Trans. Nonferrous Met. Soc. China 22(2012) 3147-3155
Effect of surfactant Tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans
PENG An-an1, LIU Hong-chang1, NIE Zhen-yuan1,2, XIA Jin-lan1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education of China, Central South University, Changsha 410083, China
Received 30 July 2012; accepted 21 November 2012
Abstract:
The effects of surfactant Tween-80 on the growth, sulfur oxidation, and expression of selected typical sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans ATCC 23270 were investigated. The results showed that in the presence of 10-2 g/L Tween-80, the growth of A. ferrooxidans and its metabolism on the insoluble substrate S0 and CuFeS2 were promoted. After 24 d of bioleaching, the copper extraction yield of chalcopyrite at 10-2 g/L Tween-80 increased by 16% compared with the bioleaching experiment without Tween-80. FT-IR spectra analysis revealed that the result was probably caused by the extracellular polymeric substances whose composition could be changed by the surfactant addition. RT-qPCR was used to analyze the differential expressions of 17 selected sulfur metabolism relevant genes in response to the addition of Tween-80. Down-regulation of the extracellular protein genes indicated the influence of Tween-80 on bacteria-sulfur adsorption. Variation of the expression level of the enzymes provided a supplement to sulfur metabolism investigation.
Key words:
Acidithiobacillus ferrooxidans; sulfur metabolism; surfactant Tween-80; RT-qPCR;
1 Introduction
Acidithiobacillus ferrooxidans is an acidophilic chemolithoautotrophic bacterium. It obtains energy from the oxidation of ferrous iron, elemental sulfur or reduced inorganic sulfur compounds (RISCs), and it is the most extensively studied bioleaching microorganism for understanding the mechanisms of bioleaching [1,2]. It is generally recognized that metal sulfide minerals are chemically attacked by Fe3+ (and H+) through the thiosulfate and/or polysulfide pathway with the assistance of sulfur/ferrous iron-oxidizing micro- organisms. These microorganisms oxidize ferrous iron into ferric iron and sulfur, via a series of reduced sulfur compounds, to sulfate, providing ferric iron and proton [3,4]. Elemental sulfur (S0) as one of sulfur intermediates may be accumulated during bioleaching when S0 is not oxidized efficiently by the sulfur- oxidizing bacteria (SOB) [5,6], implying the important roles of the oxidation of elemental sulfur by SOB in the bioleaching process.
Elemental sulfur is hydrophobic and inert at room temperature and ambient pressure. Although the sulfur oxidation biochemistry is very complex, various models for the oxidation of elemental sulfur and other RISCs in Acidithiobacillus spp. and related gram-negative sulfur- oxidizing bacteria have been developed [7,8]. According to these models, the oxidation of S0 by acidophilic SOB could be considered a three-stage process: 1) extra- cellular adsorption and activation, 2) outer membrane transportation, and 3) oxidation in the periplasmic space. The extracellular adsorption and activation stage of S0 is often considered the rate-limiting stage. Extracellular polymeric substances (EPS) involved in the process of bioleaching play a role of “bridge” in connecting minerals and cells. It was found that the EPS of S0 -grown cells contain considerably less neutral sugar and uronic acids but much more fatty acids, which assure the contact between hydrophobic S0 and SOB [9-11]. GOVENDER and GERICKE [12] suggested that the flotation of chalcopyrite (CuFeS2) could be significantly increased in the presence of bio-generated EPS.
Tween-80, formally named as polysorbate 80, is a viscous, water-soluble nonionic surfactant. It is a gentle surfactant because of the low toxicity and often used in the medical and physiological researches. Tween-80 has been investigated for orpiment and chalcopyrite bioleaching [13,14]. Addition of Tween-80 in the culture media may improve the hydrophilicity of the sulfide minerals as well as elemental S0, thus it may enhance the contact between the cells and the minerals. ZHANG et al [15] found that lower than a certain concentration of Tween-80 was conducive to growth and energy metabolism of the SOB Acidithiobacillus albertensis BY-05. But it is still unclear how the addition of Tween-80 affects the growth and sulfur oxidation of bioleaching bacteria. In order to evaluate the application potential of Tween-80 in bioleaching of metal sulfide minerals, the authors investigated the impact of Tween-80 on the growth and sulfur oxidation of the cells and the differential expression of the sulfur metabolism relevant genes of the type strain A. ferrooxidans ATCC 23270.
2 Experimental
2.1 Bacterial strain and culture media
A. ferrooxidans ATCC 23270 was purchased from American Type Culture Collection (ATCC). The media used in this work were composed by 9K basal medium added with S0 (10 g/L), FeSO4·7H2O (44.7 g/L), Na2S2O3·5 H2O (20 g/L), or chalcopyrite (10 g/L) as the energy substrate. The 9K basal medium contained the following components: 0.5 g/L MgSO4·7H2O; 0.5 g/L K2HPO4; 3.0 g/L (NH4)2SO4; 0.1 g/L KCl, 0.01 g/L Ca(NO3)2. The main components of the chalcopyrite were (mass fraction): 34.63% Cu, 25.35% Fe and 30.45% S. The S0 powder was pretreated with the method described by KONISHI et al [16]. The initial pH for thiosulfate-containing medium was adjusted to 4.0 and the initial pH for other media was adjusted to 2.0.
2.2 Cultivation of A. ferrooxidans
A. ferrooxidans was cultivated in 250 mL Erlenmeyer flask containing 100 mL of medium on a rotary shaker at 30 °C and 170 r/min. To examine the effect of Tween-80 on the cell growth on S0, Fe2+, S2O32- and CuFeS2, respectively, Tween-80 in concentrations from 10 to 10-3g/L (in 10-fold serial dilutions) was added to the culture media. Assays free of Tween-80 were used as control. Triplicate experiments were performed under identical conditions. The water evaporation was compensated with sterilized distilled water and the loss due to sampling for analyses was compensated with sterilized fresh medium. Cell number was determined by the blood corpuscle count method. The a pH values of the culture media were measured with pH meter (PHS-3C). The concentration of copper ions was determined by atomic adsorption spectrophotometry.
2.3 FT-IR spectrometry
A. ferrooxidans cells and S0 for FT-IR spectrometric analysis were prepared as follows. A. ferrooxidans cells grown on S0(with or without 10-2 g/L Tween-80) or on Fe2+ were collected at the late exponential growth phase. The cultures were filtered through Waterman No.1 filter paper. The cells in each filtrate were obtained by centrifugation at 10000 r/min for 10 min, and the S0 residue on the filter paper was re-suspended in di-distilled H2O and then centrifuged at 3000 r/min for 1 min. Both the cells and the S0 were thoroughly washed with di-distilled H2O, centrifuged, dried in vacuum, and then daubed into the KBr pellet slice, and analyzed by FT-IR spectrometer (Nexus 670, Nicolet, USA) in the range of 500-4000 cm-1.
2.4 Quantitative real-time PCR
Cells were harvested at the late exponential phase. The extraction and purification of genomic DNA were performed using TIANamp genomic DNA extraction kit (Tiangen Biotech Ltd.) and E.Z.N.A Gel extraction kit (Omega Bio-Tek, Inc.). The extraction and purification of total RNA were performed using total RNA extraction kit (Tiangen Biotech Ltd., China). The quality of total RNA was checked by agarose gel electrophoresis and ethidium bromide staining. The purity and the yield of total RNA were assessed by the A260/A280 ratio with a Thermo Scientific NanoDrop
Gel extraction kit (Omega Bio-Tek, Inc.). The extraction and purification of total RNA were performed using total RNA extraction kit (Tiangen Biotech Ltd., China). The quality of total RNA was checked by agarose gel electrophoresis and ethidium bromide staining. The purity and the yield of total RNA were assessed by the A260/A280 ratio with a Thermo Scientific NanoDrop ND-1000 spectro- photometer. Then the total RNA was immediately served as the template in reverse transcription reactions to synthesize cDNA with ReverTra Ace-α-first stand cDNA synthesis kit (Toyobo co., LTD., Osaka, Japan).
ND-1000 spectro- photometer. Then the total RNA was immediately served as the template in reverse transcription reactions to synthesize cDNA with ReverTra Ace-α-first stand cDNA synthesis kit (Toyobo co., LTD., Osaka, Japan).
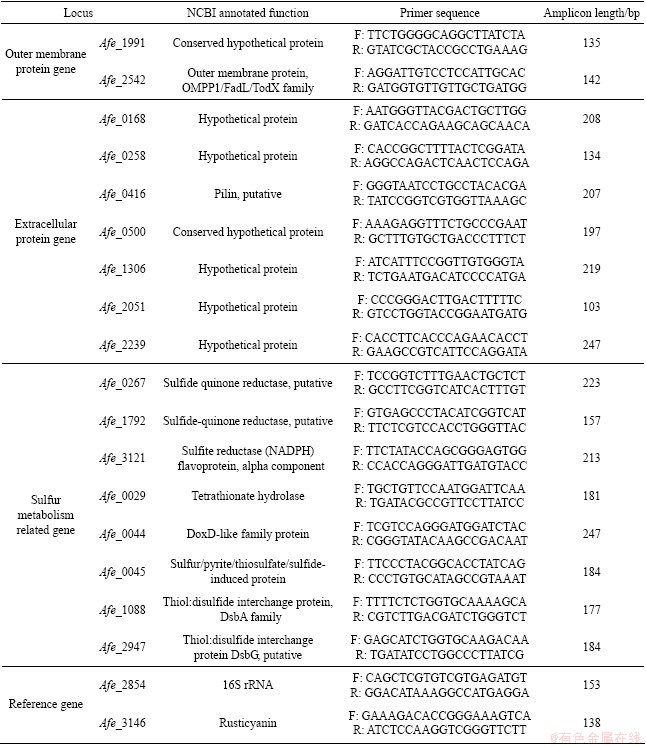
Nine putative sulfur-activation relevant protein genes derived from our results of comparative proteomics study including two outer membranes (unpublished work) and seven extracellular proteins [17] and eight genes annotated to play important roles during sulfur oxidation process were selected [8,18]. 16S rRNA (Afe_2854) was chosen as control gene for RT-qPCR analysis. In addition, Afe_3146 for ferrous iron oxidation was selected as a reference gene. The selected genes with their annotated functions, primer sequences are listed in Table 1. The primers for RT-qPCR were designed by primer premier 3.0 on-line design system (http://frodo.wi.mit.edu/primer3/), based on the genome of A. ferrooxidans ATCC 23270 from NCBI GenBank, and then synthesized by Sangon Biotech, Shanghai, China. The PCR amplification of the specific fragments was performed with Veriti 96-well fast thermal cycler and the specificity of primers and the size of PCR products were checked. The product sequencing was carried out by Sagon Biotech, and BLAST analysis was carried out with NCBI online service.
Quantitative real-time PCR was performed in triplicate with an iCycler iQTM Real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, USA) using THUNDERBIRDTM SYBR qPCR Mix (Toyobo co., LTD., Osaka, Japan). The qPCR thermal cycling program was: 95 °C for 3 min, 40 cycles of 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s, with fluorescence measurements recorded during each annealing step. A melting curve analysis was followed by raising the temperature from 55 °C to 95 °C with a step width of 0.5 °C to verify the specificity of the reactions. Efficiency of amplifications was determined by running a standard curve with serial dilutions of purified 16S rDNA amplification product. Relative quantification was used to detect the changes in the expression of the genes of interest relative to 16S rDNA and the results were expressed as lg2(S/Fe)/lg2(ST/S) of three independent experiments.
qPCR Mix (Toyobo co., LTD., Osaka, Japan). The qPCR thermal cycling program was: 95 °C for 3 min, 40 cycles of 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s, with fluorescence measurements recorded during each annealing step. A melting curve analysis was followed by raising the temperature from 55 °C to 95 °C with a step width of 0.5 °C to verify the specificity of the reactions. Efficiency of amplifications was determined by running a standard curve with serial dilutions of purified 16S rDNA amplification product. Relative quantification was used to detect the changes in the expression of the genes of interest relative to 16S rDNA and the results were expressed as lg2(S/Fe)/lg2(ST/S) of three independent experiments.
Table 1 Primers for RT-qPCR detection of genes related to sulfur metabolism, and reference genes (16S rRNA and Rusticyanin)
3 Results
3.1 Effect of Tween-80 on growth and sulfur oxidation of A. ferrooxidans ATCC 23270 grown on different substrates
The growth curves of A. ferrooxidans ATCC 23270 grown on S0 with 10-10-3 g/L Tween-80 are shown in Fig. 1(a). Compared with the state in the absence of Tween-80, the cell growth was enhanced by 10-1-10-2 g/L Tween-80. The cell growth was not affected when the concentration of Tween-80 was less than 10-3 g/L, but was obviously inhibited when the concentration of Tween-80 was higher than 1 g/L. pH curves (Fig. 1(b)) further show that 10-1-10-2 g/L Tween-80 led to apparent decrease in pH values, while 10 g/L Tween-80 led to no change in pH values. Decrease in pH could be mainly due to the sulfur oxidation. This suggests that 10-1-10-2 g/L, with the optimal of 10-2 g/L, of Tween-80 could promote the cell growth and sulfur oxidation of A. ferrooxidans ATCC 23270 grown on S0.
The growth curves and pH curves of A. ferrooxidans ATCC 23270 grown on 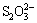 and ferrous iron with 10-10-3 g/L Tween-80 are shown in Fig. 2 and Fig. 3, respectively. The results show that the cell growth in these two culture media was inhibited when the concentration of Tween-80 was higher than 1 g/L, but was not affected by 10-1-10-3 g/L Tween-80.
and ferrous iron with 10-10-3 g/L Tween-80 are shown in Fig. 2 and Fig. 3, respectively. The results show that the cell growth in these two culture media was inhibited when the concentration of Tween-80 was higher than 1 g/L, but was not affected by 10-1-10-3 g/L Tween-80.
The growth curves and pH curves of A. ferrooxidans ATCC 23270 grown CuFeS2 with 10-10-3 g/L Tween-80 are shown in Fig. 4. The results show that the cell growth on CuFeS2could be significantly promoted by addition of 10-2 g/L Tween-80, while growth was inhibited by Tween-80 higher than 10-1 g/L, and was little affected by Tween-80 less than 10-3 g/L. And the variation of pH was inversely associated with bacterial counts. It is worthy to mention that the recovery of copper from chalcopyrite with 10-2 g/L Tween-80 was higher than that without Tween-80 from the 5th day of bioleaching (Fig. 4(c)). After 24 d of bioleaching, the copper extraction yield was about 70 % when 10-2 g/L Tween-80 was added to the bioleaching medium, while 54% was found in the control in which no Tween-80 was added. This indicated that the addition of a certain concentration range of Tween-80 may be an effective way to improve the bioleaching rate of chalcopyrite.
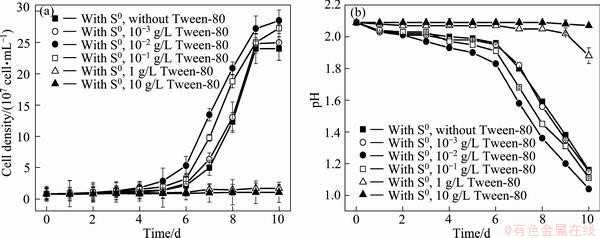
Fig. 1 Growth curves (a) and pH curves (b) of A. ferrooxidans ATCC 23270 grown on S0 with 10-10-3 g/L Tween-80
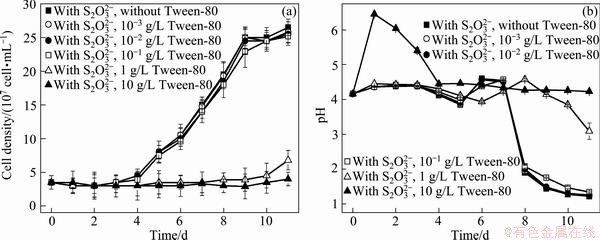
Fig. 2 Growth curves (a) and pH curves (b) of A. ferrooxidans ATCC 23270 grown on 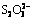 with 10-10-3 g/L Tween-80
with 10-10-3 g/L Tween-80
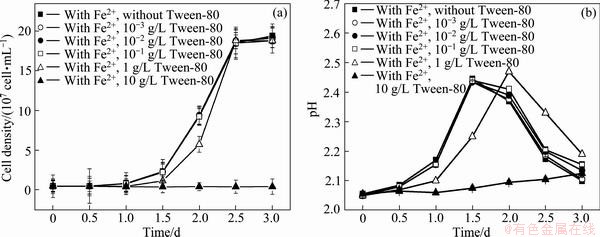
Fig. 3 Growth curves (a) and pH curves (b) of A. ferrooxidans ATCC 23270 grown on Fe2+ with 10-10-3 g/L Tween-80
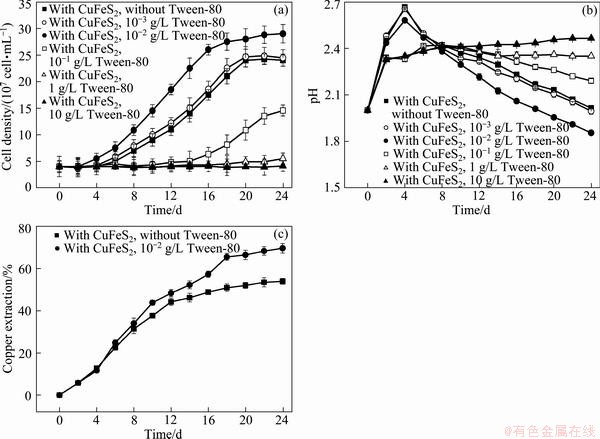
Fig. 4 Growth curves (a), pH curves (b) and copper extraction curves (c) of A. ferrooxidans ATCC 23270 grown on CuFeS2 with 10-10-3 g/L Tween-80
3.2 FT-IR spectra analysis
The FT-IR spectra of standard S0, the S0 isolated from culture without Tween-80, and the S0 isolated from the culture with 10-2 g/L Tween-80 are shown in Figs. 5(a)-(c). The FT-IR spectra of the A. ferrooxidans cells grown on S0 without and with 10-2 g/L Tween-80 are shown in Figs. 5(d) and (e), respectively. The FT-IR spectra of A. ferrooxidans cells grown on Fe2+ are shown in Fig. 5(f). The bands were assigned according to the comparison with previous publications [19,20]. The broad and strong band zone at 3100-3500 cm-1 could be assignable to —OH, and protein —NH and —NH2 groups. The sharp bands near 3068, 2942 and 2925, and 2868 cm-1 could characterize asymmetrical stretching vibration of fatty acids =C—H, —CH3 and —CH2 groups, respectively. The sharp bands at 1650-1850 cm-1 and near 1543 cm-1 were due to —C=O stretching and —NH2 bending vibration, respectively, and these two bands indicate the presence of protein amide group (—CONH—). The bands near 1450 cm-1 could be assigned to bending vibration of —CH3 and —CH2 groups, which are probably included in polysaccharide and lipids. The sharp bands at 1207-1247 cm-1 and 1040-1220 cm-1 represented stretching vibration of C—O group and S=O group, respectively. The sharp band around 845 cm-1could be the characteristic peak of orthorhombic sulfur (S8), the most stable form of elemental sulfur (S0) existence [15].
The FT-IR spectra of S0 treated by A. ferrooxidans ATCC 23270 without and with 10-2 g/L Tween-80 (Figs. 5(b) and (c)) showed the similar absorbance bands to the cellular surface (Figs. 5(d) and (e)), indicating that the surface of S0 was mainly modified by the bacteria. It is worthy, however, to note that the relative intensity of most FT-IR peaks of S0 treated by bacterial cells plus 10-2 g/L Tween-80 (Fig. 5(c)) was significantly different from that of the S0 treated only by bacterial cells (Fig. 5(b)), indicating the contribution of Tween-80 to the modification of the sulfur surface. The FT-IR spectra of the cells grown on sulfur (Figs. 5(d) and (e)) show obvious difference from those on ferrous iron (Fig. 5(f)), indicating the dependence of the chemical properties of the cell surface on the energy substrates.
3.3 Differential expressions of genes related to sulfur metabolism of A. ferrooxidans ATCC 23270 grown with or without Tween-80
Table 2 shows the differential expressions of the selected genes between the cells grown on S0 with and
without Tween-80 (in terms of lg2(ST/S)), and differential expressions between the cells grown on S0 and ferrous iron (in terms of lg2(S/Fe)). 16S rRNA was used as the internal control to adjust the systematic and random errors during the operation procedure. Statistical significance was assessed by t-test probability value p.
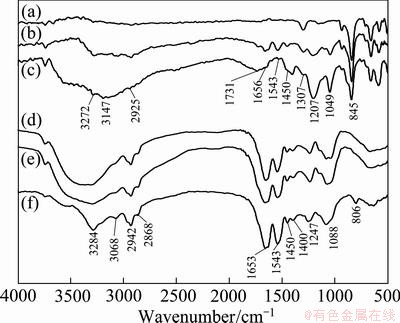
Fig. 5 FT-IR spectra of standard S0 (a), S0 isolated from culture without 10-2 g/L of Tween-80 (b), S0 isolated from culture with 10-2 g/L of Tween-80 (c), A. ferrooxidans cells grown on S0 without 10-2 g/L of Tween-80 (d), A. ferrooxidans cells grown on S0 with 10-2 g/L of Tween-80 (e), A. ferrooxidans cells grown on Fe2+ without 10-2 g/L of Tween-80 (f)
Table 2 RT-qPCR expression data for relevant validated genes
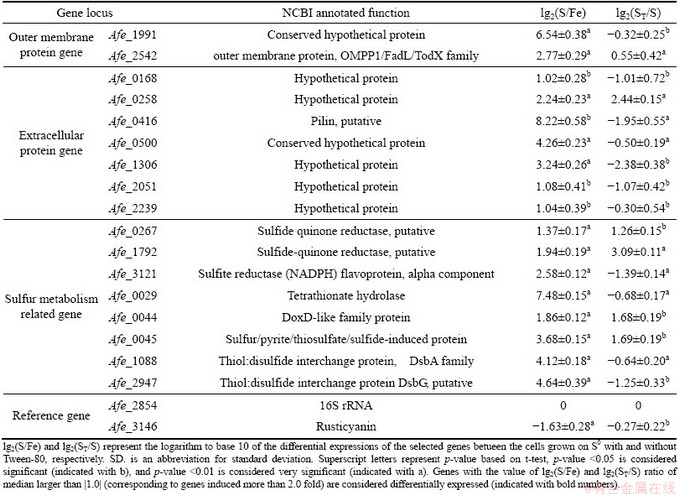
Compared with the down regulation of the rusticyanin (Afe_3146, rus), the expressions of all the genes selected, especially the hypothetical and putative protein genes from outer membrane and extracellular, were significantly up regulated when the culture was transformed from Fe2+ substrate to S0 substrate, indicating the relevance of them to sulfur metabolism [17].
Gene expressions of the cells grown on S0 were significantly different when 10-2 g/L Tween-80 was added into the culture media. The surfactant has an obvious effect on the expressions of the extracellular proteins and the sulfur metabolism related genes indicated from Table 2. Hypothetical extracellular protein gene Afe_2170 and four sulfur metabolism relevant genes (Afe_0267, Afe1792, Afe_0044, Afe_0045) were significantly up regulated. Four genes of extracellular proteins (Afe_0168, Afe_0416, Afe_1306, Afe_2051), and two genes (Afe_3121, Afe_2947) encoding sulfur metabolism related proteins were significantly down regulated. The expression of the outer membrane protein genes were not significantly influenced by Tween-80 at this concentration.
4 Discussion
It is well known that elemental sulfur could exist as one of the intermediates during bioleaching of chalcopyrite, and it could form a passivation layer when it is not oxidized efficiently by SOB [6,21]. The addition of the surfactant Tween-80 (10-2 g/L) shortened the adaption stage of bacterial growth (Fig. 4(a)), promoted sulfur oxidation (i.e., decrease of pH as shown in Fig. 4(b)) and enhanced the copper extraction yield of chalcopyrite (Fig. 4(c)). This demonstrates the addition of a certain concentration of surfactants may be an effective way to improve the bioleaching rate of chalcopyrite.
The adsorption of SOB on the hydrophobic surface is generally thought to be an essential step in sulfur oxidation process. Electrostatic interaction does not play an important role in the bacteria-sulfur interaction according to the evidence of TAN and CHEN [22]. So the affinity is affected by the wetting behavior of the hydrophobic substrates. According to the microbial population dynamics and the pH variations in this study, 10-1-10-2g/L Tween-80 showed no sign of toxicity for A. ferrooxidans ATCC 23270 grown on insoluble substrate (S0) and soluble substrates (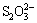 , Fe2+), and about 10-2 g/L Tween-80 could promote the cell growth on insoluble sulfur substrates (S0, CuFeS2). This is because suitable concentration of Tween-80 can efficiently modify the contact between the minerals and the cells.
, Fe2+), and about 10-2 g/L Tween-80 could promote the cell growth on insoluble sulfur substrates (S0, CuFeS2). This is because suitable concentration of Tween-80 can efficiently modify the contact between the minerals and the cells.
EPS is thought to be amphiphilic and it can mediate the contact of the hydrophilic cells and the hydrophobic sulfur or minerals, though so far little is known about the EPS composition [10]. In this work, addition of Tween-80, as one of the typical surfactants or wetting agents, altered the wettability of elemental sulfur. FT-IR spectra analysis reflected the wetting of the hydrophobic elemental sulfur (Fig. 5) by the cells, because of the appearance of the absorption brands of the organic groups, such as —OH, —NH, —NH2, —CH2, —CH3, =C—H, C—O, —C=O, and —CONH— in the FT-IR spectra of the sulfur treated by cells.
The cells grown on S0 demonstrated a different FT-IR spectra from that on Fe2+ substrates (Fig. 5), indicating the substrate specificity of EPS formation. This can be supported by the significant down-regulation of the expression of the extracellular proteins Afe_0416, Afe_0168, Afe_1306, Afe_2051 and the significant up-regulation of the extracellular protein Afe_0258 (Table 2). Most of the extracellular proteins, except Afe_0258 and Afe_2239, are cysteine-rich proteins. This indicates clearly the important role of the thiol groups of cysteine in EPS in the present study.
Pilin as the appendix of the cell surface has been considered to play an important role in the attachment of bioleaching microbes to mineral surface [23]. As shown in Table 2, the pilin relevant gene Afe_0416 was down-regulated, when 10-2 g/L Tween-80 was added to the sulfur containing medium. But this gene expression in sulfur-grown cells was significantly higher than that in ferrous iron-grown cells, indicating the important role of the pilin of A. ferrooxidans in sulfur adsorption, which enables A. ferrooxidans to attach and colonize to solid sulfur [23,24].
Extracellular conserved hypothetical protein Afe_0500 contains CXXC domains, belonging to the characteristics domain relating to oxidation and reduction of sulfur. This is assured by the fact that Afe_0500 was highly expressed in the sulfur grown A. ferrooxidans, but it seems to be not affected by 10-2 g/L Tween-80.
The effect of Tween-80 on the expression of the known proteins and enzymes involved in the sulfur oxidation process was also observed. The genes Afe_0267 and Afe_1792 encode sulfide/quinone oxidoreductase (SQR), and Afe_0044 and Afe_0045 encode thiosulfate: quinone oxidoreductase (TQR). They were highly expressed in S0 grown cells and further up-regulated in expression when 10-2 g/L Tween-80 was supplemented. This indicates the improvement of bio-oxidation of sulfur by addition of 10-2 g/L Tween-80.
Afe_3121 is a flavoprotein, subunit of sulfite reductase, which is speculated to be involved in the assimilation of sulfur into cysteine in A. ferrooxidans [23]. Afe_2947 and Afe_1088 (thiol: disulfide interchange proteins, DsbG and DsbA) are used for protein folding and stabilization by resolving incorrectly formed disulfide bonds and were considered to be related to stress response [18,24]. Down regulation of the genes Afe_3121, Afe_2947 and Afe_1088 was observed when Tween-80 was added into S0 medium. The reason for this phenomenon needs to be further investigated.
No comprehensive understanding of biochemical mechanism of the sulfur oxidation process in bioleaching system has been achieved yet. However, surfactant Tween-80 in this study has been proven its important effect on the cell adhesion, sulfur activation and the sulfur metabolism. Further investigation could not only help us establish a more effective bioleaching system but also shed light on the understanding of the regulation of sulfur-oxidizing process.
5 Conclusions
1) The growth and sulfur oxidation of A. ferrooxidans ATCC 23270 grown on S0, 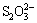 , Fe2+, and chalcopyrite with 10-10-3 g/L Tween-80 showed that 10-2 g/L Tween-80 promoted the growth of A. ferrooxidans and its metabolism on the insoluble substrates S0 and CuFeS2.
, Fe2+, and chalcopyrite with 10-10-3 g/L Tween-80 showed that 10-2 g/L Tween-80 promoted the growth of A. ferrooxidans and its metabolism on the insoluble substrates S0 and CuFeS2.
2) After 24 d of bioleaching, the copper extraction yield of chalcopyrite increased by 16 % with addition of 10-2 g/L Tween-80.
3) The results of FT-IR spectrometry and RT-qPCR demonstrated the significant effect of Tween-80 on the cell adhesion, sulfur activation and the sulfur metabolism.
References
[1] RAWLINGS D E, JOHNSON D B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia [J]. Microbiology, 2007, 153(2): 315-324.
[2] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239-248.
[3] SAND W, GEHRKE T, JOZSA P G, SCHIPPERS A. (Bio)chemistry of bacterial leaching direct vs. indirect bioleaching[J]. Hydrometallurgy, 2001, 59(2-3): 159-175.
[4] SCHIPPERS A, SAND W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur [J]. Applied and Environmental Microbiology, 1999, 65(1): 319-321.
[5] XIA J L, YANG Y, HE H, LIANG C L, ZHAO X J, ZHENG L, MA C Y, ZHAO Y D, NIE Z Y, QIU G Z. Investigation of the sulfur speciation during chalcopyrite leaching by moderate thermophile Sulfobacillus thermosulfidooxidans [J]. International Journal of Mineral Processing, 2010, 94(1-2): 52-57.
[6] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. International Journal of Mineral Processing, 2008, 86(1-4): 1-17.
[7] FRIEDRICH C G, BARDISCHEWSKY F, ROTHER D, QUENTMEIER A, FISCHER J. Prokaryotic sulfur oxidation [J]. Current Opinion in Microbiology, 2005, 8(3): 253-259.
[8] QUATRINI R, APPIA-AYME C, DENIS Y, JEDLICKI E, HOLMES D S, BONNEFOY V. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans [J]. BMC Genomics, 2009, 10: 394.
[9] GEHRKE T, HALLMANN R, KINZLER K, SAND W. The EPS of Acidithiobacillus ferrooxidans—A model for structure-function relationships of attached bacteria and their physiology [J]. Water Science & Technology, 2001, 43(6): 159-167.
[10] SAND W, GEHRKE T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157(1): 49-56.
[11] GEHRKE T, TELEGDI J, THIERRY D, SAND W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching [J]. Applied and Environmental Microbiology, 1998, 64(7): 2743-2747.
[12] GOVENDER Y, GERICKE M. Extracellular polymeric substances (EPS) from bioleaching systems and its application in bioflotation [J]. Minerals Engineering, 2011, 24(11): 1122-1127.
[13] YUAN Qiu-hong, WANG Yue-hu, ZHANG Guang-ji, LUO Zhi-xiong, YANG Chao. Bioleaching mechanism of orpiment with different bacteria strains [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(6): 1234-1240. (in Chinese)
[14] DUNCAN D W, TRUSSELL P C, WALDEN C C. Leaching of chalcopyrite with Thiobacillus ferrooxidans: Effect of surfactants and shaking [J]. Applied Microbiology, 1964, 12(2): 122-126.
[15] ZHANG C G, XIA J L, ZHANG R Y, PENG A A, NIE Z Y, QIU G Z. Comparative study on effects of Tween-80 and sodium isobutyl-xanthate on growth and sulfur-oxidizing activities of Acidithiobacillus albertensis BY-05 [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(4): 1003-1007.
[16] KONISHI Y, ASAI S, YOSHIDA N. Growth kinetics of Thiobacillus thiooxidans on the surface of elemental sulfur [J]. Applied and Environmental Microbiology, 1995, 61(10): 3617-3622.
[17] ZHANG C G, ZHANG R Y, XIA J L, ZHANG Q, NIE Z Y. Sulfur activation-related extracellular proteins of Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1398-1402.
[18]  J, PEDROSO I, QUATRINI R, DODSON R J, TETTELIN H, BLAKE R, EISEN J A, HOLMES D S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications [J]. BMC Genomics, 2008, 9: 597.
J, PEDROSO I, QUATRINI R, DODSON R J, TETTELIN H, BLAKE R, EISEN J A, HOLMES D S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications [J]. BMC Genomics, 2008, 9: 597.
[19] GU G H, SU L J, CHEN M L, SUN X J, ZHOU H B. Bio-leaching effects of Leptospirillum ferriphilum on the surface chemical properties of pyrite [J]. Mining Science and Technology, 2010, 20(2): 286-291.
[20] HE H, YANG Y, XIA J L, DING J N, ZHAO X J, NIE Z Y. Growth and surface properties of new thermoacidophilic archaea strain Acidianus manzaensis YN-25 grown on different substrates [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1374-1378.
[21] ROHWERDER T, SAND W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp [J]. Microbiology, 2003, 149(7): 1699-1710.
[22] TAN S N, CHEN M. Early stage adsorption behaviour of Acidithiobacillus ferrooxidans on minerals I: An experimental approach [J]. Hydrometallurgy, 2012, 119-120: 87-94.
[23]  J, VELOSO F, JEDLICKI E, HOLMES D. Metabolic reconstruction of sulfur assimilation in the extremophile Acidithiobacillus ferrooxidans based on genome analysis [J]. BMC Genomics, 2003, 4(1): 51.
J, VELOSO F, JEDLICKI E, HOLMES D. Metabolic reconstruction of sulfur assimilation in the extremophile Acidithiobacillus ferrooxidans based on genome analysis [J]. BMC Genomics, 2003, 4(1): 51.
[24] CHI A, VALENZUELA L, BEARD S, MACKEY A J, SHABANOWITZ J, HUNT D F, JEREZ C A. Periplasmic proteins of the extremophile Acidithiobacillus ferrooxidans [J]. Molecular & Cellular Proteomics, 2007, 6(12): 2239-2251.
表面活性剂Tween-80对Acidithiobacillus ferrooxidans硫氧化和硫代谢相关蛋白质基因表达的影响
彭安安1,刘红昌1,聂珍媛1, 2,夏金兰1, 2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:研究了表面活性剂Tween-80对Acidithiobacillus ferrooxidans ATCC 23270生长、硫氧化和硫代谢相关典型基因表达的影响。结果表明,当培养基中含有10-2 g/L Tween-80时,A. ferrooxidans的生长以及其对不溶性底物(S0和CuFeS2)的代谢得到了促进。在该条件下,经过24 d的生物浸出,黄铜矿的铜离子浸出率比对照组(不含Tween-80)高16%。FT-IR光谱分析表明,这可能是由于Tween-80的存在而导致胞外多聚物成分发生变化而引起的。用RT-qPCR来分析17个硫代谢相关基因在Tween-80存在时的表达差异。胞外蛋白质基因表达下调表明了Tween-80对细菌-硫吸附作用的影响。硫代谢相关酶基因表达水平的变化为硫代谢的研究提供了参考。
关键词:Acidithiobacillus ferrooxidans;硫代谢;表面活性剂Tween-80;RT-qPCR
(Edited by YANG Hua)
Foundation item: Projects (50974140, 51274257) supported by the National Natural Science Foundation of China; Project (20090162110054) supported by the PhD Programs Foundation of Ministry of Education of China
Corresponding author: XIA Jin-lan; Tel: +86-731-88836944; E-mail: jlxia@csu.edu.cn
DOI: 10.1016/S1003-6326(12)61767-1
Abstract: The effects of surfactant Tween-80 on the growth, sulfur oxidation, and expression of selected typical sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans ATCC 23270 were investigated. The results showed that in the presence of 10-2 g/L Tween-80, the growth of A. ferrooxidans and its metabolism on the insoluble substrate S0 and CuFeS2 were promoted. After 24 d of bioleaching, the copper extraction yield of chalcopyrite at 10-2 g/L Tween-80 increased by 16% compared with the bioleaching experiment without Tween-80. FT-IR spectra analysis revealed that the result was probably caused by the extracellular polymeric substances whose composition could be changed by the surfactant addition. RT-qPCR was used to analyze the differential expressions of 17 selected sulfur metabolism relevant genes in response to the addition of Tween-80. Down-regulation of the extracellular protein genes indicated the influence of Tween-80 on bacteria-sulfur adsorption. Variation of the expression level of the enzymes provided a supplement to sulfur metabolism investigation.


