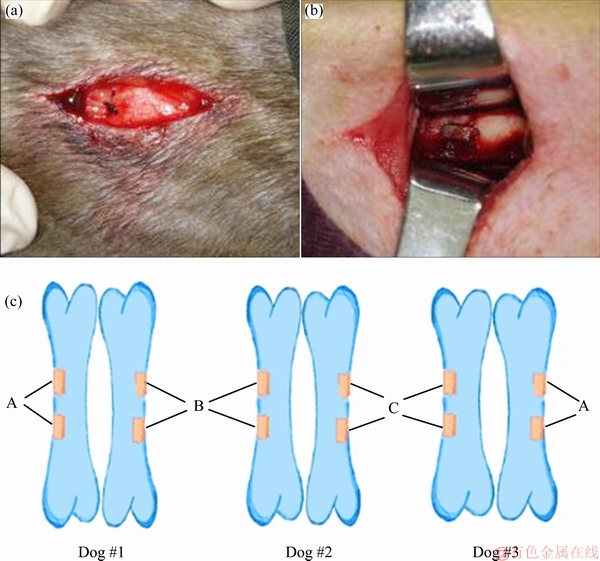
Fig. 1 Porous titanium implanted in back muscle (a) and femur (b) of hybrid male dogs, and schematic diagram of implantation of each group (c) (Orange columns indicate the implants)
The animals of three groups were anesthetized and euthanized at 28, 56 and 84 d after surgery by intravenous injection of concentrated potassium chloride. Afterwards, block sections including implants and surrounding femur bone tissues were collected, as well as the specimens of erector apinae muscle where porous titanium was implanted.
2.4 Specimens observation and analysis
2.4.1 Histomorphometry of peri-implant muscle
Specimens of the implants and the surrounding muscles were fixed in 10% formaldehyde solution for 3 d. The surrounding soft tissues were dissected carefully from the implants, and dehydrated in a series of graded alcohols (70%, 80%, 90% and 100%, volume fraction). Then, the soft tissues were embedded in paraffin and consecutively cut into films. The sectioned samples were stained with hematoxylin and eosin (H&E) stain. The morphological and histological analyses were performed and viewed under a light microscope.
2.4.2 Histomorphometry of peri-implant osseous tissue
Specimens including implants and surrounding bone tissues were fixed in 10% formaldehyde solution for 7 d and dehydrated in ascending graded alcohols (70%, 80%, 90% and 100%). Dehydrated specimens were embedded in polyester resin for 12 h. Thick sections (250 μm) were cut with a band saw (BS-3000CP; EXACT Cutting Systems, Norderstedt, Germany) perpendicularly to the axis of the implant and ground to a thickness of 15-20 μm using a grinding-sliding machine (Microgrinding MG-4000; EXACT Cutting Systems). For each specimen, two pieces of slices were made. One was directly cover-slipped with Technovit 7200VLC for fluorescence microscope (OLYMPUS) observation. Mineralization levels and directions were determined at different time points according to the situation of fluorescent markers deposition. The other was conducted by Goldner’s trichome and then used to evaluate the osseointegration, bone generation and calcification in the pores of implants under light microscope.
2.5 Statistical analysis
Results were expressed as the means ± standard deviations (SDs) for each group from at least three independent experiments. Error bars represent standard deviations. Statistical comparison was made using one-way ANOVA, followed by Bonferroni correction as a post hoc analysis. Differences of P<0.05 were considered to be statistically significant.
3 Results and discussion
3.1 Characteristics of porous titanium implants
Figure 2 shows the pore size distribution and general porosity of porous titanium implants with various volume fractions of space-holder NaCl. Despite the same particle size of NaCl (290 μm), the pore size of porous titanium varied from 20 to 300 μm. General porosity of porous titanium increased with the amount of space- holder. The general porosities of implants A30, A40, A50 were (42.4±2.1)%, (52.1±2.2)% and (62.0±1.8)%, respectively. Statistical analysis revealed that three kinds of porous titanium implants produced pore sizes from several microns to greater than 300 μm. With the increase of space-holder amount of NaCl, the proportions of aperture 50-300 μm in the three groups were 8.3%, 45.3% and 69.4%, respectively, as shown in Fig. 2. ITALA et al [13] reported that this bone fixation occurred for pores of 50-125 μm. Gradients in pore sizes were recommended for the formation of multiple tissues and tissue interfaces [23]. Additionally, the scaffolds contained a number of mesopores (2-50 nm) which allowed body fluids to circulate, while macropores provided a scaffold for bone-cell colonization.
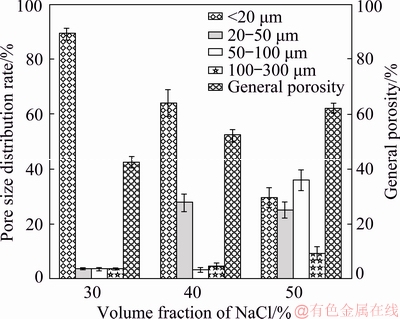
Fig. 2 Pore size distribution and general porosity of porous Ti implants with various volume fractions of space-holder NaCl
3.2 Microstructural analysis
The surface morphologies were similar among implants with different pore sizes. The particles that were loosely bonded to the body after MIM became tightly bonded by sintering and provided remarkable irregularities on the surface, as shown in Fig. 3. In terms of pore structure, pore shape was well controlled and the rectangle shape of the original NaCl was reproduced in the three groups. With the increase of additive amount of NaCl, the number of macropores (50-300 μm) on the surface of porous titanium also increased.
3.3 Mechanical properties
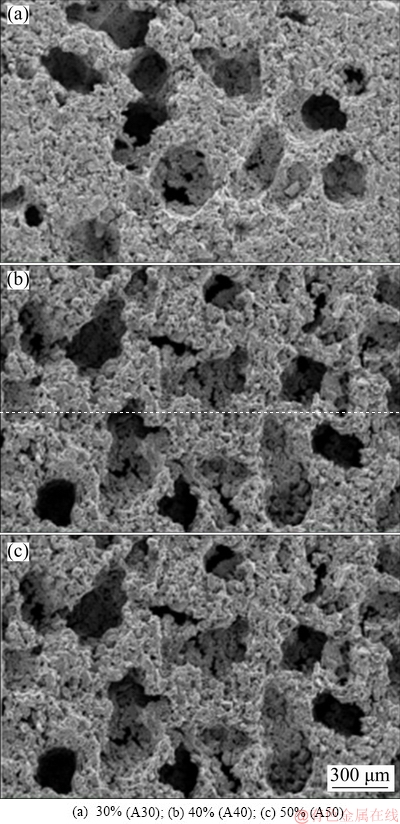
Fig. 3 SEM images of porous surface-modified titanium implants with different volume fractions of NaCl
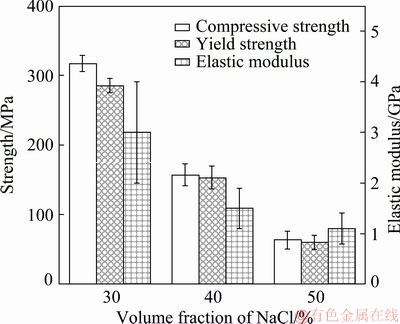
Fig. 4 Mechanical properties of three porous titanium implants
The compressive strength, yield strength and elastic modulus of the porous titanium implants are shown in Fig. 4. The mechanical properties of the porous titanium samples decreased with the increase of volume fractions of NaCl from 30% to 50%. The compressive strength decreased from (316.6±11.4) to (63.2±12.8) MPa, as well as the yield strength from (284.9±9.8) to (59.8±10.2) MPa and elastic modulus from (3.0±1.0) to (1.1±0.6) GPa. The results show that the A30 samples had the best mechanical properties, and that there were significant differences (P<0.05) among the strengths of three porous titanium implants. The A50 samples had yield strength of around 59.8 MPa, which was smaller than that of cortical bone (80-120 MPa) and close to that of human trabecular bone (0.2-80 MPa) [2]. The elastic modulus of the A50 samples (1.1 GPa) was much lower than that of cortical bone (17 GPa) [24] and close to that of human trabecular bone (0.5-4.0 GPa) [25]. The change of mechanical properties of porous scaffolds resulted in altered mechanical loading and bone regeneration under load-bearing conditions [26,27]. The fact that bony matrix infiltrated into the pores of implants may substantially reduce local stress concentration and delay the onset of plastic deformation, which in turn can reduce the risk of implant failure due to local pore wall fracture by overload or fatigue.
3.4 Surface characteristics of surface-modified porous titanium implants
After the alkali-heat treatment, the surface of the porous titanium implants exhibited a porous network structure (Fig. 5). Previous studies have reported that this network layer was sodium titanate hydrogel, formed after the NaOH treatment [28]. JALOTA et al [29] and SANDRINI et al [30] reported that cracking occurred after chemical treatment due to the difference in thermal expansion coefficients of the substrate and the surface layer after drying. However, no cracks were observed in the SEM images in this study. The results of the EDS (Fig. 6) showed that Ca and P could be detected when NaOH-treated pore wall of porous implant was soaked in SBF for 21 d. The formation of calcium and phosphorus deposition layers on the surface of bioactive titanium was believed to be the precursor to bone induction ability in the early [31-37]. TANG et al [38] and CHEN et al [39] have found that different calcium phosphate coatings have osteoinductive effects, and apatite coating has low crystallinity, easy to degrade in body fluid environment and form high calcium and phosphorus concentrations in local micro-environment, which is conducive to the mineralization of bone matrix in porous layer. However, HA coating has high crystallinity and slow degradation rate. The bonding strength between the coating and the matrix material affects the clinical application. Biomimetic deposition can form apatite coating on implant surface and pore wall of porous layer, and the coating on pore wall of porous layer can effectively avoid the damage of shear force during implantation, which lays a foundation for clinical application. However, it remains to be further studied whether the NaOH-treatment and apatite deposition of porous titanium implant are beneficial for the bone growth into scaffolds.
3.5 Histological examination of peri-implant tissue
As shown in Fig. 7, peri-implant tissue was in normal appearance. No inflammatory infiltration consisting of mononuclear cells (lymphocytes) was observed, nor was the infiltration of neutrophils and eosinophils. Ectopic osteogenesis was not found either. With the prolongation of implantation time, the number of vascular-like tissues in peri-implant tissues increased. The results demonstrated that the surface-modified porous titanium implants exhibited nontoxicity and good biocompatibility.
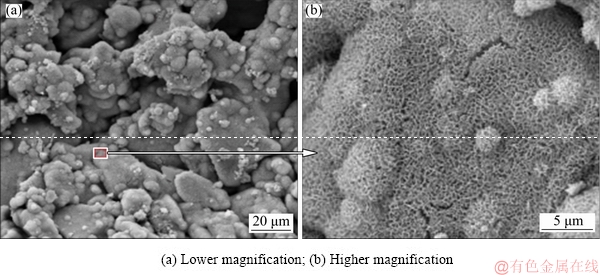
Fig. 5 SEM images of porous titanium implants after alkali-heat treatment
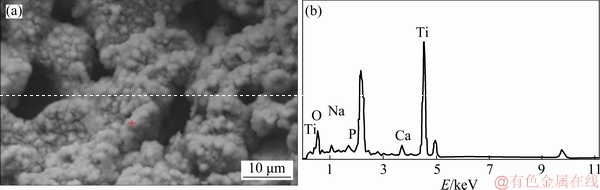
Fig. 6 SEM image (a) and EDS spectrum (b) of alkali-heat-treated porous titanium implant after soaking in SBF for 21 d
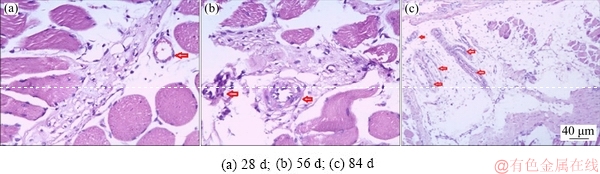
Fig. 7 Pathological analysis of tissue sections from ambient tissue of porous titanium implant (Arrow: Vascular-like tissue)
3.6 Fluorescent markers deposition of implanted femur blocks
The irradiated xylenol orange emitted green fluorescence and sodium fluorescein emitted yellow- green fluorescence excited by green light (wavelength of 490 nm) under fluorescence microscope. Both of them could be deposited in new bone tissue. The active area of new bone formation could be judged by the location of fluorescent deposition. According to the location of fluorescence colored bands, the direction of bone growth was descriptively analyzed.
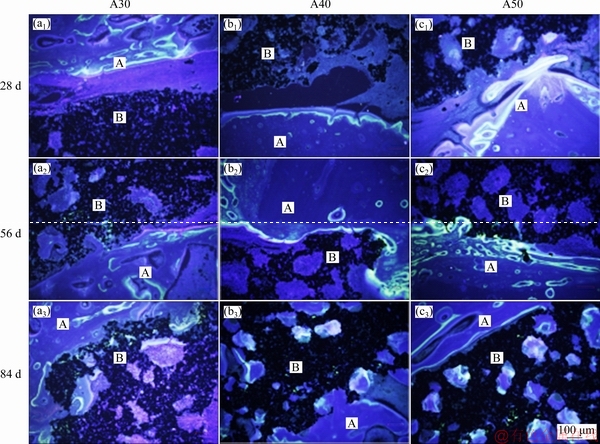
Fig. 8 Fluorescent microscopy photographs of fluorochrome labels and non-decalcified sections after being porous-titanium- implanted for 28, 56 and 84 d, respectively (A: Host bone; B: Porous titanium)
At 28 d, stripped fluorescent markers appeared in the gaps between bone and the implants and were close to the host bone (Figs. 8(a1-c1)). The green fluorescence exhibited weak intensity, while the yellow-green fluorescence was much brighter. Space between the two fluorescent markers was clear. No fluorescent marker deposition was found on the surface of implants A30 and A40. However, small clumps of fluorescence were observed both on the surface and in the superficial pores of the implant A50. At 56 d, fluorescent markers deposited on the surface of implants A30 and A40, and got stronger intensity on the surface of implant A50 (Figs. 8(a2-c2)). At 84 d, the fluorescence was distributed diffusely and irregularly with overlaps and crosses in weak intensity. It was notable that fluorescent markers deposited in the deep pores of implants A40 and A50 (Figs. 8(b3-c3)), which indicated new bone tissue formation deep into these porous titanium implants.
From 28 to 84 d, the main place for fluorescence deposition gradually transferred from the surface of host bone to the surface of the implants and the pores inside. The results indicated that the direction of new bone formation was from the natural bone to the surface of implant. At 28 d, the surface of host bone performed a stronger fluorescence than the implants due to its higher biological reactivity. Besides, fluorescence markers given by intravenous administration were difficult to cross the gap between host bone and the implants in the first few weeks. However, spots of fluorescence were observed both on the surface and in the superficial pores of implant A50. In addition, it was speculated that a bidirectional but weak bone formation existed, which initiated from the osteoblasts adhered on the implants and moved towards the host bone and the pores inside the implants. At 84 d, the fluorescence located at the bone/implant interface and the pores of the implants. It could be inferred that fluorescent labels were transported to the pores through newly established blood circulation pathways. New bone formation in the pores was slow, with alternant osteogenesis and bone resorption. When the new generated bone tissue labeled by sodium fluorescein was absorbed, bone matrix labeled by xylenol orange could deposit at the same site. Therefore, the fluorescence in the pores was irregular under observation.
3.7 Histological examination of porous implants in different periods
The bone integration of porous implants in different periods was evaluated by non-decalcified histologic section and Goldner’s trihrome staining. Goldner’s trichrome provided excellent images for osseous tissue observation with clear-cut distinction of osteoblast cell which stained orange, unmineralized bone matrix which appeared as peach-pink, mineralized bone matrix which stained light blue, mature bone matrix which was chartreuse to green, and bone marrow-like tissue which stained light orange (Fig. 9).
3.7.1 Bone formation between porous implants and host bone interface
At 28 d, there was no osseointegration between three kinds of porous implants. The host bone and connective tissues staining light orange were observed in gaps as well as the pores of the implants. Pink mineralized bone matrix was deposited on the surface of host bone near the crevice. However, there was no obvious bone matrix deposition on the surfaces of A30 and A40 specimens (Figs. 9(a1) and (b1), but there was a little unmineralized bone matrix deposition in the surface pore of A50 specimen (Fig. 9(c1)). At 56 d, the gap between implant and the host bone was narrowed obviously. Most of the new bone tissues combined with the implant surface to achieve osseointegration. There was no mineralized bone matrix deposition in the surface pore of implants. However, the deep pore of porous implants was filled with light orange connective tissue (Figs. 9(a2-c2)). At 84 d, porous implants were tightly integrated with host bone tissue. The surface and deep pore of porous implants were filled with mineralized bone matrix and vascular-like tissues were formed. The depth of bone tissue growth in A40 group (Figs. 9(b1-b3)) and A50 group (Figs. 9(c1-c3)) was higher than that of A30 group (Figs. 9(a3-c3)).
These findings indicated that the osseointegration of three porous implants with the same alkali-heat treatment and apatite deposition could be achieved over time. However, the deposition and mineralization of bone matrix in porous implants and the depth of bone tissue growth were closely related to the structural factors such as porosity, pore size and connectivity of porous titanium implants.
At 84 d, the degree of bone ingrowth into the pores was lower in the A30 implant than that in A40 and A50 implants. This can be explained by vascularization, which is essential for bone formation. BAI et al [32] reported that an increase in pore size resulted in an increase in the size of blood vessels formed. However, there was an evident increase in extent of vascularization with a pore size above 400 μm. Based on these findings, the A40 and A50 implants are more advantageous in terms of vascularization than the A30 implant. These findings indicated that the restoration of bone and soft tissue was not prevented in the pores. Good interconnectivity and well-controlled pore size are essential for vascular tissue ingrowth and bone conductivity, which leads to the restoration of bone and soft tissue.
3.7.2 Effect of porous structural factors on bone tissue formation in porous titanium implants
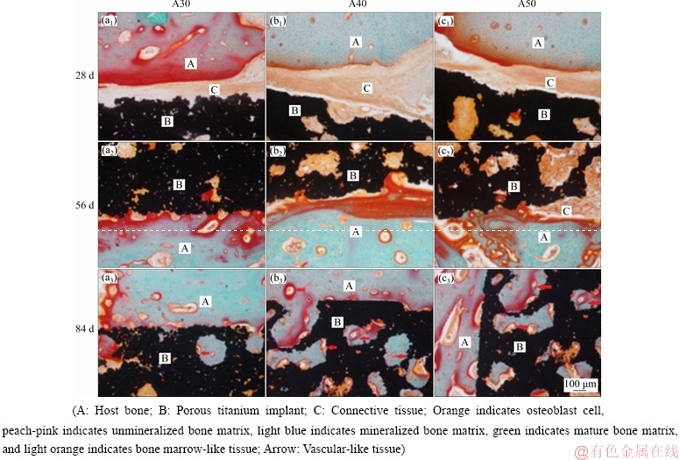
Fig. 9 Light microscope photographs of Goldner’s staining and non-decalcified sections after being porous-titanium-implanted for 28, 56 and 84 d, respectively
Histological images representative of three porous specimens after 84 d are shown in Fig. 10. In A30 implants, bone matrix or connective tissues were observed in superficial pores and deep pores that connected with superficial pores, but rarely in isolated pores (Fig. 10(a)). In A40 implant, bone matrix deposited in both superficial pores and deep pores. Vascular-like tissues were found in accompany with new generated bone. However, there were more connective tissues than one matrix in the deep pores, which indicated that the restriction of tissue differentiation due to inadequate connection of deep pores (Fig. 10(b)). In A50 implant, mineralized bone tissue took the main part in superficial and deep pores, alongside with vascular tissue. Comparing bone growth in the three types of porous titanium implants, it can be inferred that the increase of macropore and good connection of pores facilitated bone ingrowth and tissue differentiation (Fig. 10(c)). Angioid canals were observed in both superficial and deep pores. However, the large holes, which were formed by the fusion of 2-3 holes, were filled with a mass of connective tissue but a little bone matrix.
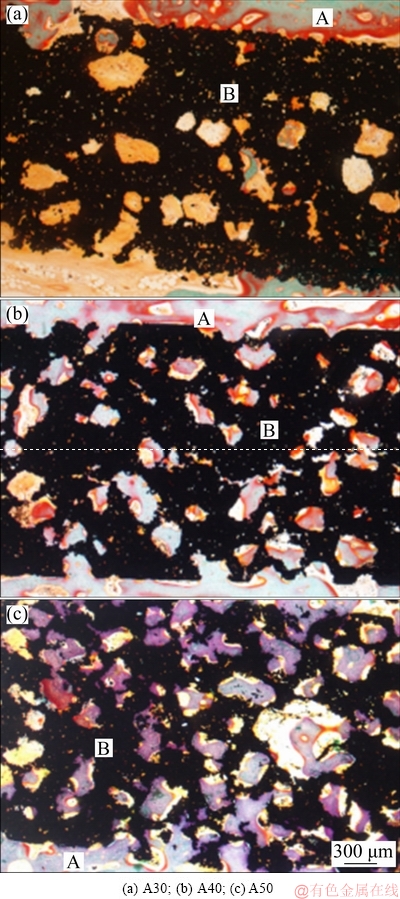
Fig. 10 Non-decalcified histologic sections of porous titanium implant with different porosities after being implanted for 84 d (A: Host bone; B: Porous titanium implant)
Histological observation of the bone-implant interaction at 84 d coincided with what was presumed theoretically from the structural factors of porosity, pore size and connectivity. Structural factors of porous titanium implants affected the depth of bone ingrowth and the differentiation of tissues inside the pores. Furthermore, sufficient blood supply is one of the basic conditions for bone growing into porous implant [11,35]. In addition to providing nutrition, capillaries in bone tissue can deliver functional factors to coordinate the migration, differentiation and performance of osseous cells [36]. Histologic sections of host bones at 84 d showed rich vascular tissues in superficial and deep pores of A40 and A50, but only in superficial pores of A30. Considering the pore size distribution of A30, the undersized interconnective pores were the limitation for capillaries stretching into adjacent pores. Connective hole was the bottle neck of the vessels growing into deep pores of the implant. Good interconnectivity and well-controlled pore size were essential for vascular tissue ingrowth and bone conductivity, which led to the restoration of bone and soft tissues. In A30 implant, pores with aperture of 50-300 μm merely accounted for 8.3%, and their porosity was 42%. The holes were relatively small and isolated, which was not conducive to the migration of osteoblasts and angiogenesis. In A40 implant, more macropores (45.3%) formed connective structure dramatically, met the basic requirement for cell penetration and vasculature formation. However, the A50 implant had a general porosity of 62%, a macropore of 69.4%, and a good-connectivity structure which was conducive to osteoblasts migration and angiogenesis. Well-controlled interconnectivity was a peculiarity of addition of pore-forming agent, and porous implants manufactured by MIM had this further advantage.
3.7.3 Evolution of tissues in pores of porous implants
High-magnification images of tissues in pore of A50 implants for each period are shown in Fig. 11. At 28 d, there were mainly marrow-like tissues staining light orange in the pores (Fig. 11(a)). During this period, a variety of cell components migrated and differentiated, capillary grew, and preosteoblast migrated into the pore from the surrounding bone. At 56 d, diverse tissues were observed, i.e. blue mineralized bone matrix, pink osteoid and marrow-like tissue (Fig. 11(b)). At 84 d, mineralized bone matrix was in the majority, accompanied with spots of osteoid, marrow-like tissue and angioid small pores (Fig. 11(c)). As a local process, the process of bone ingrowth into pore is closely related to the local bone condition, such as the existence of osteoinductive substances and the supply of blood vessels. Bone tissue formation in the pores with osteogenesis conditions is similar to fracture healing. It went through the stages of hematoma formation, cell invasion and differentiation, calcification, maturation and shaping. The evolution of tissue in the pore was also related to the structural factors like porosity, aperture, pore distribution, and connectivity of porous titanium implants affected the depth of bone ingrowth and tissue maturation.
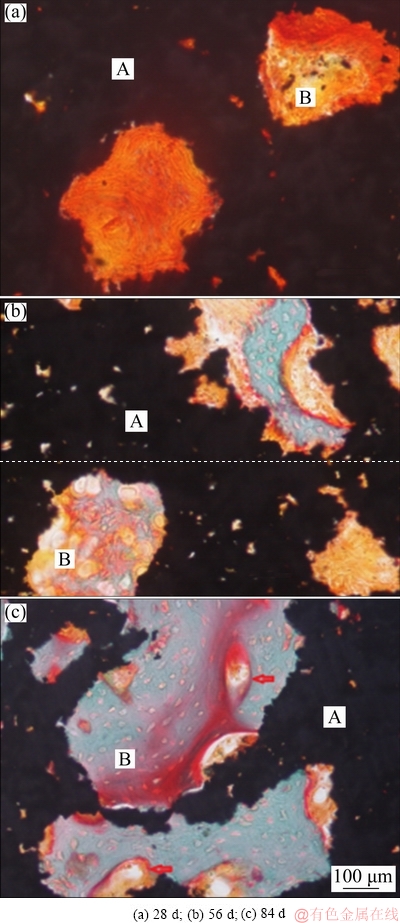
Fig. 11 High-magnification images of tissues in pore of A50 implants for different periods (A: Porous titanium implant; B: Tissue of pore; Arrow: Vascular-like tissue)
The communication between the holes should be large enough to accommodate the bone structural units. Bone unit Haversian system is the main structural unit supporting long backbone, and its aperture is usually 50-250 μm [34]. If a bone unit is formed in the pore, the pore size must be larger than that of the Haversian system. Osteoblasts are 20 μm in length and capillaries are 10-15 μm in diameter. Therefore, if the pore size is less than 100 μm (osteoblasts + capillaries), the osteogenesis is estimated to be some bone matrix, and no Haversian system is formed. The size of the interconnective pore is critical as a channel for cells and blood vessels to migrate. It is theoretically required that the pore size between the pores should be no less than 35 μm so that cells and blood vessels can pass through the pore smoothly. Because of the need for certain space in the process of cell migration and vascular growth, it is impossible to have only capillaries and osteoblasts in the interconnective pore. Thus, theoretically, the lowest requirement of interconnective pore size is larger than 50 μm. At the same time, the results of this study also confirmed that porous structure with good connectivity and pore size of 50-300 μm were conducive to the formation of bone tissue in the pores.
4 Conclusions
(1) Using NaCl as space-holder and adjusting process parameters, MIM technology can effectively control the porosity, pore size and connectivity of porous titanium implants.
(2) With the increase of the amount of space-holder, the porosity and the proportion of large pore diameter (50-300 μm) of porous titanium implant increased, and the porous structure with good connectivity was formed. Porosity affected the mechanical properties of porous titanium implant. The general porosity and percentage of macropores (>50 μm) of porous titanium implant rose from 42.4% to 62.0% and from 8.3% to 69.3%, respectively, while the compressive strength and elastic modulus of porous titanium implant decreased from 316.6 to 63.2 and from 3.0 to 1.1 MPa, respectively.
(3) Porous network structured sodium titanate was formed on surface after alkali-heat treatment. There was calcium and phosphate deposition layer with cluster structure formed on the surface after biomimetic deposition of stimulant body fluid.
(4) Modified porous titanium implanted into muscles and bones had no toxicity or rejection. Modified porous titanium was implanted into muscle for 84 d, and there was no significant ectopic bone formation.
(5) In different implantation periods, bone deposition direction and combination on implant and host bone were different. Early bone matrix deposition mainly occurred on the surface of host bone. Over time, bone matrix deposition occurred on the surface of host bone and porous implant and in the pore.
(6) The porosity, pore size and connectivity degree of porous titanium affected bone ingrowth depth and tissue differentiation. The A50 implants have a general porostiy of 62% and a macropore of 69.4%, and a good- connectivity structure was formed, which was conducive to the growth of bone tissue and neovascularization.
References
[1] MEDIASWANTI K, WEN C, IVANOVA E P, MALHERBE F, BERNDT C C, PHAM V T H, WANG J. Biomimetic creation of surfaces on porous titanium for biomedical applications [J]. Adv Mater Res, 2014, 896: 259-262.
[2] TAKEMOTO M, FUJIBAYASHI S, NEO M, SUZUKI J, KOKUBO T, NAKAMURA T. Mechanical properties and osteoconductivity of porous bioactive titanium [J]. Biomaterials, 2005, 26: 6014-6023.
[3] ARONIN C E, SADIK K W, LAY A L, RION D B, THOLPADY S S, OGLE R C, BOTCHWEY E A. Comparative effects of scaffold pore size, pore volume, and total viod volume on cranial bone healing patterns using microsphere-based scaffolds [J]. J Biomed Mater Res, 2009, 89: 632-641.
[4] PONADER S, VON W C, WIDENMYER M, LUTZ R, HEINL P, KORNER C, SINGER R F, NKENKE E, NEUKAM F W, SCHLEGE K A. In vivo performance of selective electron beam-melted Ti-6Al-4V structures [J]. J Biomed Mater Res, 2010, 92: 56-62.
[5] LPEZ-HEREDIA M A, GOYENVALLE E, AGUADO E, PILET P, LEROUX C, DORGET M, WEISS P, LAYROLLE P. Bone growth in rapid prototyped porous titanium implants [J]. J Biomed Mater Res, 2008, 85: 664-673.
[6] LI J P, HABIBOVIC P, van den DOEL M, WILSON C E, de WIJN Jr, van BLITTERSWIJK C A, DE GROOT K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition [J]. Biomaterials, 2007, 28: 2810-2820.
[7] van der STOK J, WANG H, AMIN Y S, SIEBELT M, SANDKER M, WAARSING J H, VERHAAR J, HOLGER J, ZADPOOR A A, LEEUWENBURGH S C G, WEINANS H. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors [J]. Tissue Eng: Part A, 2013, 19: 2605-2614.
[8] LOPEZ-HEREDIA M A, GOYENVALLE E, AGUADO E, PILET P, LEROUX C, DORGET M, WEISS P, LAYROLLE P. Bone growth in rapid prototyped porous titanium implants [J]. J Biomed Mater Res, 2008, 85: 664-673.
[9] BOBYN J D, WILSON G J, MACGREGOR D C, PILLIAR R M, WEATHERLY G C. Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants [J]. J Biomed Mater Res, 1982, 16: 571-584.
[10] CHANG B S, LEE C K, HONG K S, YOUN H J, RYU H S, CHUNG S S, PARK K W. Osteoconduction at porous hydroxyapatite with various pore congurations [J]. Biomaterials, 2000, 21: 1291-1298.
[11] BOYDE A, CORIS A, QUARTO R, CANCEDDA R, BIANCO P. Osteoconduction in large macroporous hydroxyapatite ceramic implants: Evidence for a complementary integration and disintegration mechanism [J]. Bone, 1999, 24: 579-589.
[12] HOLY C E, FIALKOV J A, DAVIES J E, SHOICHET M S. Use of a bio-mimetic strategy to engineer bone [J]. J Biomed Mater Res, 2003, 65: 447-453.
[13] ITALA A I, YLANEN H O, EKHOLM C, KARLSSON K H, ARO H T. Pore diameter of more than 100 microm is not requisite for bone ingrowth in rabbits [J]. J Biomed Mater Res, 2001, 58: 679-683.
[14] ST-PIERRE J P, GAUTHIER M, LEFEBVRE L P, TABRIZIAN M. Three-dimensional growth of differentiating MC3T3-E1 pre- osteoblasts on porous titanium scaffolds [J]. Biomaterials, 2005, 26: 7319-7328.
[15] OTSUKIA B, TAKEMOTOA M, FUJIBAYASHIA S, NAKAMURA T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants [J]. Biomaterials, 2006, 27: 5892-5900.
[16] RYAN G, PANDIT A, APATIDIS D P. Fabrication methods of porous metals for use in orthopaedic applications [J]. Biomaterials, 2006, 27: 2651-2670.
[17] OH IH, NOMURA N, MASAHASHI N, HANADA S. Mechanical properties of porous titanium compacts prepared by powder sintering [J]. Scr Mater, 2003, 49: 1197-1202.
[18] DAVIS N G, TEISEN J, SCHUH C, DUNAND D C. Solid state foaming of titanium by superplastic expansion of argon-filled pores [J]. J Mater Res, 2001, 16: 1508-1519.
[19] VEHOFJ W M, SPAUWEN P H M, JANSEN J A. Bone formation in calcium-phosphate-coated titanium mesh [J]. Biomaterials, 2000, 21: 2003-2009.
[20] LI J P, LI S H, de GROOT K, LAYROLLE P. Preparation and characterization of porous titanium [J]. Key Eng Mater, 2002, 218-220: 51-54.
[21] CHEN Liang-jian, LI Ting, LI Yi-min, HE Hao, HU You-hua. Porous titanium implants fabricated by metal injection molding [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 1174-1179.
[22] CHEN Liang-jian, ZHANG Si-hui, LI Yi-min, CUI Xiao-ming, ZHENG Yao, LI Ting. Effect of porosity of modified porous titanium on osteoblastic cells [J]. The Chinese Journal Nonferrous Metals Society of China, 2010, 20: 749-755. (in Chinese)
[23] KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis [J]. Biomaterials, 2005, 26: 5474-5491.
[24] DONG Xin-ni, GUO Xie. The dependence of transversely isotropic elasticity of human femoral cotical bone on porosity [J]. J Biomech 2004, 37: 1281-1287.
[25] ODGAARD A, LINDE F. The underestimation of Young’s modulus in compressive testing of cancellous bone specimens [J]. J Biomech, 1991, 24: 691-698.
[26] AMIN YAVARI S, AHMADI S, WAUTHLE R, POURAN B, SCHROOTEN J, WEINANS H, ZADPOOR A. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials [J]. J Mech Behav Biomed Mater, 2015, 43: 91-100.
[27] ZADPOOR A A. Bone tissue regeneration: The role of scaffold geometry [J]. Biomater Sci, 2015, 3: 231-245.
[28] KOKUBO T, MIYAJI F, KIM H M, NAKAMURA T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals [J]. J Am Ceram Soc, 1996, 79: 1127-1129.
[29] JALOTA S, BHADURI S B, TAS A C. Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions [J]. J Mater Sci Mater Med, 2006, 17: 697-707.
[30] SANDRINI E, GIORDANO C, BUSINI V, SIGNORELLI E, CIGADA A. Apatite formation and cellular response of a novel bioactive titanium [J]. J Mater Sci Mater Med, 2007, 18: 1225-1237.
[31] NISHIGUCHI S, KATO H, FUJITA H, KIM HM, MIYAJI F, KOKUBO T, NAKAMURA T. Enhancement of bone-bonding strengths of titanium alloy implants by alkali and heat treatments [J]. J Biomed Mater Res, 1999, 48: 689-696.
[32] BAI F, WANG Z, LU J, LIU J, CHEN G, LV R, WANG J, LIN K, ZHANG J, HUANG X. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: Aquantitative study [J]. Tissue Eng: Part A, 2010, 16: 3791-3803.
[33] VILLANUEVA A R. A new goldner one-step trichrome stain for identification of osteoid seams, bone and cells in undecalcified, plastic embedded sections of bone [J]. J Histotechnol, 1988, 11: 249-251.
[34] van OERS R F, RUIMERMAN R, van RIETBERGEN B, HILBERS P A, HUISKES R , Relating osteon diameter to strain [J]. Bone, 2008, 43: 476-482.
[35] BAROU O, MEKRALDI S, VICO L, BOIVIN G, ALEXANDRE C, LAFAGE-PROUST M H. Relationships between trabecular bone remodeling and bone vascularization: A quantitative study [J]. Bone, 2002, 30: 604-612.
[36] CHU C, CHUNG C, LIN P, WANG S. Fabrication of porous NiTi shape memory alloy for hard tissue implants by combustion synthesis [J]. Mater Sci Eng A, 2004, 336: 114-119.
[37] WANG H, ELIAZ N, XIANG Z, HSU H P, SPECTOR M, HOBBS L W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy [J]. Biomaterials, 2006, 27: 4192-4203.
[38] TANG Z, TAN Y, NI Y, WANG J, ZHU X, FAN Y, CHEN X, YANG X, ZHANG X . Comparison of ectopic bone formation process induced by four calcium phosphate ceramics in mice [J]. Mater Sci Eng C, 2017, 70: 1000-1010.
[39] CHEN Y, WANG J, ZHU X D, TANG Z R, YANG X, TAN Y F, FAN Y J, ZHANG X D. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: In vitro and in vivo evidence [J]. Acta Biomaterialia, 2015, 11: 435-448.
表面改性多孔钛植入体孔径和孔隙率对骨长入的影响
郑景璞1,陈良建1,2 ,陈代远1,邵春生1,易曼菲1,张 博1
1. 中南大学 湘雅三医院口腔科,长沙 410013;
2. 中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:研究表面改性多孔钛种植体孔隙率和孔径对骨整合的影响。用粉末注射成形技术制备3种多孔钛植入体(A30, A40和A50,造孔剂NaCl的体积分数分别为30%,40%和50%)。将改性后的多孔植入体分别植入狗的背部肌肉和股骨内28、56和84 d后,检测多孔钛植入体与宿主骨之间界面成骨活性和骨组织长入孔隙内的情况。结果表明,喂料中造孔剂NaCl的添加量从30%增至50%(体积分数),多孔钛的总孔隙率从42.4% 增至 62.0%,大孔径(>50 μm)多孔钛的质量分数从8.3% 增至 69.3%。组织学和荧光标记结果显示,A50植入体在28 d时骨界面的成骨活性明显高于其他组,在84 d时A30植入体骨长入的量低于其他组。因此,A50植入体的孔结构适合新骨组织长入多孔植入体。
关键词:多孔钛植入体;孔隙率;孔径;连通性;骨整合
(Edited by Wei-ping CHEN)
Foundation item: Project (81571021) supported by the National Natural Science Foundation of China; Projects (2015WK3012, 2018SK2017) supported by the Hunan Provincial Science and Technology Department Project, China; Project (20160301) supported by New Talent Project of the Third Xiangya Hospital of Central South University, China
Corresponding author: Liang-jian CHEN; Tel: +86-13507405799; E-mail: jian007040@sina.com
DOI: 10.1016/S1003-6326(19)65161-7
Abstract: The effects of surface-modified porous titanium implants with different porosities and pore sizes on osseointegration were investigated in vivo. Three porous titanium implants (A30, A40 and A50 containing volume fractions of space-holder NaCl being 30%, 40% and 50%, respectively) were manufactured by metal injection moulding (MIM). The surface-modified implants were implanted into muscles and femurs of hybrid male dogs. Interface osteogenic activity and histological bone ingrowth of porous titanium implants were evaluated at 28, 56 and 84 d. The results showed that when additive space-holder amount of NaCl increased from 30% to 50% (volume fraction), the general porosity and mass fraction of macropores of porous titanium rose from 42.4% to 62.0% and from 8.3% to 69.3%, respectively. Histologic sections and fluorescent labeling showed that the A50 implant demonstrated a significantly higher osteogenic capacity at 28 d than other implants. Bone ingrowth into the A30 implant was lower than that into other implants at 84 d. Therefore, the pore structure of A50 implant was suitable for new bone tissue to grow into porous implant.


