文章编号:1004-0609(2010)08-1578-05
熔盐电脱氧法制备CoSn合金
张庆军1, 2,屈梅玲2,王 岭3,戴 磊3,田 颖2,崔春翔1
(1. 河北工业大学 材料学院,天津 300130;
2. 河北理工大学 材料学院,唐山 063009;
3. 河北理工大学 化工与生物技术学院,唐山 063009)
摘 要:在850 ℃的CaCl2熔盐中,以烧结后的Co3O4-SnO2片体为阴极,高纯石墨棒为阳极,采用恒电压电解,制备CoSn合金,研究烧结温度、电解时间对电解过程的影响。采用电子扫描显微镜(SEM)和X射线衍射仪(XRD)分析样品的微观形貌和电解产物的相组成,并采用循环伏安法研究其反应机理。结果表明:经850 ℃烧结的混合氧化物试样,在2.1 V工作电压下电解12 h,可制备出海绵状纯相的CoSn合金。在电脱氧过程中,单质Co首先分步还原,锡的氧化物与熔盐反应后在单质Co表面还原,形成CoSn合金。
关键词:
中图法分类号:TF111.52 文献标志码:A
Preparation of CoSn alloy by electro-deoxidization in molten salt
ZHANG Qing-jun1, 2, QU Mei-ling2, WANG Ling3, DAI Lei3, TIAN Ying2, CUI Chun-xiang1
(1. College of Materials, Hebei University of Technology, Tianjin 300130, China;
2. College of Materials, Hebei Polytechnic University, Tangshan 063009, China;
3. College of Chemical Engineering and Biotechnology, Hebei Polytechnic University, Tangshan 063009, China)
Abstract: The CoSn alloy was prepared in molten CaCl2 at 850 ℃ by constant voltage (2.1 V) electrolysis using sintered pellet of SnO2 and Co3O4 mixture as cathode and pure graphite rod as anode. The effects of the sintering temperature and electrolysis time on the electro-deoxidization process were studied. The morphologies and phases composition of products of the electro-deoxidization were examined by SEM and XRD, respectively. The mechanism of the electro-deoxidization process was studied by cyclic voltammetry. The results show that that pure spongy CoSn can be prepared by direct electrochemical electrolysis of Co3O4/SnO2 mixture pellets sintered at 850 ℃ in molten CaCl2 at 2.1 V for 12 h. During the electro-deoxidization process, in the mixed oxide, Co3O4 is realuced to Co step by step, followed by the reaction of Sn oxide with molten on the surface of the newly-formed Co to form CoSn alloy.
Key words: electro-deoxidization; CoSn; oxide; molten salt
锡基合金作为锂离子电池的负极材料,具有比容量高、循环性能好及与电解液的相容性好等优点,引起人们的广泛关注[1-3]。目前,锡基合金常用的制备方法有机械合金化法、化学共还原法、电沉积法和碳热还原法等[4-9]。
熔盐电脱氧法制备金属或合金是一种新型的绿色冶金工艺[10]。该工艺具有操作简单、合金成分易控制、生产成本低等优点,电解产物为粉末状,非常适用于电池电极材料的制备。目前,采用该方法已成功地制备了Ti、 Cr、Nb、TbNi5、CeNi5和TbFe2等[11-16]。
本文作者使用熔盐电脱氧法,在CaCl2熔盐中,以SnO2和Co3O4混合氧化物为原料,电解制备粉末状CoSn合金,研究烧结温度和电解时间等对电解过程、产物组成和形貌的影响,探讨电脱氧过程中合金生成的机理。
1 实验
将分析纯SnO2、Co3O4粉末按n(Co)?n(Sn)为1?1配料,物料在球磨机中湿磨6 h并干燥,在30 MPa下压制成型,得到圆柱型片(直径为10 mm,质量为2.5 g),将其分别在850、1 050、1 250 ℃下烧结4 h后备用。
电解在配有程序温度控制器的铁铬铝丝电阻炉中进行。将干燥的CaCl2放入氧化铝坩埚中,置于反应器底部,待反应器升温至850 ℃后,将悬挂在铁铬铝丝上的氧化物烧结片和高纯石墨棒插入到已经熔化的CaCl2中。在Ar气氛中,分别以氧化物烧结片和高纯石墨棒作为阴极和阳极,在2.1 V电压下进行恒压电解。电解完成后将阴极产物取出,用蒸馏水洗净、 干燥。
采用KYKY-3400型扫描电镜分析阴极片电解前后的微观形貌,采用XRERT-PRO型X射线衍射仪(Cu Kα)分析电解产物的相组成。
采用纯钼薄(长20 mm, 宽10 mm,厚0.5 mm)制备成金属孔腔电极(孔径1 mm)。将球磨过的SnO2和Co3O4粉末装进小孔中作为工作电极,以高纯石墨棒作为辅助电极,以铁铬铝丝作为参比电极,在850 ℃的CaCl2熔盐中进行循环伏安测试。
2 结果及讨论
试样在不同温度烧结后的微观形貌如图1所示。由图1可看出,经850 ℃烧结后,试样的微观形貌与烧结前(见图1(a))相比,无明显差别,颗粒尺寸较小(见图1(b));随着烧结温度的升高,颗粒逐渐长大(见图1(c)和(d))。图2所示为不同温度下烧结后试样的XRD谱。由图2可看出,经850 ℃烧结后,样品的相组成未发生变化,为SnO2和Co3O4的混合物;在1 050 ℃和1 250 ℃烧结的试样中,Co3O4与部分SnO2反应生成Co2SnO4,Co3O4已完全消失。
图3所示为在2.1 V电压下电解时,不同烧结温度的试样电流随时间的变化曲线。由图3可看出,在电解开始时,迁移到样品表面的电子很快使表面的金属离子还原, 并扩散到熔盐中,因此电极表面的反应速度较快,电流较大;随着反应的进行,当样品表面电脱氧完成后,样品内部氧化物的还原需要O2-从内部扩散到熔盐中才能完成,受扩散控制,由于扩散速度较慢,所以电流迅速下降,直至达到完全电解后的残余电流[16-17]。随着烧结温度的升高,颗粒尺寸逐渐变大,与熔融CaCl2接触的面积逐渐减小,同时增大了电解过程中O2-的传输距离,因此,电解电流随烧结温度的升高而减小,不利于电脱氧过程的进行[16-17]。
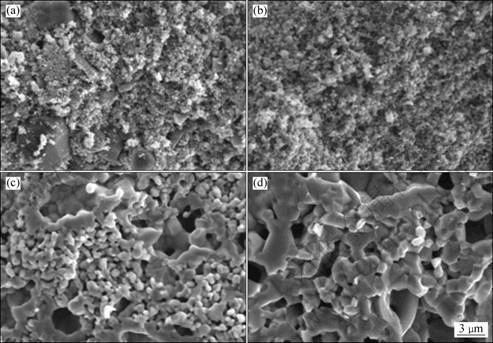
图1 不同温度下烧结4 h后Co3O4-SnO2试样的SEM像
Fig.1 SEM images of Co3O4-SnO2 pellets sintered at different temperatures for 4 h: (a) Before sintering; (b) 850 ℃; (c) 1 050 ℃; (d) 1 250 ℃

图2 不同温度下烧结4 h后Co3O4-SnO2试样的XRD谱
Fig.2 XRD patterns of Co3O4-SnO2 pellets sintered for 4 h at different temperatures
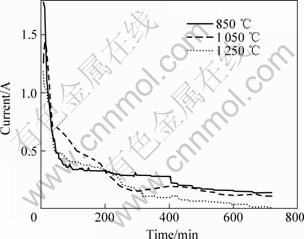
图3 不同温度烧结的试样于2.1 V电解时的电流—时间 曲线
Fig.3 Current—time curves of Co3O4 -SnO2 pellets sintered at different temperatures during electrolyzing at 2.1 V
图4为不同温度烧结后试样在2.1 V电解12 h的产物的XRD谱。由图4可见,经850 ℃烧结的试样电解产物为纯相CoSn合金;经1 050 ℃和1 250 ℃烧结的试样的电解产物主要为金属Sn和金属Co。这与图3的电流变化曲线一致,烧结温度越高,电流越小,越不利于电脱氧过程的进行。
经850 ℃、1 050 ℃和1 250 ℃烧结的试样电解产物的微观形貌如图5所示。由图5可看出,850 ℃烧结试样的电解产物呈规则的颗粒形状,界面清晰,且所得电解产物强度很小,轻轻研磨即成粉末;1 050 ℃和1 250 ℃烧结试样的电解产物的颗粒主要为层片状和块状,且形状不规则。
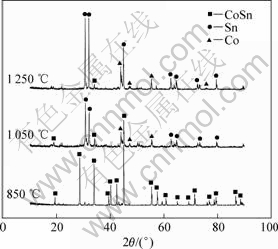
图4 不同温度烧结试样在2.1 V电解12 h后的XRD谱
Fig.4 XRD patterns of products sintered at different temperatures after electrolyzing at 2.1 V for 12 h
图6所示为经850 ℃烧结后烧结片在2.1 V电压下分别电解1、3、6、9、12 h后产物的XRD谱。由图6可看出,电解1 h后,试样中的Co3O4完全消失,一部分还原为CoO,一部分还原为单质Co。同时,试样中出现CaSnO3,这是由于电脱氧过程中,O2-从试样中扩散出来,与Ca2+结合生成CaO,进而与SnO2反应生成CaSnO3[13, 18];电解3 h后,除了少量SnO2、CaSnO3和Co之外,出现大量Co3Sn2合金,CoO已全部消失;电解6 h后,电解产物中开始出现CoSn合金;电解9 h后,试样主要为CoSn合金,但是产物不纯,仍然存在少量的CaSnO3;电解12 h后,电解产物的XRD谱中只有CoSn合金的衍射峰,表明氧化物阴极已完全电解。结果表明:电解过程中Co3O4的还原过程是分步进行的,即Co3+ →Co2+→Co。与此同时,SnO2与CaCl2发生反应生成CaSnO3,随后CaSnO3在单质Co表面还原形成Co3Sn2合金,随着CaSnO3的不断还原、合金化,最终形成CoSn合金。还原过程经历了Co→钴的氧化物→电解质和Co-Sn→CaSnO3→电解质两种三相反应过程[14, 16]。

图5 不同温度烧结的试样电解12 h后的SEM像
Fig.5 SEM images of samples sintered at different temperatures after electrolysis for 12 h: (a) 850 ℃; (b) 1 050 ℃; (c) 1 250 ℃
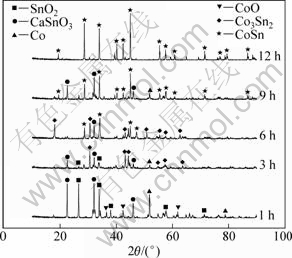
图6 2.1 V电解电压下电解不同时间后电解产物的XRD谱
Fig.6 XRD patterns of productions after electrolyzing at 2.1 V for different times
图7所示为在850 ℃的CaCl2熔盐中Co3O4粉末、Co3O4粉末与SnO2混合粉末的循环伏安曲线。由图7可看出,空白试样的电位从0 V开始负向扫描,达到-1.0 V时,电流开始增加,在-1.3 V出现还原峰c2,这是由于CaCl2的还原造成的[17]。随着电位的负移,Ca2+不断还原,电流继续增大,当电位负移至-1.5 V时,在c3峰处电流达到最大值。随着电位正向扫描,由于Ca2+不断氧化,在-1.3 V出现氧化峰a1。当电位正移到-1.2 V后,电流下降至背景电流。如Co3O4粉末循环伏安曲线所示,随着Co3O4粉末电位的负移,由于Co3O4不断还原为Co单质,在-0.4 V出现还原峰c1。对于Co3O4和SnO2混合粉末的循环伏安曲线,从0 V开始负向扫描,开始阶段电流逐渐增大,曲线变化与Co3O4粉末的曲线基本一致,表明此阶段只有Co单质还原。随着电位负移至-0.2 V,较之Co3O4粉末,混合氧化物粉末电极的还原电流明显增大,表明此时Sn在新生成的Co单质相上还原,形成Co-Sn合金,在-0.6 V附近出现还原峰c4。结合电解不同时间的试样的XRD图谱(见图6),CoSn合金的制备过程按如下过程进行:
Co3O4+e→CoO+O2- (1)
CoO+e→Co+O2- (2)
SnO2+Ca2++O2-→CaSnO3 (3)
CaSnO3+Co+4Ca2++e→Co3Sn2+CaO (4)
Co3Sn2+CaSnO3+Ca2++e→CoSn+CaO (5)

图7 不同试样的循环伏安曲线
Fig.7 CVs of different samples (CaCl2 molten; 850 ℃; Scan rate 10 mV/s)
3 结论
1) 采用熔盐电脱氧法,在850 ℃的CaCl2 熔盐体系中,当电解电压为2.1 V,电解时间为12 h 时,可直接由Co3O4和SnO2混合氧化物制备CoSn合金;经 1 050 ℃和1 250 ℃烧结的试样由于生成的Co2SnO4不利于CoSn的生成,电解产物主要为金属Sn和金属Co。
2) 根据循环伏安曲线和不同电解时间的XRD谱,可以推测电脱氧制备CoSn合金的机理为Co3O4分步还原成单质Co,随后SnO2与CaCl2熔盐反应的产物CaSnO3在单质Co表面还原,最终形成CoSn合金。
REFERENCES
[1] NEEDHAM S A, WANG G X, LIU H K. Electrochemical performance of SnSb and Sn/SnSb nanosize powders as anode materials in Li-ion cells[J]. Journal of Alloys and Compounds, 2005, 400: 234-238.
[2] HASSOUN J, PANERO S, SCROSATI B. Electrodeposited Ni-Sn intermetallic electrodes for advanced lithium ion batteries[J]. Journal of Power Sources, 2006, 160: 1336-1341.
[3] 汪 飞, 赵铭姝, 宋晓平. 锂离子电池锡基负极材料的研究进展[J]. 电池, 2005, 35(2):152–154.
WANG Fei, ZHAO Ming-shu, SONG Xiao-ping. Research progress in tin-based negative electrode materials for Li-ion batteries[J]. Battery Bimonthly, 2005, 35(2):152-154.
[4] WANG G X, AHN J H, LINDSAY M J, SUN L, BRADHURST D H, DOU S X, LIU H K. Graphite-tin composites as anode materials for lithium-ion batteries[J]. Journal of Power Sources, 2001, 97/98: 211-215.
[5] KIM D G, KIM H, SOHN H J, KANG T. Nanosized Sn-Cu-B alloy anode prepared by chemical reduction for secondary lithium batteries[J]. Journal of Power Sources, 2002,104(2): 221-225.
[6] TAMURA N, OHSHITA R, FUJITANI S, KAMINO M, FUJITAN S. Advanced structures in electrodeposited tin base negative electrodes for lithium secondary batteries[J]. Journal of The Electrochemical Society, 2003, 150 (6): A679-A683.
[7] 谭春林, 吕东升, 李伟善, 许梦清, 周代营, 胡社军. 电沉积锡镍合金作为锂离子电池负极的研究[J]. 稀有金属材料与工程, 2008, 37(2): 472-475.
TAN Chun-lin, L? Dong-sheng, LI Wei-shan, XU Meng-qing, ZHOU Dai-ying, HU She-jun. Study of electrodeposited Sn/Ni alloy as anode materials for Li-ion batteries[J]. Rare Metal Materials and Engineering, 2008, 37(2): 472-475.
[8] XIA Y, SAKAI T, FUJIEDA T, WADA M, YOSHINAGA H. Flake Cu-Sn alloys as negative electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 2001, 148(5): A471-A481.
[9] GUO H, ZHAO H L, YIN C L, QIU G H. Si/SnSb alloy composite as high capacity anode materials for Li-ion batteries[J]. Journal of Alloys and Compounds, 2006, 426: 277-280.
[10] CHEN G Z, FRAY D J, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride[J]. Nature, 2000, 407: 361-364.
[11] WANG D H, QIU G H, JIN X B, HU X H, CHEN G Z. Electrochemical metallization of solid terbium oxide[J]. Angew Chem Int Ed, 2006, 45: 2384-2388.
[12] CHEN G Z, ELENA G, FRAY D J. Direct electrolytic preparation of chromium powder[J]. Metallurgical and Materials Transactions B, 2004, 35: 223-233.
[13] 邓丽琴, 许 茜, 李 兵, 翟玉春, 黄振奇. 电脱氧法由Nb2O5直接制备金属铌[J]. 中国有色金属学报, 2005, 15(4): 541-545.
DENG Li-qin, XU Qian, LI Bing, ZHAI Yu-chun, HUANG Zhen-qi. Preparation of niobium by direct electrochemical reduction of solid Nb2O5[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(4): 541-545.
[14] ZHAO B J, WANG L, DAI L, CUI G H, ZHOU H Z, KUMAR R V. Direct electrolytic preparation of cerium/nickel hydrogen storage alloy powder in molten salt[J]. Journal of Alloy and Compounds, 2009, 468: 379-385.
[15] QIU G H, WANG D H, JIN X B, CHEN G Z. A direct electrochemical route from oxide precursors to the terbium–nickel intermetallic compound TbNi5[J]. Electrochimica Acta, 2006, 51: 5785-5793.
[16] QIU G H, WANG D H, MA M, JIN X B, CHEN G Z. Electrolytic-synthesis of TbFe2 from Tb4O7 and Fe2O3 powders in molten CaCl2[J]. Journal of Electroanalytical Chemistry, 2006, 589:139-147.
[17] GORDO E, CHEN G Z, FRAY D J. Toward optimization of electrolytic reduction of solid chromium oxide to chromium powder in molten chloride salts[J]. Electrochimica Acta, 2004,49: 2195-2208.
[18] JIANG K, HU X H, MA M, WANG D H, QIU G H, JIN X B, CHEN G Z. “Perovskitization”-assisted electrochemical reduction of solid TiO2 in molten CaCl2[J]. Angew Chem Int Ed, 2006, 45: 428-432.
基金项目:河北省自然科学基金资助项目(E2007000594,E2010000941);唐山市科技局资助项目(07150204-2)
收稿日期:2009-06-05;修订日期:2010-04-16
通信作者:王 岭,教授,博士;电话:0315-2592170;E-mail:tswling@126.com


