- Abstract:
- 1 Introduction ▲
- 2 Experimental ▲
- 3 Results and discussion ▲
- 4 Conclusions ▲
- References
- Figure
- Fig.1 Effect of concentration of 1-butanol on precipitation ratio
- Fig.2 Effect of concentration of 1,4-dioxane on precipitation ratio
- Fig.3 Effect of concentration of tetrahydrofurane on precipitation ratio
- Fig.4 Effect of type of additive on precipitation ratio
- Fig.5 Agglomeration efficiency of products
J. Cent. South Univ. Technol. (2008) 15: 622-626
DOI: 10.1007/s11771-008-0116-6
![]()
Effect of tetracarbon additives on gibbsite precipitation from
seeded sodium aluminate liquor
ZENG Ji-shu(曾纪术), YIN Zhou-lan(尹周澜), CHEN Qi-yuan(陈启元)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract:
Effects of tetracarbon additives, 1-butanol, 1,4-dioxane and tetrahydrofurane on gibbsite precipitation from caustic seeded sodium aluminate liquor were investigated. The additive was charged into supersaturated seeded sodium aluminate liquor, then the precipitation ratio of aluminate liquor in 10 h was evaluated and compared, and the particle size distribution of product was measured. The Mulliken atomic charge of oxygen in the additive molecule was calculated with DMol3 program. The results show that 1-butanol has little effect on gibbsite crystallization, while the precipitation ratios under the effect of 1,4-dioxane and tetrahydrofurane are 1.7% and 3.6% higher than that of the blank, respectively. The agglomeration efficiency of the product is also enhanced obviously by the addition of 1,4-dioxane and tetrahydrofurane. The precipitation ratio is inversely proportional to Mulliken atomic charge of oxygen atom, which implies that functional group in the additive molecule is involved in the gibbsite precipitation process more fundamentally than carbon chain.
Key words:
sodium aluminate; gibbsite; additive; precipitation;
1 Introduction
A great deal of effort has been expended in intensifying gibbsite precipitation from sodium aluminate liquor, and some progresses were achieved in the past years. Optimizing the technological parameters, activating the seed, introducing external field and adopting additive were found to be effective on accelerating gibbsite crystallization. Among these methods, adopting organic additive is more widely used for no significant change of device and technological parameters are needed when this method is adopted in the production of alumina. Investigations[1-5] indicate that some organic polymers and surfactants can promote gibbsite precipitation from sodium aluminate liquor. Hydroxyl group or ether bond is found to present in all these additives molecules. To the best of our knowledge, inert organic compounds, such as aliphatic hydrocarbons, especially with short carbon chain, are indifferent to gibbsite crystallization. So it can be concluded that the functional groups in the additive molecules play a key role in the gibbsite precipitation. Though some additives with hydroxyl group or ether bond were deemed to favor gibbsite crystallization, some other alditols were reported to inhibit gibbsite crystallization severely[6]. HELEN[6] proposed that the effect of additive is related not only to the number of hydroxyl groups of a given compound, but also to the stereochemistry. Actually, interfacial chemistry of gibbsite would change with the electric charge. For example, there is a very strong variation in charging behavior for different gibbsite crystal planes, so it is the reacting activity[7]. It can be inferred from above that the atom charges of additive, especially the charge functional group, is also an important factor that influences the behavior of additive in the seeded sodium aluminate liquor.
In this study, effect of three organic additives on gibbsite precipitation from seeded aluminate liquor under selected conditions were investigated. These additives were 1-butanol, 1,4-dioxane and tetrahydrofurane (THF). The Mulliken atomic charges of the additive molecules were calculated on C2 workstation, using Dmol3 program. The relationship between the Mulliken popula- tion of oxygen in the additive molecules and the effect of these additives on precipitation ratio were studied.
2 Experimental
2.1 Materials and preparation of solution
Additives used in present study, 1-butanol, 1,4- dioxane and tetrahydrofurane, were all analytical grade, and purchased from market. Aluminum hydroxide (industrial grade, supplied by Zhengzhou Research Institute of Aluminium Limited Corporation of China) was washed, dried and then screened with 45 μm sieve size. The fine powder was used as crystal seed.
Distilled water was used to prepare all solutions. Supersaturated sodium aluminate solution was prepared by dissolving aluminum hydroxide into hot NaOH solution. After aluminum hydroxide was completely dissolved, the solution was filtered twice and then diluted to the required volume. The concentration of alkali (Na2Ok) was 140 g/L, and the caustic molar ratio (αk, NaOH to Al(OH)3) of solution was 1.40.
2.2 Experiment method
Sodium aluminate solution was transferred to a batch crystallizer, which was bathed with temperature- controlled water and agitated. After the temperature of the solution reached the desired point, a certain amount of additive and seed were added to the solution in turn. Samples were taken out at intervals and centrifugated. Optical clean solution was used for aluminium and alkali analysis. Metallurgical industry standard of YB-817-75 was adopted for the determination of chemical components. This is a titrimetric method based on the work of Watts and Utley[8]. Precipitation ratio (η) was used to weigh the effect of additives, which is defined as
η=(1-αt/α0)×100%
where α0 and αt denote caustic molar ratio of liquor at initial time and time t, respectively. The samples were filtered and then the precipitates were washed with hot distilled water, dried at 70 ℃ for 24 h before particle size distribution analysis(Malvern Mastersizer 2000).
2.3 Apparatus and conditions
The crystallizer was a 1 L stainless steel vessel with double jacket, equipped with two baffles and a central, 3-blade and 45?-pitch overhead propeller. A hole was reserved on the cover of crystallizer for the usage of feeding and sampling, which is well sealed with a rubber plug in the rest time. During all the crystallization periods, the solution was kept at 75 ℃ and agitated at 150 r/min. A precipitation experiment, carried out without addition of any organic compound, was used as reference (the blank). In other experiments, 0.2, 0.4 or 1.0 mmol/L additives were charged.
2.4 Calculation of Mulliken population of additives
The molecular models of additives were initially constructed. The COMPASS of forcefield in Forcite Package was adopted in geometry optimization. GGA- PW91
function in DMol3 module was used for energy calculation and population analysis, and all the quality was defined as medium level.
3 Results and discussion
3.1 Effect of additives on precipitation ratio
Effects of various concentrations of 1-butanol, 1,4- dioxane and tetrahydrofurane on gibbsite precipitation from supersaturated caustic sodium aluminate liquor are shown in Figs.1, 2 and 3, respectively. Fig.1 shows that gibbsite precipitation is insensitive to the addition of 1-butanol. When concentration of 1-butanol is 0.2 mmol/L, the precipitation ratio is almost the same as that of the blank in 10 h, and a little inhibition appears when the concentration increases to 1.0 mmol/L. Fig.2 suggests that 1,4-dioxane enhances precipitation ratio in 10 h. The distinction of precipitation ratio between the blank and the experiment when 1,4-dioxane was added is invisible in initial 4 h, while the enhancing effect becomes evident after 6 h, and the precipitation ratio continuously promotes as time elapses. The precipitation ratio of the blank is 17.3% at 10 h and it gets to 18.1% and 18.8% with the addition of 0.2, 0.4 mmol/L 1,4-dioxane, respectively. The enhancing effect is very limited for it seems that the precipitation ratio does not increase any more in spite of the increase of concentration of 1,4-dioxane. Obviously, the effect of tetrahydrofurane on gibbsite precipitation is stronger than that of 1,4-dioxane (Fig.3). It is clear that tetrahydrofurane accelerates gibbsite precipitation from the starting time. The precipitation ratio rises stably with the increase of concentration of tetrahydrofurane.
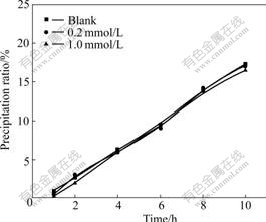
Fig.1 Effect of concentration of 1-butanol on precipitation ratio

Fig.2 Effect of concentration of 1,4-dioxane on precipitation ratio

Fig.3 Effect of concentration of tetrahydrofurane on precipitation ratio
In order to compare the effect of these three different kinds of compound on the gibbsite precipitation more clearly, the curve of precipitation ratio versus time of the blank and liquors containing the three additives at the concentration of 1.0 mmol/L is plotted and shown in Fig.4. By comparing the precipitation lines, it can be found the sequence of precipitation ratio from low to high is as follows: 1-butanol, the blank, 1,4-dioxane and tetrahydrofurane. The carbon chain presented in the molecular structure of the three additives does not affect the decomposition of aluminate ions. Variations of precipitation ratio in the presence of these additives should primarily be owned to the behavior of functional group in the sodium aluminate liquor for the interaction of functional group with seed surface is always preferential.

Fig.4 Effect of type of additive on precipitation ratio
In order to interpret the results presented above, we tried to take the atom charge of additive into account, especially oxygen atom charge, which is assumed to be a rather important factor to gibbsite precipitation rate. The Mulliken atomic charges of oxygen atoms of the additives and the corresponding precipitation ratio in 10 h are listed in Table 1.
Table 1 Oxygen atom charge of additives and precipitation ratio

Table 1 shows that the precipitation ratio is inversely proportional to Mulliken atomic charge of oxygen atom. Though the data presented here are limited, some clue can be found from this study and previous publications.
RODNEY et al[9] investigated the adsorption behavior of a number of organic compounds to kaolinite and gibbsite and concluded that the adsorption is selective. The selective adsorption seems to be related to the charge population of additive molecules for the surface of gibbsite crystal is not electrically neutral, and electric quantity on the surface is variant on different faces due to different coordinated surface groups[9]. The adsorption of electron donor on gibbsite surface would lead to an increase of Fermi level of gibbsite surface. The Fermi level of gibbsite surface becomes higher with more electron transfer from the adsorbents, and the whole adsorption system is more stable as well[10]. This indicates that the functional group with more charge population would be rather easily adsorbed, transferring more charge to the gibbsite surface, and reaching a more stable state, and therefore decreasing the crystallization rate. On the other hand, some organics in the liquor can be adsorbed on the surface of gibbsite, making the activation energy (edge free energy) for nucleation lower, thereby enhancing precipitation[11]. The action mechanism of additives to gibbsite precipitation can be summarized as follows: changing the nature of solid/solution interface and influencing the integration of growth unit. The effect of additives, enhancement or inhibition, is associated with the interaction strength of additive molecules and gibbsite surfaces. Those organics with strong polarity would be adsorbed strongly at the active growth sites on the surface of gibbsite, blocking crystal from further growth, then the crystallization is slowed down. While those additives with median polarity are adsorbed on the seed surface mildly, lowering the activation energy but not inhibiting crystal from growth severely, so the crystallization is favored.
At present, we do not clearly know what is the optimal charge population or optimal polarity to a exact additive structure. Judging from Table 1, 1-butanol is a relatively strong polarity compound, and can be grouped into inhibitors, while 1,4-dioxane and tetrahydrofurane (THF) are median polarity accelerants. Establishing a model to predict the effect of a compound would be an urgent and meaningful work.
3.2 Effect of additives on particle size distribution of product
Particle size distributions of the seed and the products evolved in the blank liquor and liquor con- taining 1.0 mmol/L additives are assembled in Table 2.
Table 2 suggests that the median size (d50) of the products enlarges obviously compared with the seed. After being evolved in the sodium aluminate liquor for 10 h, the particles less than 30 μm decease apparently and particles larger than 45 μm increase greatly, while particles in the range of 30-45 μm are relatively stable. All information indicates that the seeds have undergone an obvious agglomeration process. Referring to Refs.[12-13], agglomeration mainly occurs among the relatively small particles(usually less than 20 or 30 μm), while large particles scarcely agglomerate for kinetics reasons. Therefore, present results are in good agreement with the conclusions drawn from literatures.
The sequence of the median size of the product is: tetrahydrofurane>1,4-dioxane>1-butanol>the blank, which is approximately consistent with the sequence of precipitation ratio. Reviewing the fluctuation of the particle size distribution, it can be found the particles larger than 45 μm increase while particles in the range of 30-45 μm and 20-30 μm decrease, again obeying the order of tetrahydrofurane>1,4-dioxane>1-butanol>the blank. Particles in the range of 30-45 μm decrease apparently when tetrahydrofurane is added. Therefore, the addition of tetrahydrofurane may boost the agglomeration of relatively larger particles.
The variations of particle size distribution or median size of products are mainly due to different degree of agglomeration if no obvious second nucleation occurs[14]. Several methods have been developed to evaluate the degree of agglomeration, and there are some differences in the form of expression[15-16]. In this study, a widely accepted agglomeration efficiency was adopted to investigate the degree of agglomeration, which is presented in Eqn.(1).
I=(Ks+η)P/(KsP0)-1 (1)
where I is the agglomeration efficiency; Ks is the seed mass ratio; η is the precipitation ratio of the solution; P is the mass fraction of the particles over 45 μm in the product and P0 is the mass fraction of the particles over 45 μm in the seed.
Based on Eqn.(1), the results of agglomeration efficiency of the products evolved in the blank solution and solution containing additives are shown in Fig.5. Apparently, 1-butanol does not affect also gibbsite agglomeration, just like its performance to gibbsite precipitation. 1,4-dioxane and tetrahydrofurane promote agglomeration efficiency, and the promotion is more evident when tetrahydrofurane is added into the liquor. Concerning the enhancement mechanism of agglomeration by the additives, there are several possibilities. Firstly, agglomeration efficiency is promoted by high precipitation rate. Collisions between particles are preconditions of agglomeration but not all collisions result in agglomeration. When collisions take place, loose aggregates may form. The loose aggregates may be cemented quickly and tightly by “new” aluminium hydroxide if the precipitation rate is high enough. Otherwise, they will disintegrate back into small particles under the shear stress of fluid. The promotion of precipitation rate by tetrahydrofurane and 1,4-dioxane may lead to the promotion of agglomeration efficiency. Secondly, agglomeration efficiency is promoted by the decrease of hydration force. Many experiments proved that there is strong non-DLVO forces between gibbsite particles, which hinders particle approaching, subsequent collision and further agglomeration[17-18]. Hydration force is one kind of non-DLVO forces. With the adsorption of organic additives, gibbsite crystal surfaces have some degrees of hydrophobic character. The attraction force between hydrophobic surfaces may counteract hydration force between particles, which enhances further agglomeration of particles.
Table 2 Particle size distribution of seed and products

Fig.5 Agglomeration efficiency of products
4 Conclusions
1) 1-butanol has little effect on gibbsite precipitation while 1,4-dioxane and tetrahydrofurane promote this process, and the enhancing effect of tetrahydrofurane is more apparent than that of 1,4-dioxane.
2) Effect of additives on gibbsite precipitation process is related to the Mulliken atomic charge of the functional group of organic additive.
3) Effect of 1-butanol on particle size distribution and agglomeration of product is invisible. 1,4-dioxane and tetrahydrofurane, especially tetrahydrofurane, can enhance agglomeration efficiency and make product coarse.
References
[1] ROE W J, PERISHO J L. Use of polymers in alumina precipitation in the Bayer process of bauxite beneficiation: US, 4608237 [P]. 1986- 08-26.
[2] OWEN D O, DAVIS D C. Use of surfactants in alumina precipitation in the Bayer process: US, 4737352 [P]. 1988-10-12.
[3] CHEN Feng, ZHANG Bao-yan, BI Shi-wen, XIE Yan-li. Effect of anionics-oily additive on seed precipitation from sodium aluminate solution [J]. Journal of Northeastern University: Natural Science, 2004, 25(6): 606-609. (in Chinese)
[4] WU Yu-sheng, YU Hai-yan, YANG Yi-hong, BI Shi-wen. Effects of additives on agglomeration and secondary nucleation in seed precipitation in sodium aluminate solution [J]. Journal of Chemical Industry and Engineering, 2005, 56(12): 2434-2439. (in Chinese)
[5] ZHANG Bin, CHEN Qi-yuan, ZHOU Ke-chao. Effect of modified additives on process of seeded precipitation ratio of sodium aluminate liquors [J]. Journal of Central South University: Science and Technology, 2006, 37(5): 932-936. (in Chinese)
[6] HELEN W. Gibbsite crystallization inhibition 2: Comparative effects of selected alditols and hydroxycarboxylic acids [J]. Hydrometallurgy, 2000, 55: 289-309.
[7] HEMSTRA T, HAN Y, van RIEMSDIJK W H. Interfacial charging phenomena of aluminum (hydr)oxides [J]. Langmuir, 1999, 15: 5942-5955.
[8] WATTS H L, UTLEY D W. Volumetric analysis of sodium aluminate solutions [J]. Analytical Chemistry, 1953, 25(6): 864-867.
[9] RODNEY G H, JOHN D W, BRUCE B J. Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 180: 131-140.
[10] GUO Yang. Theoretical investigation of the on-top site adsorption of small organic molecule on γ-Al(OH)3 [D]. Changsha: Central South University, 2007.
[11] FREIJ S J, PARKINSON G M. Surface morphology and crystal growth mechanism of gibbsite in industrial Bayer liquors [J]. Hydrometallurgy, 2005, 78: 246-255.
[12] STEEMSON M L, WHITE E T, MARSHALL R J. Mathematical model for the precipitation section of a Bayer plant [C]// Light Metals: Proceedings of Sessions. Warrendale, Rennsylrania: Metallurgical Soc of AIME, 1984: 237-253.
[13] ZHANG Bin, ZHOU Ke-chao, CHEN Qi-yuan. Influences of seed size and number on agglomeration in synthetic Bayer liquors [J]. J Cent South Univ Technol, 2006, 13(5): 511-514.
[14] LI Hui-xing, JONAS A, JOHN C T, ANDREA R G. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions [J]. Journal of Crystal Growth, 2005, 279: 508-520.
[15] XIE Yan-li, LU Zi-jian. The seeded decomposition of supersaturated sodium aluminate liquors (Chapter 3) [M]. Beijing: Metallurgical Industry Press, 2003. (in Chinese)
[16] ILIEVSKIL D, LIVK I. An agglomeration efficiency model for gibbsite precipitation in a turbulently stirred vessel [J]. Chemical Engineering Science, 2006, 61: 2010-2022.
[17] ADDAI-MENSAH J, DAWE J, HAYES R, PRESTIDGE C, RALSTON J. The unusual colloid stability of gibbsite at high pH [J]. Journal of Colloid and Interface Science , 1998, 203: 115-121.
[18] PRESTIDGE C A, AMETOV I, ADDAI-MENSAH J. Rheological investigations of gibbsite particles in synthetic Bayer liquors [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 157: 137-145.
Foundation item: Project(2005CB623702) supported by Major State Basic Research Development Program of China
Received date: 2008-03-25; Accepted date: 2008-05-29
Corresponding author: YIN Zhou-lan, Professor, PhD; Tel: +86-731-8877364; E-mail: xhli@csu.edu.cn
- Effect of tetracarbon additives on gibbsite precipitation from seeded sodium aluminate liquor


