Trans. Nonferrous Met. Soc. China 24(2014) 279-284
Effects of Fe-Al intermetallic compounds on interfacial bonding of clad materials
Qian WANG, Xue-song LENG, Tian-hao YANG, Jiu-chun YAN
State Key Laboratory of Advanced Welding and Joining, Harbin Institute of Technology, Harbin 150001, China
Received 29 October 2012; accepted 13 March 2013
Abstract:
The growth of intermetallic compounds at the interface between solid Al and Fe and the effects of intermetallic compound layers on the interfacial bonding of clad materials were investigated. The results showed that the interface between the solid Fe and Al formed by heat-treatment consisted of Fe2Al5 and FeAl3 intermetallic compound layers, which deteriorated the interfacial bonding strength. Fractures occurred in the intermetallic compound layer during the shear testing. The location of the fracture depended on the defects of microcracks or voids in the intermetallic compound layers. The microcracks in the intermetallic compound layer were caused by the mismatch of thermal expansion coefficients of materials during cooling, and the voids were consistent with the Kirkendall effect. The work will lay an important foundation for welding and joining of aluminum and steel, especially for fabrication of Al-Fe clad materials.
Key words:
Al-Fe clad materials; interfacial bonding; Fe-Al intermetallic compounds; interface structure; mechanical properties;
1 Introduction
Aluminum can effectively improve the properties of Fe with excellent corrosion resistance, reduced mass and durability. To achieve the combined properties of Al and Fe, it is necessary to join these metals. Some different methods are used to produce Al/Fe clad materials [1-3]. The methods of hot-dipped-aluminum and roll bonding are important production method commonly used to manufacture Al-Fe cladding materials [4-7]. However, intermetallic compound (IMC) layers composed of FeAl3, Fe2Al5 and others were observed at the interface of Al coating and the steel substrate in the process [1-7].
The microstructure and phase evolution of the interface of hot-dipped-aluminized mild steel (at 700 °C for 180 s) during high-temperature treatment (at 750 °C for 5 min to 480 h) have been studied [5]. Fe2Al5 and FeAl3 are observed at the interface of materials. During high-temperature treatment, FeAl2 is formed at the interface of Fe2Al5-steel substrate in the hot-dipped- aluminized mild steel. Increasing the diffusion treatment time, Fe2Al5 and FeAl3 are gradually replaced by FeAl2 and FeAl. Finally, the intermetallic compound layers are composed of FeAl2 and FeAl. The growth of the IMC layers has been found to be controlled by diffusion, and the solutions of diffusion equations have been presented [4].
The liquid-solid roll bonding (750 and 850 °C) methods of joining Al-Sn-Pb alloy and steel have been studied previously [6]. Al-clad Fe sheet is produced by solid-solid (450 and 600 °C) rolling process [7]. In these researches, the Fe-Al intermetallic compound layers were observed at the interface.
The intermetallic compound layers are important to obtain materials with excellent performance. Thus, it is essential to understand the effect of different types of intermetallic compounds at the interface. In this study, solid Al and Fe were heat-treated, and the microstructure of intermetallic compounds was studied. The reaction between solid Al and solid Fe as well as influence of the Al-Fe intermetallic compound layers on interfacial bonding was discussed. This work provided guidelines for the prediction and control of interface intermetallic compounds during the welding and joining processes, including producing Al-clad Fe material by roll-bonding under solid-phase conditions, and it also provided a reference for the influence on the interfacial bonding of clad materials.
2 Experimental
The 1.5 mm-thick plate of pure Fe coated with a 0.07 mm-thick film of Al was used as core material in this study. The plates were cut to dimensions of 45 mm× 10 mm×1.5 mm. Figure 1 shows the interfacial microstructure of the Fe coated with Al produced by roll-bonding. The interface was clean and not composed of Al-Fe intermetallic compounds.
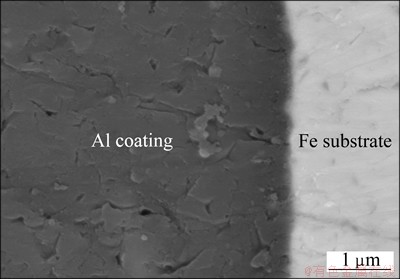
Fig. 1 Backscattered electron image of Al-Fe clad materials
In this work, the solid Fe and solid Al were heated to 620 °C or (630±2) °C and held for 120 min in air. Thereafter, they were cooled to room temperature.
The mechanical properties of the interfacial bonding were measured using a special method for shear strength tests. A 5A06 aluminum plate with dimensions of 45 mm×10 mm× 4 mm was joined to a Fe-Al clad materials plate, as shown in Fig. 2. The bonding strength between the 5A06 Al and the pure Al coating layer is stronger than that between the Fe substrate and the Al coating layer. Tensile shear strength tests were carried out at room temperature at a cross-head speed of 1 mm/min using a tensile testing machine (Instron-5569).
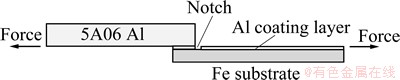
Fig. 2 Schematic diagram of tensile shear samples
The specimens were cross sectioned for metallographic analyses using an electrical-discharge cutting machine. The cross sections of the metallographic specimens were polished with a diamond polishing agent. The interfacial structure and elemental distribution in the joint were analyzed by SEM equipped with an EDX spectroscopy analysis system (HITACHI S-4700). The phase structure on the surface of fracture of the joints was determined by XRD (D/max-rB).
3 Results and discussion
3.1 Interface structure of Al-Fe clad materials heated at 630 °C
Figure 3 shows the microstructure and elemental distributions of the interface of Al-Fe clad materials heated at 630 °C for 120 min. There were defects (Fig. 3(a)), such as microcracks and voids, in the intermetallic compound layers. Most of the microcracks ran along almost the length of the interface direction. Figure 3(b) shows the lap joints consisted of four parts: an un-reacted Al coating, a darker gray phase, a lighter phase layer and a Fe substrate. There were continuous lines in Fig. 3(b), indicating that intermetallic compound layers existed at the interface. The darker gray phase was Al-rich, and the lighter phase was Fe-rich. The Al-rich and Fe-rich intermetallic compound layers were termed IMC1 and IMC2, respectively. A small amount of IMC1 existed close to the Al coating. Majority of the intermetallic compounds was IMC2.
Figure 4 shows the cross section of the fracture of Al-Fe clad materials heated at 630 °C for 120 min after the tensile test. The fracture location was in the IMC2,
and the fracture was along almost the length of the interface direction with IMC2.
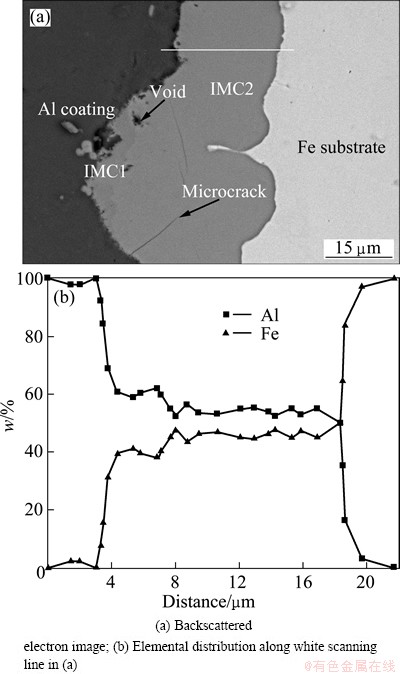
Fig. 3 Microstructure and elemental distributions of Al-Fe clad materials heat treated at 630 °C for 120 min
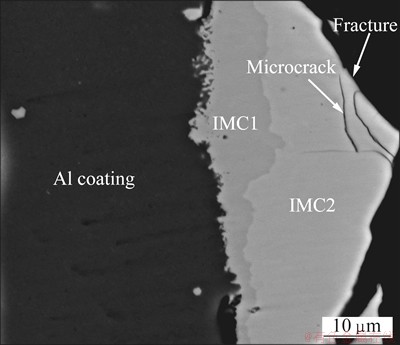
Fig. 4 SEM image showing fracture location of Al-Fe clad materials heat treated at 630 °C for 120 min after tensile test
Figure 5 (a) shows the secondary electron image of the fracture surface of Al-Fe clad materials heat treated at 630 °C for 120 min after the tensile test. In Fig. 5(a), the bright and dark zones occurred because the fracture occurred in different compound layers. The fracture location of the black zones was deeper from the Fe side and closer to the Fe substrate than the light zones.
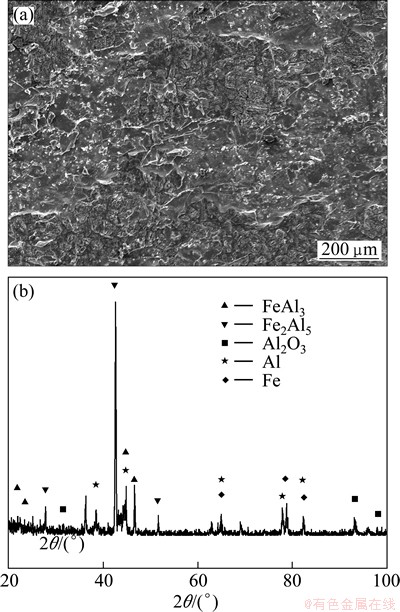
Fig. 5 Secondary electron image (a) and XRD pattern (b) of tensile fracture surface morphology of Fig. 4
To further determine the type of intermetallic compound layers formed at the interface of Al-Fe clad materials heated at 630 °C for 120 min, the XRD pattern from the fractured surfaces of the Fe sides was analyzed (Fig. 5(b)). Fe2Al5, FeAl3, Fe, Al and Al2O3 were detected in the XRD pattern and majority of the intermetallic compounds was Fe2Al5 phase. Therefore, the interface of Al-Fe clad materials heated at 630 °C for 120 min mainly consisted of Fe2Al5 and FeAl3 layer. IMC1 was FeAl3 and IMC2 was Fe2Al5. The fractures mainly occurred at the Fe2Al5 intermetallic compound layer during the tensile-shear test.
3.2 Interface structure of Al-Fe clad materials heated at 620 °C
Figure 6 shows the microstructure and elemental distribution at the interface of Al-Fe clad materials heated at 620 °C after 120 min. The continuous lines were observed in Fig. 6(b), indicating that intermetallic compound layers were generated. Figure 6(a) shows that the lap joints consisted of four parts: an un-reacted Al coat, a darker gray phase, a lighter phase layer and a base material of Fe. The darker gray phase was Al-rich, and the lighter phase was Fe-rich, which were termed IMC3 and IMC4, respectively. The IMC3 layer was thicker than the IMC4 layer. There were no obvious microcracks or voids in IMC4. However, there were some defects such as voids and microcracks in IMC3.
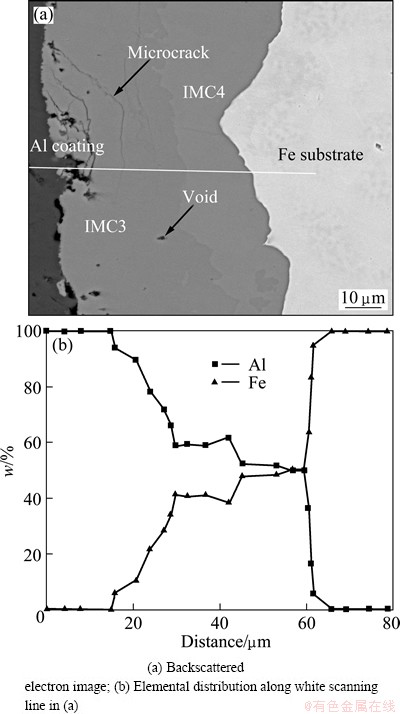
Fig. 6 Microstructure and elemental distribution of Al-Fe clad materials heat treated at 620 °C for 120 min
Figure 7 shows the cross section of the fracture of Al/Fe clad materials heated at 620 °C for 120 min after the tensile test. The fracture existed along almost the length of the interface direction, and occurred at IMC3. SEM micrograph of the fracture surface is shown in Fig. 8(a). The bright and dark zones were observed because the fracture occurred in different compound layers. The fracture location of the light zones was much shallower from the Fe substrate and closer to the Al coat layer than the dark zones.
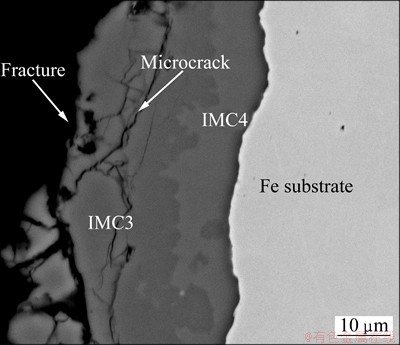
Fig. 7 SEM image showing fracture location of Al-Fe clad materials heat treated at 620 °C for 120 min after tensile test
The intermetallic compound layers formed at the interface of Al-Fe clad materials heat treated at 620 °C for 120 min were analyzed by XRD from the fractured surface of the Fe sides. The results from the fractures are shown in Fig. 8(b). The intermetallic compound of FeAl3 with Fe2Al5, Al and Al2O3 elements was found to be present. IMC3 was FeAl3 and IMC4 was Fe2Al5. The light zones were FeAl3 phase. The overwhelming majority of fractures occurred in the FeAl3 intermetallic compound layer during the tensile test and only a few fractures occurred in the Fe2Al5 intermetallic compound layer.
3.3 Reaction of solid Al and solid Fe
From the above analysis and Fe-Al binary diagram, it was concluded that the interface of solid Al coating and solid Fe substrate involved the formation of Fe-Al intermetallic compounds. The layer composition gradually approached the composition of the Al-rich intermetallic compounds from the Fe substrate to the Al coating.
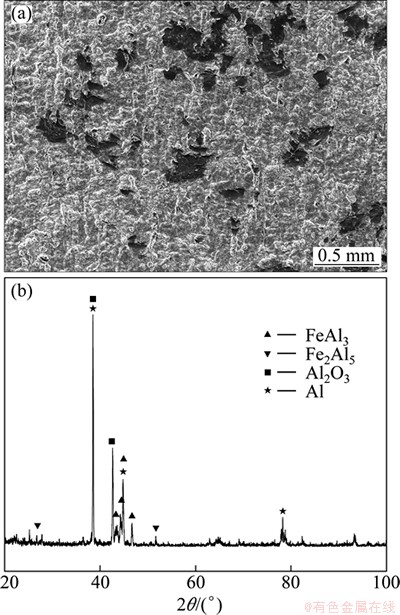
Fig. 8 Secondary electron image (a) and XRD patterns (b) of tensile fracture surface morphology in Fig. 7
During the reaction between solid Al and solid Fe, the formation of the Fe2Al5 and FeAl3 phase was detected and other Fe-Al phases were not found, which was consistent with the reaction between liquid Al and solid Fe as observed by other authors [4,5,8]. However, the morphology of intermetallic compounds at the solid–solid interface was different from that at the solid–liquid interface.
The Fe2Al5 phase was slightly tongue-like and regular with peaks oriented towards Fe. However, the FeAl3 layer was slightly thicker than the Fe2Al5 layer in this work, and the morphology of the FeAl3 phase was not thin needles or platelets uniformly dispersed in the solidification aluminum matrix which was reported by other authors [4,5,8]. The thickness of the two intermediate phases was 20-50 μm. However, the thickness of the two intermediate phases was more than 100 μm when the solid–solid interface reacted only a few minutes [4,5,8]. The inter-diffusion controlled their growth, and the growth rate of the two intermediate at solid–solid interface was slower than that at solid–liquid interface.
3.4 Effect of Fe-Al intermetallic compounds
Figure 9 shows that the shear strength to fracture occurred at different locations after the tensile-shear test. The shear strength of the original Al-Fe clad materials was about 70 MPa. However, the shear strength was only about 20 MPa when the fracture occurred at the intermetallic compound layers. Both Fe2Al5 and FeAl3 caused deterioration of the Al-Fe clad materials bonding.
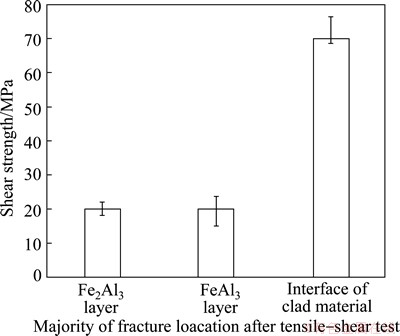
Fig. 9 Shear strength to fractures occurring at different locations
In this study, the defects at the interface of Fe-Al reaction layers were observed [3,9,10]. However, the fractures mainly occurred at the intermetallic compound layer of Fe2Al5 or FeAl3, which was different from other reports [11-15]. The location of the fracture depended on the defects in Fe2Al5 and FeAl3 layers. The intermetallic compounds led to the fracture of the weakest part of the interface. Therefore, the intermetallic compound layer significantly deteriorated the mechanical properties of the interface.
The thermal expansion coefficients for Fe (12.2× 10-6 K-1), Al (23.5×10-6 K-1) and Al-Fe intermetallic compounds (18.94×10-6 K-1 for Fe2Al5, 19.68×10-6 K-1 for FeAl3) [16,17] were different from each other. The intermetallic compound layers usually formed at a higher temperature. During cooling, the different thermal expansion coefficients of the Fe substrate, Al coating and intermetallic compounds caused residual stress along the interface direction. This further caused internal microcracks which were found in the intermetallic compound layer. Microcracks were the main factor of the fracture.
According to results of CHENG and WANG [5], the formation of internal voids resulted from the Kirkendall effect. The different diffusion rates of Fe and Al caused a net flux of vacancies to the interface where voids condensed out. The Kirkendall effect was a rational mechanism for voids observed at the interface. The voids resulted in stress concentration which accelerated the expansion of the crack.
4 Conclusions
1) Intermetallic compounds of Fe2Al5 and FeAl3 were detected at the interface of Al-Fe clad materials with heat-treatment. The interface between the solid Fe and solid Al with heat-treatment consisted of Fe2Al5 intermetallic compound layer and FeAl3 intermetallic compound layer. The morphology of intermetallic compounds at the solid-solid interface was different from that at the solid–liquid interface.
2) The intermetallic compound layers significantly deteriorated the mechanical properties of Al-Fe clad materials. Shear strength decreased from 70 to 20 MPa. The fractures mainly occurred at the Fe2Al5 or FeAl3 intermetallic compound layer after tensile test.
3) The defects in the Fe2Al5 and FeAl3 layers are the dominant element of controlling the location of the fracture. The formation of internal microcracks in the intermetallic compound layers was caused by the thermal expansion coefficient mismatch between the materials, and the formation of internal voids was consistent with the Kirkendall effect.
References
[1] ZHU Xiao-lin, YAO Zheng-jun, GU Xue-dong, CONG Wei, ZHANG Ping-ze. Microstructure and corrosion resistance of Fe-Al intermetallic coating on 45 steel synthesized by double glow plasma surface alloying technology [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 143-148.
[2] ZHAN Zhao-lin, HE Ye-dong, WANG De-ren, GAO Wei. Preparation of aluminide coatings at relatively low temperatures [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 647-653.
[3] LEE Jung-Han, KIM Jong-Do, OH Jin-Seok, PARK Seo-Jeong. Effect of Al coating conditions on laser weldability of Al coated steel sheet [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(4): 946-951.
[4] BOUCHE K, BARBIER F, COULET A. Intermetallic compound layer growth between solid iron and molten aluminium [J]. Materials Science and Engineering A, 1998, 249(1-2): 167-175.
[5] CHENG W, WANG C. Study of microstructure and phase evolution of hot-dipped aluminide mild steel during high-temperature diffusion using electron back scatter diffraction [J]. Applied Surface Science, 2011, 257(10): 4663-4668.
[6] XU G, LI B, CUI J. Interfacial microstructure of Al-Sn-Pb alloy-steel during liquid-solid bonding rolling [J]. Journal of Iron and Steel Research International, 2006, 13(4): 40-43.
[7] DANESH MANESH H, KARIMI TAHERI A. Bond strength and formability of an aluminum-clad steel sheet [J]. Journal of Alloys and Compounds, 2003, 361(1-2): 138-143.
[8] AGUDO L, EYIDI D, SCHMARANZER C H, ARENHOLZ E, JANK N, BRUCKNER J. Intermetallic FexAly-phases in a steel/Al-alloy fusion weld [J]. Journal of Material Science, 2007, 42(12): 4205-4214.
[9] CHANG Y, TSAUR C C, ROCK J C. Microstructure studies of an aluminide coating on 9Cr-1Mo steel during high temperature oxidation [J]. Surface and Coatings Technology, 2006, 200(22-23): 6588-6593.
[10] BOUAYAD A, GEROMETTA CH, BELKEBIR A, AMBARI A. Kinetic interactions between solid iron and molten aluminium [J]. Materials Science and Engineering A, 2003, 363(1-2): 53-61.
[11] BORRISUTTHEKUL R, YACHI T, MIYASHITA Y, MUTOH Y. Suppression of intermetallic reaction layer formation by controlling heat flow in dissimilar joining of steel and aluminum alloy [J]. Materials Science and Engineering A, 2007, 467(1-2): 108-113.
[12] PEYER P, SIERRA G, DESCHAUX-BEAUME F, STUART D, FRAS G. Generation of aluminium-steel joints with laser-induced reactive wetting [J]. Materials Science and Engineering A, 2007, 444(1-2): 327-338.
[13] SIERRA G, PEYRE P, DESCHAUS-BEAUME F, STUART D, FRAS G. Steel to aluminium key-hole laser welding [J]. Materials Science and Engineering A, 2007, 447(1-2): 197-208.
[14] SIERRA G, PEYRE P, DESCHAUX-BEAUME F, STUART D, FRAS G. Galvanised steel to aluminium joining by laser and GTAW processes [J]. Materials Characterization, 2008, 59(12): 1705-1715.
[15] CHEN Y C, KOMAZAKI T, KIM Y G, TSUMURA T, NAKATA K. Interface microstructure study of friction stir lap joint of AC4C cast aluminum alloy and zinc-coated steel [J]. Materials Chemistry and Physics, 2008, 111(2-3): 375-380.
[16] MASAHASHI N, WATANABE S, NOMURA N, SEMBOSHI S, HANADA S. Laminates based on an iron aluminide intermetallic alloy and a CrMo steel [J]. Intermetallics, 2005, 13(7): 717-726.
[17] LIU Jin-bang. Steel hot dipped aluminum [M]. Beijing: Metallurgy Industry Press, 1995: 14-15. (in Chinese).
Al-Fe金属间化合物对复合板界面结合的影响
王 谦,冷雪松,杨天豪,闫久春
哈尔滨工业大学 先进焊接与连接国家重点实验室,哈尔滨 150001
摘 要:对固态铝和固态铁界面金属间化合物的生长及金属间化合物对界面结合的影响进行了研究。结果表明,固态铝和固态铁热处理后的界面主要包括Fe2Al5和FeAl3化合物层,金属间化合物恶化了界面结合强度。在拉剪测试中,断裂主要发生在Fe2Al5或FeAl3化合物层,断裂的位置主要取决于化合物层内部的缺陷,包括微裂纹和空洞。热膨胀系数不匹配产生的应力导致内部微裂纹产生,内部孔洞产生的原因是Kirkendall效应。该研究对铝和铁的焊接与连接,尤其是对铝钢复合板的制备,奠定了一定的基础。
关键词:Al-Fe复合板;界面结合;Al-Fe金属间化合物;界面结构;力学性能
(Edited by Xiang-qun LI)
Foundation item: Project (2011DFR50630) sponsored by the International S&T Cooperation of China
Corresponding author: Jiu-chun YAN; Tel: +86-451-86416607; E-mail: jcyan@hit.edu.cn
DOI: 10.1016/S1003-6326(14)63058-2
Abstract: The growth of intermetallic compounds at the interface between solid Al and Fe and the effects of intermetallic compound layers on the interfacial bonding of clad materials were investigated. The results showed that the interface between the solid Fe and Al formed by heat-treatment consisted of Fe2Al5 and FeAl3 intermetallic compound layers, which deteriorated the interfacial bonding strength. Fractures occurred in the intermetallic compound layer during the shear testing. The location of the fracture depended on the defects of microcracks or voids in the intermetallic compound layers. The microcracks in the intermetallic compound layer were caused by the mismatch of thermal expansion coefficients of materials during cooling, and the voids were consistent with the Kirkendall effect. The work will lay an important foundation for welding and joining of aluminum and steel, especially for fabrication of Al-Fe clad materials.


