Atomic states and properties of Ru-electrocatalyst
PENG Hong-jian(彭红建)1, 2, XIE You-qing(谢佑卿)2,WEI De-liang(魏得良)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Materials and Science Engineering, Central South University, Changsha 410083, China
Received 7 June 2005; accepted 5 April 2006
Abstract:
Using the one-atom theory(OA), the atomic states of Ru-electrocatalyst with hcp structure was determined as [Kr](4dn)3.78(4dc)2.22(5sc)1.77(5sf)0.23. The potential curve, elasticity and the temperature dependence of linear thermal expansion coefficient and bulk modulus of hcp-Ru were calculated quantitatively. The atomic states of this metal with fcc and bcc structure and liquid state were also studied. According to its atomic states, the relationship between the atomic states and catalytic performance was explained qualitatively and these supplied Ru-metal and electrocatalyst with complete data for optimum designation in accordance with metal material systematic sicence.
Key words:
Ru-electrocatalyst; atomic states; catalytic performance;
1 IntroductionIn recent years, because the consumption of irreplaceable fossil fuels which meet the bulk of power needs nowadays is non-sustainable and worldwide attention focus on processes that convert these precious resources into electricity via efficient, economical and environmentally sound manner. Proton exchange membrane fuel cell(PEMFC) has the characters of no pollution, no-noise, high efficiency, high power and low temperature operation, therefore, more and more nations attach importance to it. The high reliability and friendly environment make them particularly attractive as a power sources in space is very in important in reducing the cost of proton exchange membrane fuel cell stack and find a new type catalyst which is cheap and made up of disprecious metal to substitute precious metal as electrocatalyst. Now, Pt-Ru alloy is still used very widely and has good CO-tolerance ability[1-5]. In the framework of the metallic materials systematic science, there are three parts: pure metal systematic science, alloy physics and chemistry and alloy statistical thermo- dynamics. The core of the pure metal systematic science is the one atom theory(OA)[6-9] of pure metals, and that of the alloys physics and chemistry and alloys statistical thermodynamics is the characteristic crystal theory(CC) [10-13] of alloys. Now, few scientists reveal the essences of catalytic performance of Ru from atomic state level. In this paper, we have applied metallic materials systematic science to calculate the atomic states, physical properties and catalytic performance of pure metal Ru and supplied Ru-metal and electrocatalyst with complete data for optimum designation.
2 Basic atomic states of electrocatalyst Ru
In the OA theory, the atomic states of pure metals is described by the quasi-electron-occupation(QEO) number of the one-atom state ψa which is made up of some basic atom states φk as
![]() (1)
(1)
At the outer shell of Ru atom, there are covalent electrons nc, near-free electrons nf and no-bond electrons nn. In each basic atom state, the distribution of electrons follows the Pauli exclusion principle. The characteristic properties (lattice constant a and cohesive energy Ec) of the temperature, pseudo-crystals formed by atoms in each kind of basic atom state of Ru metal can be obtained by a series of established formulas[8]. Using one-atom state self-consistency method and schematic procedure shown in Fig.1, the lattice constant, cohesive energy and Debye temperature of experimental value can be used to further calculate the physical properties. The results of pure Ru metal are listed in Table 1.

Fig.1 Schematic procedure for determining atomic states and properties of hcp, fcc, bcc and liquid pure Ru-metals (‘p’ denotes phase, ‘h’ denotes hcp structure, ‘*’ denotes experi- mental value)
3 Determination of atomic state of hcp-Ru
In this research, firstly the two characteristic properties (lattice constant a and cohesive energy Ec) were taken as the criterion and perform the three-state hybridization, Multiple-Properties-Determining-State method was used in OA theory. Secondly, after a systematic analysis mainly taking the lattice constant and cohesive energies into consideration, the three-state combination of hcp-Ru are determined as: (c1=0.11, c5=0.88, c7=0.01), then the atomic states of hcp-Ru can be determined as: [Kr](4dn)3.78(4dc)2.22(5sc)1.77(5sf)0.23. The atomic state parameters, bond parameters and characteristic properties of hcp-Ru crystal are listed in Table 2, where r1, r2, r3 denote covalent bond lengths, n1, n2, n3 denote covalent electron pair numbers and ![]() ,
, ![]() ,
, ![]() denote covalent bond energies.
denote covalent bond energies.
4 Atomic states of fcc-Ru, bcc-Ru
4.1 Lattice constant and cohesive energy of fcc-Ru, bcc-Ru
Under the isopiestic condition, the Gibbs energy of pure metal is a function of the capacity of heat cp(T) and volume V(T):
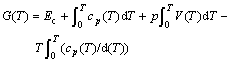 (2)
(2)
![]() (3)
(3)
According to the cohesive energies of hcp-Ru and the G′(T) data of hcp-Ru supplied in SGTE database, the cohesive energies of fcc-Ru, bcc-Ru can be obtained approximately as following: Ec(fcc-Ru)=637.50 kJ/mol, Ec(bcc-Ru)=623.50 kJ/mol.
By OA theory and schematic procedure in Fig.1, the lattice constants of fcc-Ru, bcc-Ru can be obtained approximately as follows: a(fcc-Ru)=0.379 01 nm, a(bcc-Ru)=0.300 82 nm.
4.2 Atomic states of fcc-Ru, bcc-Ru
Similar to determining the atomic states of hcp-Ru, atomic states of fcc-Ru, bcc-Ru can be determined by the Multiple-Properties-Determining-State method as
ψa(fcc-Ru)=[Kr](4dn)3.70(4dc)2.44(5sc)1.42- (5sf)0.44
ψa(bcc-Ru)=[Kr](4dn)4.00(4dc)2.22 (5sc)1.56(5sf)0.22
4.3 Atomic state of liquid-Ru
Similar to the case of fcc-Ru bcc-Ru, the cohesive energies of L-Ru can be determined through SGTE database: Ec(L-Ru)=632.80 kJ/mol.
Supposing Ru still has hcp structure after melted, according to their densities[11] before and after melted and the lattice constants of solid hcp-Ru (ρs=12.36 g/cm3, ρl=10.99 g/cm3), the lattice constants of L-Ru can be obtained: a(L-Ru)= 0.279 47 nm. By the way, it has been confirmed by modern X-ray experiments that liquid metals really have the short distance ordering similar to crystals.
Table 1 Basic atomic states and corresponding pseudo crystal characteristic properties of Ru metal

Now, similar to the case above, it is not difficult to determine the atomic states of L-Ru: ψa(L-Ru)=[Kr] (4dn)4.00 (4dc)2.00 (5sc)1.52(5sf)0.48.
5 Interpretation catalytic performance of Ru from atomic state level
According to solid energy-level theory, we delineate d-state of transition metal to adopt the concept called as d-orbital vacancy. It is advantageous to catalytic reaction. Generally, the more the d-orbital vacancy is, the more advantageous the catalytic reaction is. When Ru atoms get together, 1 s electrons transform into d electrons, the number of d-electrons decreases, so the d-orbital vacancies increases. In the PtRu alloys, Ru atom substitutes Pt atom in fcc structure to form PtRu solid solution mixture because their bond radius and volume are almost the same, d electrons of Pt transfer to Ru atoms due to the effect related to the electron withdrawing properties of Ru[19], therefore the d-orbital vacancies of Pt increase and lead to chemiadsorb substances, and then electrocatalyst has high catalytic activity. We think that under the metal material systematic science instruction, to realize the optimum designation electrocatalyst PtxRu1-x, to obtain basic information of characteristic atom sequences and corresponding characteristic crystal sequences, then according to objective information, formulate designation plan about catalyst alloy of gradient, structure and state and prepare a new alloy catalyst, it will lead to optimum value d-orbital vacancy of Pt and the inhibition of carbon monoxide. In a large scale to adsorb substances in catalyst surface, then catalyst has best catalytic properties.
6 Quantitative calculations of physical pro- perties of hcp-Ru
As a whole, one of the critical parts of the OA theory is the determination of atomic states, the other is the qualitative explanations and quantitative calculations of various physical properties based on the atomic states obtained above. The object of researching atomic states is to perform these kinds of explanations and calculations, and to understand the change rules of various physical properties in essence and control them effectively. On the other hand, these kinds of qualitative explanations and calculations can also be used to judge whether the atomic states we obtain are proper. The potential curves, elasticity and the changes of linear thermal expansion with temperature of Ru with hcp structure will be calculated.
According to MAI potential function, theoretical potential curves of hcp-Ru can be obtained as shown in Fig. 2.
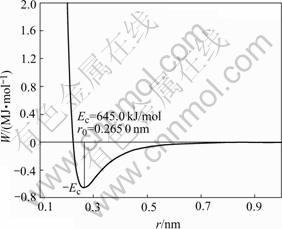
Fig.2 Theoretical potential curves of hcp-Ru
6.1 Elasticity
According to the elasticity calculating formula in OA theory, bulk modulus B=322.8 GPa is very close to experimental value 320.8 GPa, elastic modulus Y=320.59 GPa, modulus of rigidity μ=120.45 GPa and Poisson’s ratio σ=0.330 8 of hcp-Ru can be obtained. And Bulk modulus as a function of temperature for hcp-Ru can also be obtained as shown in Fig.3.
6.2 Linear thermal expansion coefficient as function of temperature for hcp-Ru
According to the expression between linear thermal expansion coefficient and temperature in OA theory, the change curves of linear thermal expansion coefficient as a function of temperature of hcp-Ru can be obtained as shown in Fig.4. The constants in Grüneisen equation are, respectively, n=1.3, j=8.6, K=1.43, Q=302.20 kJ/mol.

Fig.3 Bulk modulus as function of temperature for hcp-Ru

Fig.4 Linear thermal expansion coefficient as function of temperature for hcp-Ru
7 Conclusions
1) When Ru atoms get together, 1s electrons transform into d electrons. So, the atomic states of hcp-Ru can be described as follows: [Kr](4dn)3.78(4dc)2.22- (5sc)1.77(5sf)0.23. Based on the atomic states of hcp-Ru, the relationship between the difference of the physical properties of hcp-Ru and their atomic states was explained qualitatively; the lattice constant, cohesive energy, potential curve, elasticity and change curves of linear thermal expansion coefficient as a function of temperature are calculated quantitatively, and the theoretical values and experimental values are in good accordance.
2) Atomic states of fcc-Ru, bcc-Ru and L-Ru are as follows:Ψa (fcc-Ru) = [Kr] (4dn)3.70(4dc)2.44(5sc)1.42- (5sf)0.44, Ψa(bcc-Ru)=[Kr](4dn)4.00(4dc)2.22(5sc)1.56(5sf)0.22;Ψa (L-Ru)=[Kr](4dn)4.00(4dc)2.00 (5sc)1.52(5sf)0.48.
3) According to atomic states, we initially interpret the relationship between atomic states of Ru and its catalytic performance.
References
[1] RADMILOVIC V, GASTEIGER H A, ROSS P N. Structure and chemical composition of a supported Pt-Ru electrocatalyst for methanol oxidation [J]. Journal of Catalysis, 1995, 154: 98-106.(in Chinese)
[2] LU Ling-hong, JIN Li-hua, WANG Jun-tao. Oxygen electrode for proton exchange membrane fuel cell [J]. Chinese Journal of Powder Sources, 2001, 25(2): 69-71.
[3] BRANKOVIC S R, WANG J X, ZHU Y, SABATINI R, MCBREEN J. Electrosorption and catalytic properties of bare and Pt modified single crystal and nanostructured Ru surface [J]. Journal of Electroanalytical Chemistry, 2002, 524: 231-241.
[4] HOU Zhong-jun, YU Hong-mei, YI Bao-lian, HAN Ming. The development of carbon monoxide tolerant catalyst for proton exchange membrane fuel cell [J]. Electrochemistry, 2000, 6(4): 379-384.
[5] LIZCANO-VALBUENA W H, PAGANIN V A, LEITE C A P. Catalysts for DMFC: relation between morphology and electrochemical performance [J]. Electrochimica Acta, 2003, 48: 3869-3878.
[6] XIE You-qing. The framewok of metallic materials systematic science [J]. Mater Rev, 2001, 15(4): 12-15. (in Chinese)
[7] XIE You-qing, MA Liu-ying. The theoretical lattice parameter of valence electron structure of crystal [J]. J Cent South Inst of Mining and Metall, 1985(1): 1-10. (in Chinese)
[8] XIE You-qing. A new potential function with many-atom interactions in solid [J]. Science in China(Series A),1993, 36(1): 90-99.
[9] XIE You-qing. One atom self-consistency method for determining atomic states of crystal [J]. Chinese Science Bulletin, 1992, 37(16): 1529-1532.
[10] XIE You-qing. Atomic states and properties of pure iron [J]. Acta Metall Mater, 1994, 42(11): 3705-3715.
[11] XIE You-qing. Atomic energies and gibbs energy functions of Ag-Cu alloys [J]. Science in China (Series E), 1998, 41(2): 146-156.
[12] XIE You-qing, ZHANG Xiao-dong. Atomic volumes and volume functions for Ag-Cu alloys [J]. Science in China(Series E), 1998, 41(2): 157-168.
[13] XIE You-qing, ZHANG Xiao-dong. Atomic states of Ag-Cu alloys [J]. Science in China (Series E), 1998, 41(3): 225-236.
[14] ASM International Handbook Committee, Metals handbook (vol. 2): Properties and Selection: Nonferrous Alloys and Special-Purpose Materials [M]. 10th ed. OH: Materials Park, 1990. 1108.
[15] KITTEL C. Introduction to Solid State Physics [M]. 6th ed. New York: John Wiley & Sons, Inc, 1986. 55, 57.
[16] DINSDALE A T. SGTE data for pure elements [J]. CALPHAD, 1991, 15(4): 317-425.
[17] American Institute of Physics, American Institute of Physics Handbook [M]. 3rd ed. New York: McGraw-Hill Book Company, 1972. 4-123.
[18] COSTANAGNA P, SRINIVASAN S M. Quantum jumps in the PEMC science and technology from the 1960s to the year 2000: Part I, Fundamental scientific aspects [J]. J Powder Sources, 2001, 102: 242-251.
[19] CAMARA G A, GIZ M J, PAGAMIN V A, TICIANELLI E A, Correlation of electrochemical and physical properties of PtRu alloy electrocatalysts for PEM fuel cells [J]. Journal of Electroanalytical Chemistry, 2002, 537: 21-29.
(Edited by LONG Huai-zhong)
Foundation item: Project(50271085; 50471058) supported by the National Natural Science Foundation of China; Project(04FJ2002) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: PENG Hong-jian; Tel: +86-731-8879287; E-mail: phj108@163.com



