
Solvothermal synthesis of grain size controlled nanocrystalline SnO2
XU Gang(徐 刚), XU Sheng-ming(徐盛明), CHEN Song-zhe(陈崧哲), WU Fang(吴 芳), LI Lin-yan(李林艳)
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
Received 20 April 2006; accepted 30 June 2006
Abstract:
Uniform nanocrystalline SnO2 with different particle sizes was prepared by a solvothermal method using SnCl4×5H2O as the starting material and ethanol as the solvent in the presence of different concentrations of HCl or KOH. The as-prepared powders were characterized by X-ray diffraction(XRD), transmission electron microscopy(TEM), thermo-gravimetry and differential thermal analysis(TG-DTA), and nitrogen BET measurements. With increasing alkalinity of the precursor solution, the crystallite size of the as-prepared SnO2 nanocrystals decreases from 7.1 nm to 3.2 nm, while the specific surface area of the as-prepared powders increases from 101 m2/g to 195 m2/g. When calcined at 500 ℃ for 2 h in air, the crystallite size of the as-calcined powders increases slightly (12-5.9 nm) and the specific surface area of the as-calcined powders is 42-63 m2/g.
Key words:
SnO2; solvothermal synthesis, nanocrystalline particle;
1 Introduction
In recent years, nanocrystalline materials with size-dependent optical, electronic and magnetic properties, due to the reduction of grain size in the nanometer range and/or due to the large fraction of atoms in the grain boundary regions, have attracted increasing interest. Tin dioxide (SnO2) is a wide-bandgap semiconductor (with a room temperature bandgap of about 3.6 eV) and has received intensive interest for its applications in solar cells[1], catalysis[2], and transparent electrodes[3], especially in combustible and toxic gas detection devices (gas sensors)[4]. For most of these applications, using nanocrystalline SnO2 with controlled grain size and surface area is regarded as a promising way to fabricate the catalytic and electronic devices (such as gas sensors) with superior performance.
So far, many techniques including sol-gel[5], anodic oxidation[6], thermal oxidation[7], physical/chemical vapor deposition[8], tin salt decomposition[9], and hydrothermal approaches[10] have been developed to fabricate ultrafine SnO2 powders. However, it is still difficult to control the crystallite size of nanocrystalline SnO2 powders precisely. In this study, we reported a facile solvothermal treatment to prepare nanocrystalline SnO2 with different particle sizes. The morphology, structure, and calcination behavior of the as-prepared SnO2 powders were also investigated.
2 Experimental2.1 Synthesis
All chemicals were analytically pure. For the synthesis of SnO2 nanocrystals, SnCl4×5H2O was used as the starting material to prepare six precursor mixtures in the presence of different concentrations of HCl or KOH in ethanol: 1) 0.10 mol/L HCl (denoted as S1); 2) 0.05 mol/L HCl (S2); 3) 0.05 mol/L KOH (S3); 4) 0.10 mol/L KOH (S4); 5) 0.15 mol/L KOH (S5); and 6) 0.20 mol/L KOH (S6). The above six precursors were transferred into Teflon-lined stainless steel autoclaves, and subjected to solvothermal treatment at 180 ℃ for 24 h. The obtained precipitates were carefully washed with water and ethanol repeatedly, and dried in a vacuum oven at about 60 ℃. The as-prepared powders were then calcined in a muffle furnace at 500-800 ℃ for 2 h.
2.2 Characterization methods
The crystal structures were identified by a powder X-ray diffractometer (XRD, Rigaku D/max-2000, Tokyo,Japan), employing Cu Ka radiation (l=0.154 18 nm). The transmission electron microscopy(TEM) measurements were conducted employing a JEOL 200CX electronic microscope working at 200 kV. The BET specific surface area was measured by nitrogen physisorption at 77.5 K using an ASAP 2010 analyzer (Micromeritics Co Ltd, Norcross, USA). The combined thermo-gravimetry and differential thermal analysis (TG-DTA, SDT2960, Thermal Analysis) were performed at a heating rate of 10 ℃/min from room temperature to 1 000 ℃, using α-Al2O3 as a reference.
3 Results and discussion
Fig.1 shows the typical TEM image of the as-prepared SnO2 powder (sample S4). The primary particles of the as-prepared SnO2 nanocrystallites are highly uniform and well-dispersed, showing no definite and tight agglomeration with a crystallite size of less than 5 nm. The selected area electron diffraction(SAED) pattern, shown in the inset of Fig.1, confirms the formation of tetragonal SnO2 nanocrystals for the as-prepared powders.
Fig.2 depicts the typical combined TG-DTA curves
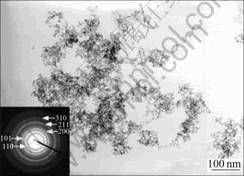
Fig.1 Typical TEM image of as-prepared SnO2 powder (S4) and corresponding SAED pattern
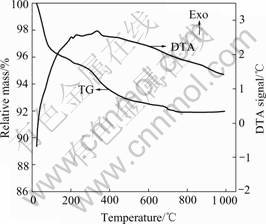
Fig.2 Typical combined TG-DTA curves of as-prepared SnO2 powders
of the as-prepared SnO2 powder (sample S4), indicating a two-step mass loss process on the TG curve. The first mass loss beginning from 25 to 200 ℃ can be attributed to the liberation of physically adsorbed water and ethanol molecules, and the second one between 200 and 400 ℃ can be ascribed to the elimination of residuals through oxidation-reduction reaction. However, no obvious exothermic (exo) peak for the transition from amorphous to crystalline at 400-500 ℃ is detected[11], indicating that the hydrolytic products have already been crystallized well.
Fig.3 shows the XRD patterns of the as-prepared SnO2 powders. It is observed from Fig.3 that the main diffraction peaks are broadened, indicating the formation of nanocrystals under solvothermal treatment at 180 ℃, and can be indexed as tetragonal SnO2 (JCPDS 41-1445, P42/mnm). When increasing alkalinity of the precursor solution from 0.1 mol/L HCl (S1) to 0.2 mol/L KOH (S6), the intensity of the diffraction peaks become weaker and more broadened, suggesting smaller crystals formed under alkaline condition. When being calcined at 500 ℃ for 2 h in air, as noted from Fig.4, the intensities of the diffraction peaks of rutile SnO2 happen to be stronger and more sharpened, indicating that the as-prepared nanocrystals become well-crystallized at that calcination temperature.
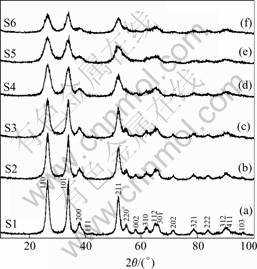
Fig.3 XRD patterns of as-prepared SnO2 powders: (a) 0.10 mol/L HCl; (b) 0.05 mol/L HCl; (c) 0.05 mol/L KOH; (d) 0.10 mol/L KOH; (e) 0.15 mol/L KOH; (f) 0.20 mol/L KOH
The average grain size D of both the as-prepared and the as-calcined SnO2 powder was estimated according to the Scherrer equation[12]:
![]() (1)
(1)
where q is the diffraction angle of the (110) peak of
Table 1 Grain size D and BET specific surface area SBET of SnO2 powders
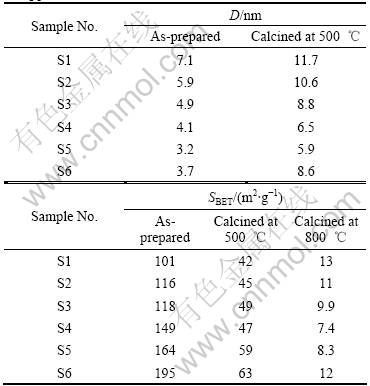
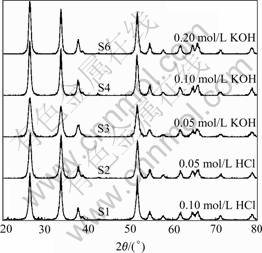
Fig.4 XRD patterns of SnO2 powders calcined at 500 ℃ for 2 h
the tetragonal phase, b is the full width at half maximum (FWHM) of the (110) peak (in radian), which is calibrated from high purity silicon. All results are collected in Table 1, and the dependence of crystallite size on the acidity and alkalinity is elucidated. It is noted that the mean grain size reduces gradually with the increase of the alkalinity. The grain size of the as-prepared samples in the presence of HCl media is relatively larger, and it is 7.1 nm and 5.9 nm for samples S1 and S2, respectively. When using KOH media, the grain size of the as-prepared samples is much smaller, from 4.9 nm for S3 to 3.7 nm for S6. After calcining at 500 ℃ for 2 h, the grain size increases slightly from 11.7 nm for S1 to 8.6 nm for S6.
The BET specific surface area, SBET, of the nano- crystalline SnO2 powders is also summarized in Table 1. The variation sequence of the SBET is opposite to that of the grain size, i.e. the SBET of the as-prepared samples increases monotonously from 101 m2/g for S1 to 195 m2/g for S6. This is in accordance with the fact that smaller particle can provide larger specific surface area. When calcined at 500 ℃ for 2 h, the as-calcined samples still remain high specific surface area from 42 m2/g for S1 to 63 m2/g for S6. When calcined at 800 ℃ for 2 h, the SBET of the as-calcined samples inclines to be the same, which is 7-13 m2/g. Thus, the particle size and the specific surface area can be controlled by simply modifying the acidity/alkalinity of the precursor solution, and the study on the essential of this influential factor is in progress.
4 Summary
The grain size controlled nanocrystalline SnO2 powders were successfully prepared by a facile solvothermal synthetic approach. The concentration of the acid or alkali has a direct influence on the grain size of the nanocrystalline powders, i.e. with decreasing the acidity and increasing the alkalinity of the precursors, the grain size and the specific surface area of the nanocrystals become smaller and larger, respectively. The well- dispersed, uniform, and well-crystallized powders are favorable for gas-sensing and catalysis applications. The as-developed solvothermal crystalliz- ing process can be extended to prepare other oxide nanoparticles.
References
[1] PARK N G, KANG M G, KIM K M, RYU K S, CHANG S H, KIM D K, VAN DE LAGEMAAT J, BENKSTEIN K D, FRANK A J. Morphological and photoelectrochemical characterization of core-shell nanoparticle films for dye-sensitized solar cells: Zn-O type shell on SnO2 and TiO2 cores [J]. Langmuir, 2004, 20(10): 4246- 4253.
[2] HARRISON P G, BAILEY C, AZELEE W. Modified tin (IV) oxide (M/SnO2, M=Cr, La, Pr, Nd, Sm, Gd) catalysts for the oxidation of carbon monoxide and propane [J]. Journal of Catalysis, 1999, 186(1): 147-159.
[3] VOLKOV A G, MARKIN V S, LEBLANC R M, GUGESHASHVILI M I, ZELENT B, MUNGER G. Light energy-conversion with pheophytin—A monolayer at the SnO2 optically transparent electrode [J]. Journal of Solution Chemistry, 1994, 23(2): 223-248.
[4] XU G, ZHANG Y W, SUN X, XU C L, YAN C H. Synthesis, structure, texture, and CO sensing behavior of nanocrystalline tin oxide doped with scandia [J]. Journal of Physical Chemistry B, 2005, 109(8): 3269-3278.
[5] ZHANG J R, GAO L. Synthesis and characterization of nanocrystalline tin oxide by sol-gel method [J]. Journal of Solid State Chemistry, 2004, 177(4-5): 1425-1430.
[6] SHIN H C, DONG J, LIU M L. Porous tin oxides prepared using an anodic oxidation process [J]. Advanced Materials, 2004, 16(3): 237.
[7] NAYRAL C, VIALA E, FAU P, SENOCQ F, JUMAS J C, MAISONNAT A, CHAUDRET B. Synthesis of tin and tin oxide nanoparticles of low size dispersity for application in gas sensing [J]. Chemistry-A European Journal, 2000, 6(22): 4082-4090.
[8] DENG H M, LAMELAS F J, HOSSENLOPP J A. Synthesis of tin oxide nanocrystalline phases via use of tin (II) halide precursors [J]. Chemistry of Materials, 2003, 15(12): 2429-2436.
[9] YU K N, XIONG Y H, LIU Y L, XIONG C S. Microstructural change of nano-SnO2 grain assemblages with the annealing temperature [J]. Physical Review B, 1997, 55(4): 2666-2671.
[10] HE Y P, LI Y D, YU J, QIAN Y T. Chemical control synthesis of nanocrystalline SnO2 by hydrothermal reaction [J]. Materials Letters, 1999, 40(1): 23-26.
[11] EPIFANI M, ALVISI M, MIRENGHI L, LEO G, SICILIANO P, VASANELLI L. Sol-gel processing and characterization of pure and metal-doped SnO2 thin films [J]. Journal of the American Ceramic Society, 2001, 84(1): 48-54.
[12] GUINIER A. Theorie et Technique de la Radiocristallographie [M]. 3rd Ed. Paris: Dunod Editeur, 1964.
Foundation item: Project(50274045) supported by the National Natural Science Foundation of China; Project supported by Hubei Key Laboratory of Novel Reactor and Green Chemical Technology(NRGCT)
Corresponding author: XU Sheng-ming; Tel: +86-10-89796082; Fax: +86-10-62773585; E-mail: smxu@tsinghua.edu.cn


