
Corrosion resistance of 2195 aluminum alloy treated by
multi-step-heating-rate controlled process
XU Yue(许 越), LIU Yu-feng(刘玉峰), GENG Ji-ping(耿季平)
School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics,
Beijing 100083, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
2195 aluminum-lithium alloy was widely applied in the aviation and aerospace industry, but it is highly susceptible to pitting and intergranular corrosion undergoing sever corrosive circumstance and moisture atmosphere. To solve this problem and consequently to prolong its service life, a multi-step-heating-rate(MSRC) process was carried out. Investigations were carried out to find the effect of the MSRC process on the alloys corrosion resistance. It is found that the MSRC process is more favorable for the uniform phase precipitation by comparing the corrosion resistance of samples treated by traditional heat treatments. The potential difference between phases can be reduced and intergranular corrosion is able to be prohibited efficiently. Besides, the rare earth infiltration is beneficial to improving the corrosion resistance. As heating time increases, the corrosion resistance declines gradually, samples treated by artificial aging and solid solution also exhibit a better corrosion resistance.
Key words:
2195 aluminum-lithium alloy; multi-step-heating-rate controlled process; corrosion resistance; rare earth;
1 Introduction
2195 aluminum-lithium alloy is considered to be a kind of the most favorable structural materials in the aviation and aerospace industry attributed to its supereminent mechanical properties such as low density, and high strength-to-mass ratio. However, some of the drawbacks including its low facture toughness, insufficient strength and sever anisotropy have greatly limited its further application as a structural material. Universal studies dedicated for solving these problems have been carried out from several aspects, for instance, the alloy design; new casting and molding methods; and heat treatment[1-5]. By far, most of these investigations were focused on improving the mechanical properties. On the other hand, 2195 Al-Li alloy is highly susceptible to pitting, intergranular corrosion as well as stress corrosion crack undergoing moisture atmospheres or salt spray. This will lead to deterioration of its mechanical properties and decrease of its durability.
Amongst all those methods for improving the properties of 2195 alloy, a better heat treatment will be preferable for promoting its strength and corrosion resistance in corrosive media. This work adopted the multi-step heating-rate controlled(MSRC)[6] treatment to improve the corrosion resistance of 2195 alloy, which is still not widely reported in this field.
2 Experimental
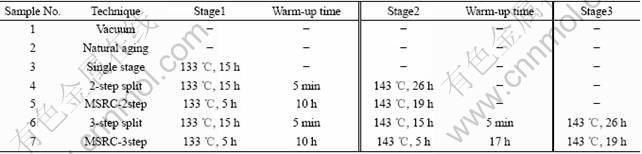
The material adopted in this work was 2195 Al-Li plates with a size of 20 mm×15 mm×2 mm, the solid solution was processed at 530 ℃ within the fluctuation of 1 ℃ for 35 min, quenched in mild water, and then placed in furnace for artificial aging. The parameters of aging techniques are illustrated in Table 1.
Samples chosen for rare earth infiltration were polished by abrasive paper, kept in the cementation furnace filled with evaporated cerium slat at 470 ℃ for 2 h, and then quenched in mild water.
The immersion tests were carried out in the 3.5%NaCl neutral aqueous solution (pH=7) at (35±2)℃. The volume of corrosive media was 20 mL in term of the proportion of working electrode, which was 1 cm2.
Table 1 Technique parameters of aging
The samples were weighted before and after removal of corrosion products and the corrosion rates were calculated by the mass lose versus exposed proportion plus immersion time.
The intergranular corrosion was tested according to the national standard GB7998—2005[7]. The corrosive media was the aqueous solution involving 1 mol/L NaCl+1.94 mol/L H2O2, and the samples were immersed in this solution at (35±2)℃ for 6 h. Then the exposed area were polished, deoiled, rinsed by de-ionized water, and dried in blast orderly for observing the morphology of intergranular corrosion by metallographic microscope. Polarization curves were worked out using CHI604 electrochemical analyzer of a three-electrode system in solution containing 3.5%NaCl. A saturated calomel electrode and a platinum electrode were adopted as the reference and counter electrode respectively. The exposed area of samples for testing was 1 cm2. 3 Results and discussion
The corrosion rate of samples with different heat treatment techniques were calculated and shown in Fig.1.
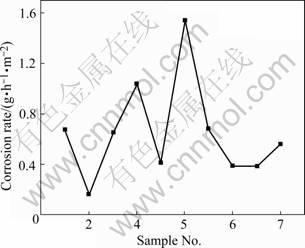
Fig.1 Corrosion rate curve of immersion test
Fig.1 indicates that the corrosion resistance exhibits great improvements after being treated by solid solution and natural aging (sample 2), and there is a sharp decrease around 75% of the corrosion rate in comparison with vacuum ones (sample1). However, the corrosion in the sample treated by 2-step split aging seems to be even more serious (sample 4), and their corrosion rates exceed the vacuum ones greatly. In addition, the corrosion rate treated by 2-step MSRC aging (sample 5) is 58.5% lower than that by 2-step split aged ones (sample 4).
Uniform precipitations of δ′ phase of 2195 Al-Li alloy formed during natural aging processes have fine grain size, so the natural aged sample is more likely to be suffered from uniform corrosion rather than intergranular corrosion. In this case, initially the T1 phase precipitated uniformly along with grain boundary, and the precipitation free zone(PFZ) is still not so obvious. Then as the aging time increases, the coarse of grain size becomes even sever and PFZ grows wider along grain boundary so that the corrosion becomes even faster. Inversely, if treated by MSRC aging, the distribution and the grain size of the precipitated phase turn out to be more uniform, and consequently the corrosion rate is slower than that treated by split aging.
Sample 8 has an inferior corrosion resistance in comparison with sample 2, which suggests that the sample treated by both rare earth infiltration and aging performs worse in corrosive media than those treated solely by a aging process. This can be attributed to the reason that the repeated process involving these two steps actually prolongs the heating time and this aggravates the difference of grain size and the complicacy of phase distribution. However, the sample treated by rare earth infiltration and single-step aging reveals a sharp decrease of 13.7% in corrosion rate than the one that is firstly treated by solid solution in atmospheres without gasified Ce salts. This can be ascribed to the chemical activity of Ce atoms, and because it has a larger atom diameter as well good thermo resistance, it can easily capture the position where lattice dislocation and basal panel slip occur. Then the ingress of corrosion products and detrimental ions along with electrode and oxygen can be retarded. So the oxidization of anode can be prohibited, namely, the corrosion is alleviated.
The depth and the degree of intergranular corrosion are illuminated in Table 2. And the morphologies of intergranular corrosion of samples treated by 3-step split aging and artificial aging respectively are displayed in Fig.2.
Table 2 Depth and degree of IG corrosion
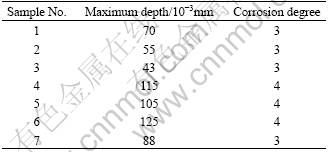
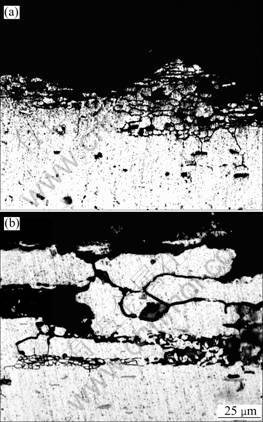
Fig.2 Morphologies of IG corrosion: (a) Natural aging; (b) 3- step split aging
The facts illustrated above show that the prolongation of aging time promotes the susceptibility to intergranular corrosion. The corrosion resistance of the samples treated by 2-step split aging and 3-step split aging is debased and even worse than the bare one. Nonetheless, if treated by MSRC aging, the resistance against intergranular corrosion turns out to be better than that by the spilt aging. Because MSRC process allows a uniform precipitation of each phase, the potential difference is narrowed.
The anodic polarization testing of different samples in 3.5% NaCl solution is plotted and illustrated in Figs.3-5.
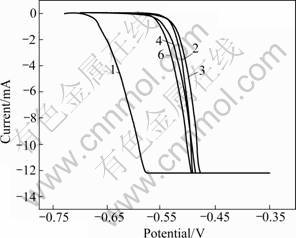
Fig.3 Anodic polarization of split aged samples
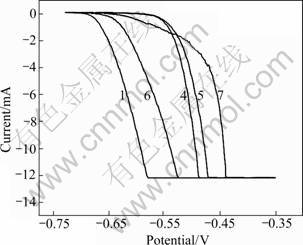
Fig.4 Anodic polarization of MSRC aged samples

Fig.5 Effect of rare earth infiltration on breakage potential
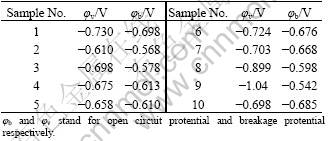
The susceptibilities to pit corrosion were studied from the anodic polarization curves (Figs.3-5). The susceptibility to pit corrosion mainly depends on the φb values. The data in Table 3 read from the anodic polarization curve show that φb shifts positively compared with that of vacuum sample after being treated by various process paths involving RE infiltration and heat treatment. Similarly, from comparison of φb values between sample 4 and sample 5 as well as between sample 6 and sample 7, it can be concluded that the samples treated by MSRC aging exhibit preferable resistance to pit corrosion. Meanwhile, the resistance towards pit corrosion debases with the increase of total time for heat treatment. RE infiltration has a positive effect on the resistance to pit corrosion and this is supported by the fact that sample 9 has a mild increase of φb compared with sample 2. However, the anti corrosion ability is greatly deteriorated since the heating duration is prolonged by repeated procedure, the corrosion resistance against pitting declines. This is supported either by the obvious decrease of φb between sample 10 and sample 9 or by the fairly negative φb value of sample 10 which is almost equal to that of the vacuum sample. It is once evidenced that RE elements as the alloying composition have a superior effect on the refinement and purification of grains which can efficiently inhibit pit corrosion[8]. However, on the other hand, the excessive duration of heat treatment is likely to generate the non-uniform and complex precipitations along grain boundaries which can induce localized corrosion including pitting[9]. Hence, the genuine effects of rare earth infiltration also depend on the technique parameters of heat treatment. So the whole process should be balanced to avoid the complex and non-uniform phase precipitations and meanwhile have the reformative effect of RE elements exerted ultimately[10].
Table 3 Breakage potential φb and φv of samples
4 Conclusions
1) The MSRC process is more favorable for the uniform phase precipitation than the traditional split aging. Consequently, the micro galvanic reaction along grain boundaries as well as intergranular corrosion can be prohibited. As a result, corrosion rate of sample treated by 2-step MSRC process is 8.7% lower than the one treated by 2-step split aging and the intergranular corrosion degree alter from 3 to 2.
2) After being treated by solid solution and artificial aging, the corrosion rate can be reduced by as much as 75% comparing to the vacuum one. This can also be evidenced by the fact that the corrosion rate of sample treated by 2-step MSRC aging decreases by 58.8% whilst corrosion rate of the sample treated by 3-step MSRC is 55.7% lower than the vacuum one. However, the prolongation of heating time can result in the deterioration of corrosion resistance. Pitting can also be retarded by MSRC aging and solid solution with appropriate heating time, and all the breakage potential of samples treated by heating process without overfiring fluctuates positively.
References
[1] HALES S J, HAFLEY R A. Texture and anisotropy in Al-Li alloy 2195 plate and near-net-shape extrusions [J]. Material Science and Engineering A, 1998, 257: 153-164.
[2] KALU P N, ZHANG Lan. Texture evolution in Al-Li 2195 alloy during net shape roll forming [J]. Scripta Materialia, 1998, 39(2): 175-180.
[3] BAI P C, ZHOU T T, LIU P Y. Effects of lithium addition on precipitation in Li-containing Al-Zn-Mg-Cu alloy [J]. Materials Letters, 2004, 58: 3084-3087.
[4] CHEN P S., KURUVILLA A K, MALONE T W. The effects of artificial aging on the microstructure and fracture toughness of Al-Cu-Li alloy 2195 [J]. ASM International, 1998, 7: 682-690.
[5] CHATURVEDI M C, CHEN D L. Effect of specimen orientation and welding on the fracture and fatigue properties of 2195 Al-Li alloy [J]. Mater Sci Eng A, 2004, A387/389: 465-469.
[6] GB/T 7998—2005. Testing Methods for IG Corrosion of Aluminum alloy [S].
[7] MENG L, ZHENG X L. Overview of the effects of impurities and rare earth elements in Al-Li alloys [J]. Mater Sci Eng A, 1997, 237: 109-115.
[8] YOSHIMURA R, KONNO T J, ABE E. Transmission electron microscopy study of the early stage of precipitates in aged Al-Li-Cu alloys [J]. Acta Materialia, 2003, 51: 2891-2903.
[9] LI J F, ZHENG Z Q, JIANG N. Study on localized corrosion mechanism of 2195 Al-Li alloy in 4.0% NaCl solution [J]. Materials and Corrosion, 2005, 56(3): 192-196.
[10] CHATURVEDI M C, CHEN D L. Effect of specimen orientation and welding on the fracture and fatigue properties of 2195 Al–Li alloy [J]. Mater Sci Eng A, 2004, 387/389: 465-469.
(Edited by LI Xiang-qun)
Foundation item: Project(51471050105HK0101) supported by the National Key Laboratory of Precision Thermal Treatment, Harbin Institute of Technology, China
Corresponding author: XU Yue; Tel: +86-10-82316161; E-mail: xuyue@buaa.edu.cn


