
Low temperature synthesis and luminescence properties of YAG:Eu nanopowders prepared by modified sol-gel method
S. A. HASSANZADEH-TABRIZI
Department of Materials Engineering PO Box 851414313/517,
Islamic Azad University, Najafabad Branch, Isfahan, Iran
Received 6 January 2011; accepted 25 April 2011
Abstract:
Europium-doped yttrium aluminum garnet nanopowders were synthesized through an aqueous sol-gel method. The structure, morphology and luminescence spectra were investigated by using X-ray diffraction, thermogravimetric and differential thermal analysis, scanning electron microscopy, transmission electron microscopy and photoluminescence spectroscopy measurements. The results show that YAG:Eu with an average particle size of 50 nm is formed. The activation energy of YAG:Eu nanocrystallite growth during calcination is 24.1 kJ/mol. The crystalline YAG:Eu nanopowders show an orange-red emission with 5D0–7F1 as the most prominent group.
Key words:
nanostructured material; Y3Al5O12 (YAG); sol-gel method; luminescence;
1 Introduction
Yttrium aluminum garnets (Y3Al5O12, YAG) doped with a small amount of element such as Eu, Cr and Tb are now widely used as optical host materials for their good optical properties in cathode-ray tubes (CRTs), field emission displays (FED) and scintillation and electro-luminescent applications [1-5]. Owing to such a wide and diverse application potential for YAG-based materials, new synthesis methods of pure and homogeneously doped yttrium aluminum garnets are highly desirable. Conventional methods suffer from problems of abnormal grain growth and compositional inhomogeneity in YAG [6]. For instance, Y3Al5O12 prepared by conventional solid state reaction requires mechanical mixing and extensive heat-treatment at high temperature (>1 600 °C) with a prolonged heating period to achieve the desired phase purity [7]. In addition, due to insufficient mixing and low reactivity of the raw materials, several intermediate phases such as Y4Al2O9 (YAM) and YAlO3 (YAP) easily exist in the products [8]. It is well recognized that wet-chemical processing of multi cation oxides provides considerable advantages of good mixing of the starting materials and excellent chemical homogeneity of the final product. Various wet chemical methods have been developed and successfully used in recent years for the production of YAG powders, including sol-gel processing [9-10], precipitation [11], co-precipitation [12], spray pyrolysis [13], combustion [14] solvo-thermal [15], polyacrylamide gel [16] and hydrothermal synthesis [17].
Recently, new and economical wet chemical methods have been developed for synthesis of alumina nanopowder using Al powder and AlCl3·6H2O [18-19]. In the present study, YAG:Eu nanopowders were synthesized using the same raw materials of Y2O3 and EuCl3·6H2O through a sol-gel method. The photoluminescence characteristics of synthesized nanoparticles were investigated. Sodium dodecyl sulfate (SDS), as a common anionic surfactant, was selected as a dispersant to reduce agglomeration.
2 Experimental
The precursor solutions for YAG:Eu nanopowder were prepared by a sol-gel method using AlCl3·6H2O (Merck), Al powder (>99.6%), Y2O3 (Aldrich), EuCl3·6H2O (Aldrich), sodium dodecyl sulfate (Aldrich) and HCl (Merck). The aluminum powder exhibited a spherical shape with an average diameter about 37.5 μm. The nominal composition was (Y0.9Eu0.1)3Al5O12. Y2O3 powder was first dissolved in aqueous HCl. The main solution was prepared by dissolving aluminum chloride hexahydrate, aluminum powder, europium chloride hexahydrate and yttrium oxide into deionized water. The ratios of Al to AlCl3·6H2O and HCl to H2O were chosen under the conditions that were found to be optimum in the work by SHOJAIE-BAHAABAD and TAHERI-NASSAJ [18]. According to their work, the molar ratios of Al to AlCl3 and HCl to H2O were 1.81 and 0.18, respectively. The precursor solution was then continuously stirred at 100 °C for 3 h to completely dissolve the starting materials. Sodium dodecyl sulfate (SDS) was added to the resultant sol, then the sol was aged at 60 °C. The viscosity of the batch gradually increased and finally the batch was set to a rigid gel. It was then dried at 80 °C for 48 h. The dried gel was calcined in a muffle furnace at various temperatures for 3 h and wet milled in an ethanol medium. The powder was again dried at 80 °C.
The crystalline structure of the powders was determined by X-ray diffraction using a Philips X-pert model with Cu Kα radiation. The average crystallite size (d) of the powder was estimated from the Scherrer equation as:
![]() (1)
(1)
where λ is the wavelength; θ is the diffraction angle and βsample is the full-width for the half-maximum (FWHM) intensity peak of the powder. For instrumental correction, a Gaussian-Gaussian relationship was used:
![]() (2)
(2)
where βexp and βins are the measured FWHMs of the powder and the standard sample, respectively. Differential thermal (DTA) and thermogravimetric (TG) analyses were used in a range of 100-975 °C at a rate of 10 K/min with a STA equipment (PL Thermal Sciences STA 1500, UK). The microstructure of the powders was observed by scanning electron microscope (SEM, XL30-Phillips, Netherlands) and transmission electron microscope (TEM, CM200-FEG-Phillips, Netherlands). The photoluminescence of the samples was measured using a fluorescence spectrophotometer with a 150W Xe lamp (Hitachi F-4600, Japan).
3 Results and discussion
The decomposition of the as-dried gel is studied by DTA and TG, as shown in Fig. 1. The TG curve shows that the as-dried gel displays a total mass loss of 63% up to 750 °C. The precursor undergoes several stages of decomposition upon heating because of the evaporation of adsorbed water, dehydration of the dried gel, decomposition of sodium dodecylsulfate and removal of chloride components. It has been reported that the alkyl chain of the surfactant, sodium dodecyl sulfate, is completely removed below 200 °C, whereas the sulfate head group is lost in the region between 400 and 600 °C [20]. However, the major portion of the thermal decomposition of the precursor is completed at about 750 °C. The decomposition of the volatile species is also reflected in the DTA curve of the powder by endothermic peaks around 170 and 644 °C.

Fig. 1 DTA and TG curves of as-dried gel
The X-ray diffraction patterns of the gel heat-treated for 3 h at temperatures ranging from 600 to 1 400 °C are shown in Fig. 2. There are no diffraction peaks for the samples calcined up to 700 °C, indicating that the powders are amorphous below this temperature. The characteristic peaks of the YAG phase appear at 800 °C and no other crystalline phase such as Y4Al12O9 (YAM) or YAlO3 (YAP) can be detected. The main peak of the cubic YAG:Eu structure is centered at 2θ=33.4° and corresponds to the crystalline plane with Miller indices of {4 2 0}. The crystallization temperature of YAG powder prepared by the sol-gel method is lower than that in the solid-state reaction from constituent oxide mixtures [21]. The lower crystallization temperature of YAG could be related to the fine crystallite size of the powder and mixing of the components on a molecular scale. The low crystallization temperature is important to achieve a single-phase pure material because it is very important to obtain high luminescence efficiency [22]. It is well known that only the pure YAG phase is favorable for the luminescent properties of phosphors, but it normally needs high temperatures to synthesize the pure YAG phase by the solid-state reaction of Al2O3 and Y2O3. As a result, the powder is inhomogeneous and large in size, which brings negativity for luminescent properties. The width of the diffraction peaks decreases and their intensity increases with an increase of the calcination temperature. This shows that the YAG:Eu crystallites grow in size as the calcination temperature increases. The mean crystallite size is determined from the XRD results (Fig. 3). As can be seen, with increasing the temperature, crystallite size increases. Increasing crystallite size is attributed to the typical effect of temperature on crystal growth. In addition, the nanocrystallites grow slowly up to 1000 °C and then grow rapidly. In the case of a low calcination temperature, the porosity is quite high and the pores are interconnected to maintain smaller crystal sizes [23]. For the specimens calcined at higher temperatures, continuous grain boundary networks have been formed due to the bridging of fine particles to increase the crystal sizes.
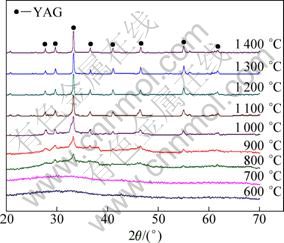
Fig. 2 XRD patterns of samples heat treated at various temperatures for 3 h

Fig. 3 Effect of calcination temperature on crystallite size of YAG:Eu
A straight line of ln D against 1/T is plotted in Fig. 4 according to the Scott equation given below on the assumption that the nanocrystallite growth is homogeneous [24], which approximately describes the nanocrystallite growth during annealing as:
![]() (3)
(3)
where D is the XRD crystal size; C is a constant; E is the activation energy for nanocrystallite growth; R is the molar gas constant; T is the thermo dynamic temperature of the heat treatment. The activation energy for YAG:Eu nanocrystallite growth during calcination was calculated to be about 24.1 kJ/mol. It can be considered that the crystallite grows by means of an interfacial reaction [25].
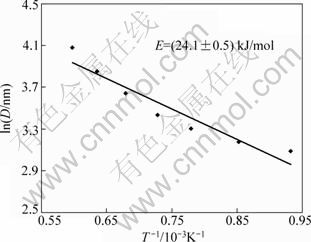
Fig. 4 Plot of lnD against 1/T
Figure 5(a) shows a SEM image of the powders heat treated at 900 °C. As can be seen, some agglomerates exist in the powders, which are attributed to the uncontrolled coagulation during gelation and formation of necks during calcination. Most of the agglomerates are in the range of 80-250 nm. A TEM micrograph of primary particles is shown in Fig. 5(b). The size of primary particles are in the range of 20-60 nm.
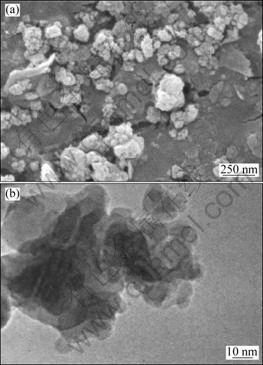
Fig. 5 SEM (a) and TEM (b) images of powders heat treated at 900 °C for 3 h
Figure 6 presents the emission spectra of YAG:Eu powders calcined at 900 and 1 100 °C for 3 h. For emission spectra measurement, YAG:Eu powder is excited with a 254 nm-wavelength from a xenon lamp. Three major emission peaks are observed at 590, 606 and 628 nm, corresponding to the 5D0→7F1 and 5D0→7F2 transitions, which are the typical emission properties of Eu3+ activators. From the luminescence spectra, it can be observed that the YAG:Eu powder shows an orange–red emission. In the cubic YAG phase, the Y3+ is coordinated by eight oxygen ions with D2 point symmetry [22]. The doped Eu3+ is substituted for Y3+ and it also exhibits a D2 point symmetry. However, the exact local symmetry is only a small distortion of the centrosymmetric D2h point symmetry. As a result, the luminescent intensity is concentrated mainly on the 7D0–7F1 magnetic dipole transition (590 nm) rather than the 5D0–7F2 forced electric dipole transition (606 and 628 nm). The emission intensity increases with an increase in calcination temperature. This may be due to the improvement of crystallinity [26]. In addition, the perfect incorporation of Eu3+ ions into the YAG lattice also leads to the improvement of the emission efficiency and emission intensity [11].
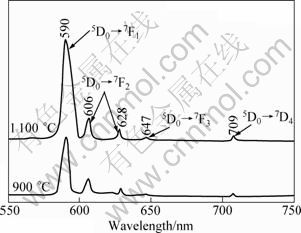
Fig. 6 Emission spectra of samples calcined at 900 and 1 100 °C for 3 h
5 Conclusions
1) A YAG:Eu nanopowder is synthesized through a modified aqueous sol-gel method by using AlCl3·6H2O, Al and Y2O3 powders as raw materials, the total mass loss of 63% appears in the gel after heat treatment at 750 °C. Crystallization of YAG:Eu nanopowdsers occur above 700 °C without the formation of any intermediate phases.
2) The activation energy of YAG:Eu nanocrystallite growth during calcination is measured to be 24.1 kJ/mol. The crystalline YAG:Eu nanopowders show an emission with 5D0–7F1 as the most prominent group. The emission intensity is increased by increasing the calcination temperature.
References
[1] SIM S M, KELLER K A. Phase formation in yttrium aluminum garnet powders synthesized by chemical methods [J]. J Mater Sci, 2000, 35(3): 713-717.
[2] ZHANG X D, LIU H, HE W, WANG J Y, LI X, BOUGHTON R I. Synthesis of monodisperse and spherical YAG nanopowder by a mixed solvothermal method [J] J Alloys Compd, 2004, 372(1-2): 300-303.
[3] ZHANG N, WANG D J, LI L, MENG Y S, ZHANG X S, MING N. YAG:Ce phosphors for WLED via nano-pesudoboehmite sol-gel route [J]. J Rare Earth, 2006, 24(3): 294-297.
[4] LI X, LIU H, WANG J Y, CUI H M, ZHANG X D, HAN F. Preparation of YAG:Nd nano-sized powder by co-precipitation method [J]. Mater Sci Eng A, 2004, 379(1-2): 347-350.
[5] RODRIGUEZ R A, De la ROSA E, DIAZ-TORRES L A, SALAS P, MELENDREZ R, BARBOZA-FLORES M. Thermoluminescence characterization of Tb3+ and Ce3+ doped nanocrystalline Y3Al5O12 exposed to X- and β-ray irradiation [J]. Opt Mater, 2004, 27(2): 293-299.
[6] HOU J G, KUMAR R V, QU Y F, KRSMANOVIC D. Crystallization kinetics and densification of YAG nanoparticles from various chelating agents [J]. Mater Res Bull, 2009, 44(8): 1786-1791.
[7] WARSHAW I, ROY R. Stable and metastable equilibria in the systems Y2O3-Al2O3 and Gd2O3-Fe2O3 [J]. J Am Ceram Soc, 1959, 42(9): 434-438.
[8] SHIKAO S, JIYE W. Combustion synthesis of Eu3+ activated Y3Al5O12 phosphor nanoparticles [J]. J Alloy Compd, 2001, 327(1-2): 82-86.
[9] PEREIRA P F S, CAIUT J M A, RIBEIRO S J L, MESSADDEQ Y, CIUFFI K J, ROCHA L A, MOLINA E F, NASSAR E J. Microwave synthesis of YAG:Eu by sol-gel methodology [J]. J Lumin, 2007, 126(2): 378-382.
[10] FUJIOKA K, SAIKI T, MOTOKOSHI S, FUJIMOTO Y, FUJITA H, NAKATSUK M. Luminescence properties of highly Cr co-doped Nd:YAG powder produced by sol-gel method [J]. J Lumin, 2010, 130(3): 455-459.
[11] FADLALLA H M H, TANG C C. YAG:Ce3+ nano-sized particles prepared by precipitation technique [J]. Mater Chem Phys, 2009, 114(1): 99-102.
[12] SU J, ZHANG Q L, SHAO S F, LIU W P, WAN S M, YIN S T. Phase transition, structure and luminescence of Eu:YAG nanophosphors by co-precipitation method [J]. J Alloy Compd, 2009, 470(1-2): 306-310.
[13] ZHOU Y H, LIN J, YU M, HAN S M, WANG S B, ZHANG H J. Morphology control and luminescence properties of YAG:Eu phosphors prepared by spray pyrolysis [J]. Mater Res Bull, 2003, 38(8): 1289-1299.
[14] FU Y P, WEN S B, HSU C S H. Preparation and characterization of Y3Al5O12:Ce and Y2O3:Eu phosphors powders by combustion process [J]. J Alloy Compd, 2008, 458(1-2): 318-322.
[15] WU Z, ZHANG X, HE W, DU Y, JIA N, XU G. Preparation of YAG:Ce spheroidal phase-pure particles by solvo-thermal method and their photoluminescence [J]. J Alloy Compd, 2009, 468(1-2): 571-574.
[16] LIU Chun-jia, WANG Rui-min, XU Zhi-wei, CAI Jing, YAN Xing-huang, LUO Xue-tao. Crystallization, morphology and luminescent properties of YAG:Ce3+ phosphor powder prepared by polyacrylamide gel method [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(5): 1093-1099.
[17] YANG H, YUAN L, ZHU G, YU A, XU H. Luminescent properties of YAG:Ce3+ phosphor powders prepared by hydrothermal- homogeneous precipitation method [J]. Mater Lett, 2009, 63(27): 2271-2273.
[18] SHOJAIE-BAHAABAD M, TAHERI-NASSAJ E. Economical synthesis of nano alumina powder using an aqueous sol-gel method [J]. Mater Lett, 2008, 62(19): 3364-3366.
[19] HASSANZADEH-TABRIZI S A, TAHERI-NASSAJ E. Economical synthesis of Al2O3 nanopowder using a precipitation method [J]. Mater Lett, 2009, 63(27): 2274-2276.
[20] SICARD L, LLEWELLYN P L, PATARIN J, KOLENDA F. Investigation of the mechanism of the surfactant removal from a mesoporous alumina prepared in the presence of sodium dodecylsulfate [J]. Micro Meso Mater, 2001, 44-45: 195-201.
[21] YADA M, OHYA M, MACHIDA M, KIMA T. Synthesis of porous yttrium aluminium oxide templated by dodecyl sulfate assemblies [J]. Chem Commun, 1998, 19(18): 1941-1942.
[22] RAVICHANDRAN D, ROY R, CHAKHOVSKOI A G, HUNT C E, WHITE W B, ERDEI S. Fabrication of Y3Al5O12:Eu thin films and powders for field emission display applications [J]. J Lumin, 1997, 71(4): 291-297.
[23] LAI T, SHU Y, HUANG G, LEE C, WANG C. Microwave-assisted and liquid oxidation combination techniques for the preparation of nickel oxide nanoparticles [J]. J Alloy Compd, 2008, 450(1-2): 318-322.
[24] SCOTT M G. Amorphous metallic alloys [M]. London: Butterworths, 1983: 151.
[25] YANG Hua-ming, HUANG Cheng-huan, TANG Ai-dong, ZHANG Xiang-chao, YANG Wu-guo. Microwave-assisted synthesis of ceria nanoparticles, Mater Res Bull, 2005, 40(10): 1690-1695.
[26] XIA Guo-dong, ZHOUA Sheng-ming, ZHANG Jun-ji, XU Jun. Structural and optical properties of YAG:Ce3+ phosphors by sol-gel combustion method [J]. J Cryst Growth, 2005, 279(3-4): 357-362.
改进溶胶-凝胶法低温合成YAG:Eu纳米粉末
及其发光性能
S. A. HASSANZADEH-TABRIZI
Department of Materials Engineering, Islamic Azad University, Najafabad Branch, Isfahan, Iran
摘 要:通过水溶胶-凝胶法合成Eu掺杂钇铝石榴石纳(YAG:Eu)米粉末。采用X射线衍射仪、热重和差热分析仪、扫描电子显微镜、透射电子显微镜和发光谱仪等研究粉末的结构、形貌和发光光谱。结果表明:合成的YAG:Eu纳米粉末平均粒径为50 nm,在煅烧过程中其活化能为24.1 kJ/mol,YAG:Eu纳米粉末晶体表现出橙-红发射特征,对应的5D0–7F1最为显著。
关键词:纳米结构材料;YAG;溶胶-凝胶法;发光性
(Edited by FANG Jing-hua)
Foundation item: Project supported by the Islamic Azad University, Najafabad Branch, Iran
Corresponding author: S.A. HASSANZADEH-TABRIZI; Tel: +98-331-2291111; Fax: +98-331-2291016; E-mail: tabrizi1980@gmail.com
DOI: 10.1016/S1003-6326(11)61034-0
Abstract: Europium-doped yttrium aluminum garnet nanopowders were synthesized through an aqueous sol-gel method. The structure, morphology and luminescence spectra were investigated by using X-ray diffraction, thermogravimetric and differential thermal analysis, scanning electron microscopy, transmission electron microscopy and photoluminescence spectroscopy measurements. The results show that YAG:Eu with an average particle size of 50 nm is formed. The activation energy of YAG:Eu nanocrystallite growth during calcination is 24.1 kJ/mol. The crystalline YAG:Eu nanopowders show an orange-red emission with 5D0–7F1 as the most prominent group.


