
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1693-1700
Effects of polyaniline on electrochemical properties of
composite inert anodes used in zinc electrowinning
ZHAN Peng1,2, XU Rui-dong1,2, HUANG Li-ping3, CHEN Bu-ming1,2, ZHOU Jian-feng1,2
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. State Key Laboratory Breeding Base of Complex Nonferrous Metal Resources Cleaning Utilization in
Yunnan Province, Kunming 650093, China;
3. Key Laboratory of Inorganic Coating Materials, Chinese Academy of Sciences, Shanghai 200050, China
Received 8 July 2011; accepted 19 March 2012
Abstract:
In order to search for a suitable anode material used in zinc electrowinning in place of Pb-Ag alloy, Al/Pb-PANI (polyaniline)-WC (tungsten carbide) composite inert anodes were prepared on aluminum alloy substrate by double pulse electrodeposition (DPE) of PANI and WC particles with Pb2+ from an original plating bath. Thereafter, anodic polarization curves, cyclic voltammetry curves and Tafel polarization curves for the composite inert anodes obtained under different PANI concentrations in the original plating bath were measured, and the microstructural features were also investigated by scanning electron microscopy (SEM). The results show that Al/Pb-PANI-WC composite inert anode obtained under PANI concentration of 20 g/L in the original plating bath possesses uniform microstructures and composition distributions, higher electrocatalytic activity, better reversibility of electrode reaction and corrosion resistance in a synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃. Compared with Pb-1%Ag alloy, the overpotential of oxygen evolutions for the composite inert anode are decreased by 185 mV and 166 mV, respectively, under 500 A/m2 and 1000 A/m2.
Key words:
composite inert anodes; double pulse electrodeposition; anodic polarization curves; cyclic voltammetry curves; Tafel polarization curves; microstructures;
1 Introduction
Zinc is mainly extracted from its sulfide ores, oxide ores or other secondary resources by means of electrowinning process for hydrometallurgy. Generally, the electrode materials used in zinc electrowinning must meet the following requirements of good electroconductivity, mechanical strength and electrocatalytic activity, excellent corrosion resistance and long service life. At present, only Pb-Ag alloy anode in which Ag content ranges form 0.5% to 1.0% (mass fraction), is used extensively in zinc electrowinning, but it also has some shortages of higher overpotential of oxygen evolution, worse conductivity and mechanic performance [1-5]. The overpotential of oxygen evolution, current efficiency and corrosion rate for Pb-Ag-Sb, Pb-Ca-Sn, Pb-Ag-Ca and Pb-Co alloy anodes used in zinc electrowinning were investigated and some valuable results have been obtained [6-8].
PANI particle has been intensively researched in recent years owing to its high conductivity, good electrocatalytic activity and environment stability, and has a large variety of applications in the fields of light-emitting materials, electronic device materials, chemical sensor materials and electrode materials [9-13]. WC particle can increase distortion resistance and decrease overpotential of oxygen evolution in zinc electrowinning when deposited into matrix metal Pb or metal oxidation PbO2. Compared with direct current electrodeposition (DCE), pulse electrodeposition (PE) with higher instantaneous current density can increase cathodic activation polarization and decrease cathodic concentration polarization, which is easier to make out metal matrix composite materials in the fine grained structures by means of relaxation of pulse current [14-18]. In order to search for a suitable anode material used in zinc electrowinning in place of Pb-Ag alloy, a new kind of Al/Pb-PANI-WC composite inert anode was prepared by double pulse electrodeposition (DPE) and it has been found to a potential anode material. In this research, effects of PANI concentrations in an original plating bath on electrochemical properties and microstructural features for the composite inert anodes were studied.
2 Experimental
2.1 Preparation of composite inert anodes
Al/Pb-PANI-WC composite inert anodes were prepared on aluminum alloy substrate by DPE from an original plating bath. The dimensions of plating cell were 80 mm(L)×60 mm(W)×130 mm(H); a pair of electrolytic lead sheets with dimensions of 30 mm(L) × 3 mm(W) × 100 mm(H) were used as electrodepositing anodic materials, and were connected with anodic wire of DPE supply; aluminum alloy sheet with dimensions of 30 mm(L)×2 mm(W)×60 mm(H) was used as cathodic material, and was connected with cathodic wire of DPE supply; the electrode spacing between anodic material and cathodic material was 40 mm.
The original plating bath compositions used for electrodepositing Al/Pb-PANI-WC composite inert anodes were as follows: 180 g/L Pb(AC)2, 220 mL/L HBF4, 20 g/L H3BO3, 1.0 g/L gelatin, 0.2 g/L thiourea, 5 mL/L polyethylene glycol, 20 g/L WC and 0-25 g/L PANI. The plating temperature was maintained at 35 ℃, the plating pH value was about 1.0. The waveforms of DPE supply used for electrodepositing the composite inert anode from the original plating bath were as follows: forward and reverse pulse duty cycles were 10% and 30% respectively, forward and reverse pulse average current densities were 4 A/dm2 and 0.4 A/dm2 respectively, and forward and reverse pulse working time were 200 ms and 20 ms, respectively. The electrodeposition time was 1.5 h. Thereafter, the composite inert anodes were used for measuring of electrochemical properties and surface microstructural features.
To guarantee better dispersion of PANI and WC particles in the original plating bath and in the composite inert anodes, the original plating bath was dispersed by ultrasonic device with 2 A for 30 min before DPE. After that, the mechanical stirring was used for the original plating bath in DPE experiments and the stirring speed was controlled at 150 r/min.
2.2 Measurement and analysis
The electrochemical workstation (CHI760C) with three-electrode system was used for measuring the anodic polarization curves, cyclic voltammetry curves and Tafel polarization curves for the composite inert anodes in a synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃. The auxiliary electrode was graphite, the reference electrode was a saturated calomel electrode, the working electrode was the composite inert anode and the working areas were 1.0 cm2. The working electrode and auxiliary electrode were connected with KCl agar salt bridge, and their electrode spacing was 30 mm. Scanning electron microscope (SEM, XL30 ESEM-TEP+EDAX) was used for determining surface microstructures and composition distributions of the composite inert anodes.
3 Results and discussion
3.1 Anodic polarization curves and kinetic parameters of oxygen evolution
At a constant scan rate of 5 mV/s, anodic polarization experiments for Al/Pb-PANI-WC composite inert anodes obtained under different PANI concentrations in the original plating bath, were carried out in a synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, and the results are shown in Fig. 1.
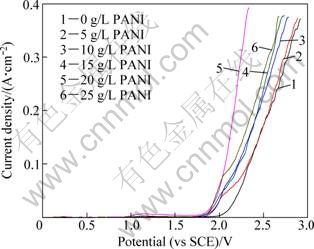
Fig. 1 Anodic polarization curves for composite inert anodes obtained under PANI concentrations in original plating bath
As shown in Fig. 1, the relationships of PANI concentrations in the original plating bath and anodic polarization curves have identical characteristics in the potential ranges of 0-2.9 V, but PANI concentrations in the original plating bath have no obvious effects on the initial anodic behavior for the composite inert anodes in the potential ranging from 0 V to 1.8 V. It can also be seen that the potentials of oxygen evolution for the composite inert anodes obtained under PANI concentrations of 0 g/L and 5 g/L in the original plating bath, are higher in the potential ranging from 1.8 V to 2.9 V. Increasing PANI concentrations from 0 to 20 g/L in the original plating bath leads to the decrease of the potential of oxygen evolution in the range of high potential, and it reaches the lowest value under PANI concentration of 20 g/L, displaying better electrocatalytic activity. Thereafter, the potential of oxygen evolution begins to increase when PANI concentration is increased from 20 g/L to 25 g/L in the original plating bath.
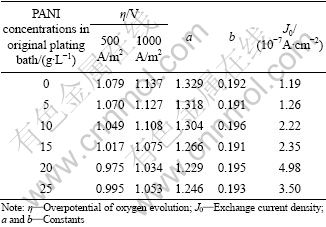
According to Fig. 1, the kinetic parameters of oxygen evolution calculated by Tafel equation (η=a+blgJ0) are listed in Table 1. The value of J0 for the composite inert anode obtained under PANI concentration of 20 g/L in the original plating bath, is the highest (4.98×10-7 A/cm2), and higher than that for other composite inert anodes, displaying that the anodic reaction is easier to happen and its electrocatalytic activity is the highest when current passes through the anode. In addition, it can be seen that in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, the values of η for the composite inert anodes obtained under PANI concentrations of 20 g/L in the original plating bath, are 0.975 V and 1.034 V under 500 A/m2 and 1000 A/m2, respectively. Under above current density conditions, the values of η for Pb-1%Ag alloy prepared in laboratory are 1.160 V and 1.200 V, respectively. Therefore, compared with Pb-1%Ag alloy, the values of η for the composite inert anodes are decreased by 185 mV and 166 mV under 500 A/m2 and 1000 A/m2, respectively.
Table 1 Oxygen evolution kinetic parameters of composite inert anodes obtained under different PANI concentrations in original plating bath
3.2 Cyclic voltammetry curves
At a constant scan rate of 10 mV/s, cyclic voltammetric experiments for Al/Pb-PANI-WC composite inert anodes obtained under different PANI concentrations in the original plating bath, were carried out in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, and the results are shown in Fig. 2.
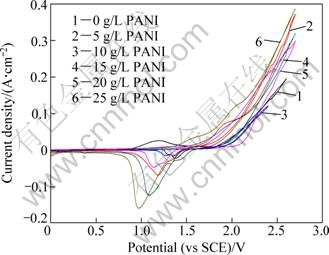
Fig. 2 Cyclic voltammetry curves for composite inert anodes obtained under different PANI concentrations in original plating bath
As shown in Fig. 2, one anodic oxidation peak in the forward scan and one cathodic reduction peak in the reverse scan appear in the cyclic voltammetry curves for Al/Pb-PANI-WC composite inert anodes obtained under different PANI concentrations in the original plating bath. The anodic oxidation peak should be the conversion peak for Pb/PbO2, and the cathodic reduction peak should be the conversion peak for PbO2/Pb. The potential difference between Pb/PbO2 oxidation peak and PbO2/Pb reduction peak for the composite inert anode obtained under the PANI concentration of 20 g/L in the original plating bath, is only 0.41 V, and it is also the lowest among these composite inert anodes, which can be concluded that the reversibility of electrode reaction is better in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃.
3.3 Tafel polarization curves
At a constant scan rate of 10 mV/s, Tafel polarization experiments for Al/Pb-PANI-WC composite inert anodes obtained under different PANI concentrations in the original plating bath, were carried out in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, and the results are shown in Fig. 3, the corrosion potential and corrosion current are listed in Table 2.
As shown in Table 2, the corrosion potential for the composite inert anode increases with the rise of PANI concentrations from 0 g/L to 20 g/L in the original plating bath and it reaches the highest value (-0.533 V) under the PANI concentration of 20 g/L. In the meantime, the corresponding corrosion current of the composite inert anode is also the lowest (1.05 mA), displaying better corrosion resistance in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 and 35 ℃. Thereafter, the corrosion potential begins to decrease and corrosion current begins to increase with further increasing PANI concentrations from 20 g/L to 25 g/L in the original plating bath, showing that the corrosion resistance for the composite inert anode becomes bad.
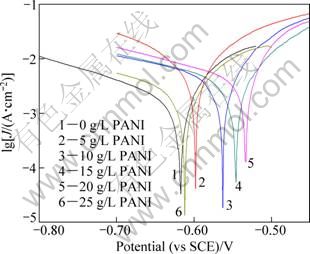
Fig. 3 Tafel polarization curves for composite inert anodes obtained under different PANI concentrations in original plating bath
Table 2 Corrosion potential and corrosion current for composite inert anodes obtained under different PANI concentrations in original plating bath

3.4 Microstructural features and composition distributions
Surface microstructural features of Al/Pb-PANI-WC composite inert anodes obtained under different PANI concentrations in the original plating bath, are shown in Fig. 4, and the composition distribution results of line scanning are shown in Fig. 5 and Table 3.
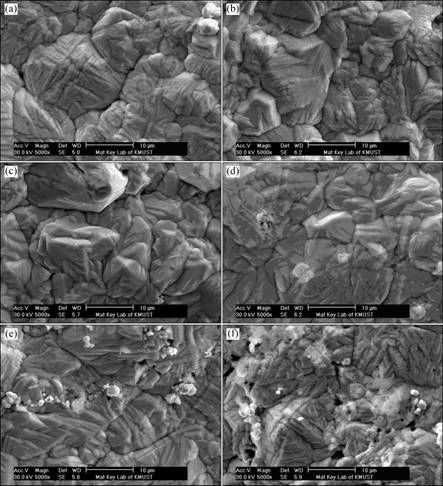
Fig. 4 Surface microstructural features for composite inert anodes obtained under different PANI concentrations: (a) 0 g/L; (b) 5 g/L; (c) 10 g/L; (d) 15 g/L; (e) 20 g/L; (f) 25 g/L
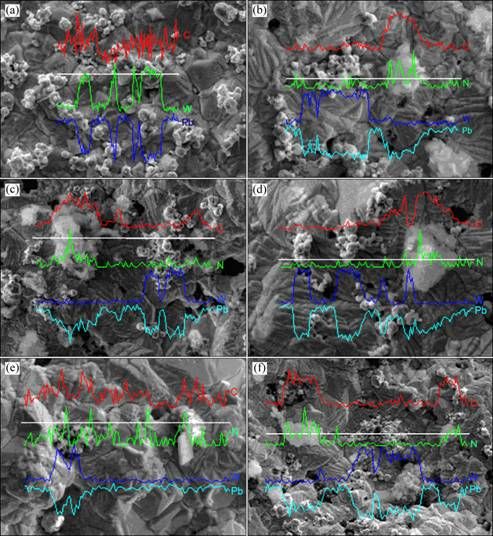
Fig. 5 Composition distributions for composite inert anodes obtained under different PANI concentrations: (a) 0 g/L; (b) 5 g/L; (c) 10 g/L; (d) 15 g/L; (e) 20 g/L; (f) 25 g/L
Table 3 Results of line scanning for composite inert anodes obtained under different PANI concentrations in bath
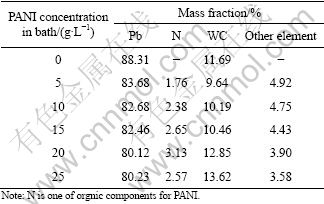
It can be seen that increasing PANI concentration from 0 g/L to 20 g/L in the original plating bath leads to the refinement in the grained structures and the improvement of composition fluctuation. In the meantime, it also increases mass fractions for PANI particles and WC particles in the composite inert anodes. The main reasons may be that increasing PANI concentration from 0 g/L to 20 g/L in the original plating bath is favorable for their enrichment and deposition as the core on the cathodic surface under the function of mechanical stirring and electrical forces, which brings on a large amount of nucleation points for matrix metal Pb, increases deposition rate and deposition amount of solid particles, inhabits the continuous growth of Pb grains and refines the grains. While when PANI concentration is increased from 20 g/L to 25 g/L in the original plating, higher particle concentration in the original plating bath results in serious coacervation for solid particles in the composite inert anodes owing to the fact that the increase of electrolyte viscidity and the coacervation of solid particles absorbed on the cathodic surface. Therefore, the structural defects such as cavities and cracks and composition fluctuations, are obvious, showing that too higher PANI concentration in the original plating bath is not favorable to preparing the uniform composite materials.
3.5 Discussion
Composite electrodeposition is a valuable new surface intensification technology to obtain metal-matrix composite materials by making inorganic and organic particles co-deposit with metal or alloy [19-21]. The composite materials are made up of matrix metal and the second phase particles which are dispersed evenly within matrix metal, possessing comprehensive performances of matrix metal and the second phase particles. Especially, pulse electrodeposition is found to be an effective means of controlling microstructures and properties of the composite materials by varying pulse parameters owing to higher instantaneous current density [22-25], and has received a considerable attention in recent years [26,27].
The above research results show that Al/Pb-PANI-WC composite inert anode obtained under PANI concentration of 20 g/L in the original plating bath possesses a higher electrocatalytic activity and lower overpotential of oxygen evolution in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, which is related to the characteristics of solid particles, the distribution of solid particles in the composite inert anodes and the electrochemical active sites amounts used for oxidation-reduction transition on the surface of the composite inert anodes. On one hand, PANI particle is a typical conducting polymer with organic conjugated structure, and its macroscopic conductivity is affected by conjugate degree and mutual stack mode of molecular chain [28,29]. PANI doped with inorganic acid possesses a higher electrical conductivity owing to the fact that the extension of molecular chain structure leads to high conjugate degree and close chain packing is favorable to the transmission of charge in the chain and between the chain [30]. The conductive mechanism of doped PANI differs from metal and semiconductor, in which the carriers are composed of “delocalization” π electrons and solitons, polarons or bipolarons formed by doping [31], which has been described by peace model [32,33], granular metal island model [34-36] and monopole and bipolar sub-transformation model [37]. Meanwhile, PANI has an active surface that exceeds geometric surface and can play a role in selective catalysis to some certain when used as the electrode materials, such as the electrocatalytic oxidation of ascorbic acid at polyaniline film modified glassy carbon electrodes [38], the electrocatalysis of polyaniline film on Fe [II] and Sb [II], the synergistic effects of electrocatalytic oxidation of polyaniline on small organic molecules [39-41] and the same electrocatalytic activity for Pb/PANI and Pt/PANI in acid solutions [42]. On the other hand, WC particle has the electrochemical characteristics that is similar to platinum owing to the fact that the existing of carbide changes the electronic surface properties of tungsten, possessing a higher electrochemical activity, better catalytic activity and conductivity in a acidic medium low temperature [43-46]. For example, the reduction of activation energy in the reaction control steps improves the electrocatalytic activity of hydrogen evolution for Pb-WC composite anode [47], the deposition of WC particles into Cu/PbO2 composite anode changes deposition mode and preferred orientation of PbO2, leading to that the electrocatalytic activity of oxygen evolution reaction for PbO2-WC composite anode in 0.5 mol/L H2SO4 solution is increased nearly by one-foId [48].
Therefore, the deposition of PANI particles and WC particles as conductive matrix and electrocatalytic activity substance and their dispersion distributions obviously improve the electrocatalytic activity and decrease the overpotential of oxygen evolution for Al/Pb-PANI-WC composite anode in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃. In addition, high deposition amounts of PANI particles and WC particles in the composite inert anode obtained under PANI concentration of 20 g/L, provide more electrochemical active sites used for oxidation- reduction transition on the surface of the composite inert anodes, displaying a higher electrocatalytic activity and lower overpotential of oxygen evolution in the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃.
4 Conclusions
1) Al/Pb-PANI-WC composite inert anode obtained by DPE under PANI concentration of 20 g/L in the original plating bath, is a suitable anode material used in zinc electrowinning in place of Pb-Ag alloy, possessing uniform microstructures, composition distributions and less microstructural defects.
2) In the synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃, the composite inert anode possesses a higher electrocatalytic activity, lower overpotential of oxygen evolution, better reversibility of electrode reaction and corrosion resistance. Compared with Pb-1%Ag alloy, the overpotentials of oxygen evolution for the composite inert anode are decreased by 185 mV and 166 mV under 500 A/m2 and 1000 A/m2, respectively.
References
[1] YANG Sheng-hai, TANG Mo-tang, CHEN Yi-feng, TANG Chao-bo, HE Jing. Anodic relection kinetics of electrowing zinc in system of Zn(Ⅱ)-NH3-NH4Cl-H2O [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(3): 626-630.
[2] ZHONG Shui-ping, LAI Yan-qing, JIANG Liang-xing, TIAN Zhong-liang, LI Jie, LIU Ye-xiang. Anodization behavior on Pb-Ag-Ca-Sr alloy during zinc electrowinning [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(7): 1342-1346. (in Chinese)
[3] ROCCA E, STEINMETZ J. Mechanism of passivation of Pb(Ca)-Sn alloys in sulfuric acid: Role of tin [J]. Electrochimica Acta, 1999, 44(25): 4611-4618.
[4] HEIN K, SCHIERLE T. Oxygen overvoltage at insoluble anodes in the system Pb-Ag-Ca [J]. Erzmetall, 1991, 44(9): 447-451.
[5] ZHANG Qi-bo, HUA Yi-xin. Effect of the ionic liquid additive-[BMIM] H2SO4 on the kinetics of oxygen evolution during zinc electrowinning [J]. Acta Physico-Chimica Sinica, 2011, 27(1): 149-155.
[6] STEFANOV Y, DOBREV T S. Potentiodynamic and electronmicroscopy investigations of lead-cobalt alloy coated lead composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 296-299.
[7] ZHONG Shui-ping, LAI Yan-qing, JIANG Liang-xing, TIAN Zhong-liang, LI Jie, LIU Ye-xiang. Research development of new anode and technics for zinc electrowinning [J]. Materials Review, 2008, 22(2): 86-89.
[8] RASHKOV S,STEFANOV Y, NONCHEVA Z, PETROVA M,DOBREV T, KUNCHEV N, PETROV D,VLAEV S, MIHNEV V, ZAREV S, GEORGIEVA L, BUTTINELLI D. Investigation of the processes of obtaining plastic treatment and electrochemical behaviour of lead alloys in their capacity as anodes during the electroextraction of zinc [J]. Hydrometallurgy, 1996, 40(3): 319-334.
[9] THANPITCHA T, SIRIVAT A, JAMIESON A M, RUJIRAVANIT R. Synthesis of polyaniline nanofibrils using an in situ seeding technique [J]. Synthetic Metals, 2008, 158(17-18): 695-703.
[10] ROUT T K, JHA G, SINGH A K, BANDYOPADHYAY N, MOHANTY. Development of conducting polyaniline coating: A novel approach to superior corrosion resistance [J]. Surface and Coatings Technology, 2003, 167(1): 16-24.
[11] JANG J, HA J, KIM K. Organic light-emitting diode with polyaniline-poly(styrene sulfonate) as a hole injection layer [J]. Thin Solid Films, 2008, 516(10): 3152-3156.
[12] PUD A, OGURTSOV N, KORZHENKO A, SHAPOVAL G. Some aspects of preparation methods and properties of polyaniline blends and composites with organic polymers [J]. Progress in Polymer Science, 2003, 28(12): 1701-1753.
[13] BHADRA S, KHASTGIR D, SINGHA N K, LEE J H. Progress in preparation, processing and applications of polyaniline [J]. Progress in Polymer Science, 2009, 34(8): 783-810.
[14] XU Rui-dong, WANG Jun-li, GUO Zhong-cheng, WANG Hua. High temperature oxidation behavior of CeO2-SiO2/Ni-W-P composites [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(5): 1190-1195.
[15] XU Rui-dong, WANG Jun-li, HE Li-fang, GUO Zhong-cheng. Study on the characteristics of Ni-W-P composite coatings containing nano-SiO2 and nano-CeO2 particles [J]. Surface and Coatings Technology, 2008, 202(8): 1574-1579.
[16] WANG Jun-li, XU Rui-dong, ZHANG Yu-zhi. Influence of SiO2 nano-particles on microstructures and properties of Ni-W-P/CeO2-SiO2 composites prepared by pulse electrodeposition [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 839-843.
[17] XU Rui-dong, WANG Jun-li, GUO Zhong-cheng, WANG Hua. Effects of rare earth on microstructures and properties of Ni-W-P-CeO2-SiO2 nano-composite coatings [J]. Journal of Rare Earths, 2008, 26(4): 579-583.
[18] WANG Jun-li, ZHANG Yu-zhi, XU Rui-dong, ZHAN Peng. Probe into deposition mechanism of double pulse electrodepositing Ni-W-P matrix composite coatings containing CeO2 and SiO2 nano-particles [J]. Journal of Rare Earths, 2010, 28(S1): 437-441.
[19] ZHITOMIRSKY I. Electrochemical processing and characterization of nickel hydroxide–polyelectrolyte coatings [J]. Materials Letters, 2004, 58(3-4): 420-424.
[20] WANG Z, SHEMILT J, XIAO P. Novel fabrication technique for the production of ceramic/ceramic and metal/ceramic composite coatings [J]. Scripta Materialia, 2000, 42(7): 653-659.
[21] MACRO M. Electrodeposition of composites: an expanding subject in electrochemical materials science [J]. Electrochimica Acta, 2000, 45(20): 3397-3402.
[22] RICHOUX V, DILIBERTO S, BOULANGER C, LECUIRE J M. Pulsed electrodeposition of bismuth telluride films: Influence of pulse parameters over nucleation and morphology [J]. Electrochimica Acta, 2007, 52(9): 3053-3060.
[23] KIM D J, ROH Y M, SEO M H, KIM J S. Effects of the peak current density and duty cycle on material properties of pulse-plated Ni-P-Fe electrodeposits [J]. Surface and Coatings Technology, 2005, 192(1): 88-93.
[24] EL-SHERIK A M, ERB U, PAGE J. Microstructural evolution in pulse plated nickel electrodeposits [J]. Surface and Coatings Technology, 1996, 88(1-3): 70-78.
[25] DENNY T M, RONNY L, ANDREAS B. Influence of pulse plating parameters on the electrocodeposition of matrix metal nanocomposites [J]. Electrochimica Acta, 2007, 52(25): 7362-7371.
[26] ZIMMERMAN A F, CLARK D G, AUST K T. Pulse electrodeposition of Ni-SiC nanocomposite [J]. Materials Letters, 2002, 52(1-2): 85-90.
[27] MISHRA R, BALASUBRAMANIAM R. Effect of nanocrystalline grain size on the electrochemical and corrosion behavior of nickel [J]. Corrosion Science, 2004, 46(12): 3019-3029.
[28] OU R Q, SAMUELS R. Investigation of fundamental molecular parameters of polyaniline films [J]. Journal of Polymer Science: Part B, 1999, 37(24): 3473-3487.
[29] TALAIE A, ROMAGNOLI J A. An integrated artificial neural network/polymer-based pH sensor: A new engineering perspective to conducting polymer technology [J]. Synthetic Metals, 1996, 82(3): 231-235.
[30] JAVADI H H S, ANGELOPOULOS M, MACDIARMID A G, EPSTEIN A J. Conduction mechanism of polyaniline—Effect of moisture [J]. Synthetic Metals, 1998, 26(1): 1-8.
[31] LI Ying, ZHAO Di-shun. Conducting polymer materials [J]. Journal of Hebei University of Science and Technology, 2000, 21(2): 9-12. (in Chinese)
[32] NECHTSCHEIN M, SANTIER C, TRAVERS J P, CHROBOCZEK J, ALIX A,RIPERT M. Water effects in polyaniline: NMR and transport properties [J]. Synthetic Metals, 1987, 18(1-3): 311-316.
[33] WANG Li-xiang, WANG Fo-song. Progress in polyaniline as a novel conducting polymer [J]. Chinese Journal of Applied Chemistry, 1990, 7(6): 1-8. (in Chinese)
[34] LUNDBERG B, SALANECK W R. Temperature and field dependence of hopping conduction in polyaniline [J]. Synthetic Metals, 1987, 21(1-3): 143-147.
[35] JAVADI H H S, LAVERSANNE R, EPSTEIN A J, KOHLI P K, SCHERR E M, MACDIARMID A G. ESR of protonated “emeraldine”: Insulator to metal transition [J]. Synthetic Metals, 1989, 29(1): 439-444.
[36] NECHTSCHEIN M, GEOND F, MENARDO C. Water absorption study in polyaniline [J]. Synthetic Metals, 1989, 29(1): 457-462.
[37] WANG Hui-zhong, WANG Rong-shun, ZHAO Cheng-da, HUANG Zong-hao, TANG Jin-song, WANG Bao-chen, WANG Fo-song. Studies on the electronic energy band structure and conducting mechanism for doped polyaniline [J]. Chemical Journal of Chinese Universities, 1991, 12(9): 1229-1233. (in Chinese)
[38] DONG Shao-jun, SONG Fa-yi. Electrocatalytic oxidation of ascorbic acid at polyaniline film modified glassy carbon electrodes [J]. Acta Physico-Chimica Sinica, 1992, 8(1): 82-86.
[39] DUI? L, GRIGI? S. The effect of polyaniline morphology on hydroquinone/quinone redox reaction [J]. Electrochinica Acta, 2001, 46(18): 2795-2803.
[40] MIKHAYLOVA A A, MOLODKINA E B,KHAZOVA O A,BAGOTZKY V S. Electrocatalytic and adsorption properties of platinum microparticles electrodeposited into polyaniline films [J]. Journal of Electroanalytical Chemistry, 2001, 24(2): 119-127.
[41] RAJENDRAPRASAD K,MUNICHANDRAIAHN. Fabrication and evaluation of 450 F electrochemical redox supercapacitors using inexpensive and high performance polyaniline coated stainless steel electrodes [J]. Journal of Power Sources, 2002, 112(2): 443-451.
[42] CHERAGHIA B, FAKHARIB A R, BORHANIA S, ENTEZAMIC A A. Chemical and electrochemical deposition of conducting polyaniline on lead [J]. Journal of Electroanalytical Chemistry, 2009, 626(1-2): 116-122.
[43] LEVY R B, BOUDART M. Platimun-like behavior of tungsten carbide in surface catalysis [J]. Science, 1972, 181(4099): 547-549.
[44] ZENG Jiang-hua, YUAN Ding-sheng, LIU Ying-liang, CHEN Jing-xing, TAN San-xiang. Synthesis of tungsten carbide nanocrystals and its electrochemical properties [J]. Chinese Journal of Catalysis, 2008, 29(7): 607-611. (in Chinese)
[45] MCINTYRE D R, BURSTEIN G T, VOSSEN A. Effect of carbon monoxide on the electrooxidation of hydrogen by tungsten carbide [J]. Journal of Power Sources, 2002, 107(1): 67-73.
[46] ROSENBAUM M, ZHAO F, QUAAS M, WULFF H, SCHR?DER U, SCHOLZ F. Evaluation of catalytic properties of tungsten carbide for the anode of microbial fuel cells [J]. Applied Catalysis B, 2007, 74(3-4): 261-269.
[47] LIU Zhao-lin, ZHU Song-ran. A study of the mechanism of electrocatalysis of Pb-WC composite anode [J]. Plating and Finishing, 1990, 12(1): 11-17. (in Chinese)
[48] LIU Shu-lan,YU De-long, QIN Qi-xian. Investigation on the PbO2-WC composite anode [J]. Chinese Journal of Applied Chemistry, 1995, 12(5): 46-49. (in Chinese)
聚苯胺对锌电积用复合惰性阳极材料电化学性能的影响
詹 鹏1,2,徐瑞东1,2,黄利平3,陈步明1,2,周建峰1,2
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 云南省复杂有色金属资源清洁利用国家重点实验室培育基地,昆明 650093;
3. 中国科学院 特种无机涂层重点实验室,上海 200050
摘 要:为了寻找一种可以替代锌电积用Pb-Ag合金的阳极材料,通过PANI(聚苯胺)、WC(碳化钨)颗粒与Pb2+的双脉冲电沉积,在Al合金基体上制备了Al/Pb-PANI-WC复合惰性阳极材料。测试了镀液中不同PANI浓度下制备的惰性阳极材料的阳极极化曲线、循环伏安曲线和塔菲尔极化曲线,采用扫描电镜考察复合惰性阳极材料的微观组织特征。结果表明:当将制备镀液中PANI浓度控制在20 g/L时,Al/Pb-PANI-WC复合惰性阳极材料的微观组织和成分分布均匀,在含50 g/L Zn2+、150 g/L H2SO4的35 ℃锌电积液中具有较高的电催化活性、较好的电极反应可逆性和耐腐蚀性,在电流密度500 A/m2和1000 A/m2下的析氧过电位与Pb-1%Ag合金相比分别降低了185 mV和166 mV。
关键词:复合惰性阳极材料;双脉冲电沉积;阳极极化曲线;循环伏安曲线;塔菲尔极化曲线;微观组织
(Edited by YANG Hua)
Foundation item: Project (51004056) supported by the National Natural Science Foundation of China; Project (KKZ6201152009) supported by the Opening Foundation of Key Laboratory of Inorganic Coating Materials, China; Projects (2011239, 2011240) supported by Analysis and Measurement Research Fund of Kunming University of Science and Technology, China
Corresponding author: XU Rui-dong; Tel: +86-871-5157903; E-mail: rdxupaper@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61375-7
Abstract: In order to search for a suitable anode material used in zinc electrowinning in place of Pb-Ag alloy, Al/Pb-PANI (polyaniline)-WC (tungsten carbide) composite inert anodes were prepared on aluminum alloy substrate by double pulse electrodeposition (DPE) of PANI and WC particles with Pb2+ from an original plating bath. Thereafter, anodic polarization curves, cyclic voltammetry curves and Tafel polarization curves for the composite inert anodes obtained under different PANI concentrations in the original plating bath were measured, and the microstructural features were also investigated by scanning electron microscopy (SEM). The results show that Al/Pb-PANI-WC composite inert anode obtained under PANI concentration of 20 g/L in the original plating bath possesses uniform microstructures and composition distributions, higher electrocatalytic activity, better reversibility of electrode reaction and corrosion resistance in a synthetic zinc electrowinning electrolyte of 50 g/L Zn2+, 150 g/L H2SO4 at 35 ℃. Compared with Pb-1%Ag alloy, the overpotential of oxygen evolutions for the composite inert anode are decreased by 185 mV and 166 mV, respectively, under 500 A/m2 and 1000 A/m2.


