Trans. Nonferrous Met. Soc. China 26(2016) 1878-1884
Microstructure and evolution of (TiB2+Al2O3)/NiAl composites prepared by self-propagation high-temperature synthesis
Xiao-jie SONG1, Hong-zhi CUI1, Li-li CAO1, P. Y. GULYAEV2
1. School of Materials Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, China;
2. Polytechnic Institute, Yugra State University, Rhanty-Mansiysk 628012, Russian Federation
Received 5 June 2015; accepted 3 March 2016
Abstract:
(TiB2+Al2O3)/NiAl composites were synthesized by self-propagation high-temperature synthesis, and their phase compositions, microstructures and evolution modes were studied. The microstructures and shapes vary with the TiB2+Al2O3 content in the NiAl matrix. TiB2 particles take a great variety of elementary shapes such as white bars, plates, herringbones, regular cubes and cuboids. These results outline a strategy of self-assembly processes in real time to build diversified microstructures. Some TiB2 grains in sizes of 2-5 μm are embeded in Al2O3 clusters, while a small number of TiB2 particles disperse in the NiAl matrix. It is believed that the higher the TiB2+Al2O3 content is, the more the regular shapes and homogeneous distributions of TiB2 and Al2O3 will be present in the NiAl matrix.
Key words:
(TiB2+Al2O3)/NiAl composites; self-propagation high-temperature synthesis; microstructure; evolution mechanism;
1 Introduction
Due to its low density (5.59 g/cm3), high melting point (1911 K), good thermal conductivity and good corrosion resistance [1], the NiAl intermetallic compounds have a wide application for components in high-temperature and corrosive environments, such as aero-engines, blades of gas turbine, thermal barrier coatings, and corrosion-resisting coatings. However, the low plasticity at room temperature and low strength at high temperature limit their applications in industry and production [2,3].
Up to now, many publications have involved in improving the room temperature plasticity and high temperature strength of Ni-Al intermetallic compounds. Adding different alloy elements such as Mo [4], Ti [5], Cr [6], and rare-earth metals [7] in NiAl is an efficient way to improve its properties. By adding or forming ceramics particles, such as Al2O3 [8,9], TiB2 [10], NbB2 [11] and TiC [12,13], the strength and creep properties of Ni-Al composite material can also be greatly improved. MICHALSKI et al [14] fabricated a NiAl-Al2O3 composite with different amounts of Al2O3 in NiAl through spark plasma sintering and self- propagation high-temperature synthesis (SHS) of Ni and Al, and the results showed that the hardness of NiAl-Al2O3 increased and the fracture toughness of it was almost twice that of NiAl. The fabrication of NiAl matrix composites was carried out by a variety of novel processes such as combustion synthesis [11,14], mechanical alloying [13] and high pressure reaction sintering. It has been proved that combustion synthesis has significant advantages, such as low energy consumption, inexpensive fabrication equipment, simplicity of operation and in situ synthesis of composite components, compared with other conventional methods. Via combustion synthesis procedure, a series of NiAl matrix composites reinforced by TiB2, NbB2, TiC, Al2O3, ZrB2 [15] and TiN [16] were obtained conveniently.
At present, many researches focus on TiB2 and Al2O3 ceramics as a duplex reinforced phase in different matrices because of their similar thermal expansion coefficient, chemical and physical compatibility [17]. By adding TiB2 into Al2O3 ceramics, the growth of Al2O3 grains and the propagation of cracks in the matrix can be prevented. Consequently, the fine and well-distributed TiB2 phase would help to enhance the strength and fracture toughness of the composite material. Thus, it would contribute to improving the abrasion resistance and fracture toughness in cutting tool materials [18,19].
The study on the previous work indicates that the fabrication of TiB2+Al2O3/NiAl composite materials through combustion synthesis has not been fully investigated. The fundamental objective of the present study is to investigate the microstructures and their evolution modes when different TiB2+Al2O3 contents were formed in the NiAl matrix, using Ni, Al, B2O3 and TiO2 as raw materials, via combustion synthesis. Also, the correlation between the TiB2+Al2O3 content, microstructure and properties of the composites was investigated.
2 Experimental
The raw materials are commercial pure Ni powder (99.9%, 38-50 μm), Al powder (99.9%, 75 μm), B2O3 powder (98%, 75 μm), and TiO2 powder (98%, 45 μm). The powder mixtures were considered as two parts: one was Ni+Al in which the mole ratio was fixed to n(Ni):n(Al)=1:1, the other was Al+TiO2+B2O3 in which the mole ratio was fixed to n(Al):n(TiO2):n(B2O3)= 10:3:3, and the latter was added into the former in mass fractions of 0, 5%, 10%, 15%, 20%, 25% and 30%, respectively. In order to produce a homogeneous mixture, all the powders were mixed in a three– dimension blender for about 6 h. Then, the mixtures were poured into metal molds with dimensions of d20 mm × 20 mm and pressed at a pressure of 150 MPa into cylindrical compacts. Seven typical green compacts were fabricated with different contents of Al+TiO2+B2O3. In order to make the reactants react completely, the green compacts were preheated in an electric furnace at 300 °C for 30 min. After that, the compacts together with the molds were taken out and a small amount of Mg powders was poured onto the surface as the initiating combustion agent. At last the compacts were ignited by live tungsten wire immediately. The reactions between powders in raw materials were as follows:
Ni+Al=NiAl (1)
10Al+3TiO2+3B2O3=3TiB2+5Al2O3 (2)
Under the present condition, the reaction in green compact could self-sustain due to its high exothermic heat (SHS), the process is shown in Fig. 1.
The crystalline phases of the products were characterized by X-ray diffraction (XRD, Model D/Max 2500PC Rigaku, Japan). The morphologies of the fracture and microstructure were observed by scanning electron microscopy (SEM, Model KYKY2800B) and field emission scanning electron microscopy (FESEM, NOVA NANOSEM 450, FEI). The compositions of samples were analyzed using electron probe microanalysis (EPMA, JXA-8230). The microhardness was measured by FM-700 with the load of 1 N and sustaining for 10 s. For each compound, five indentations were taken in the microhardness test and made an average of these points with a consideration of relative errors.
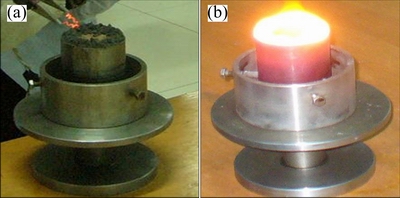
Fig. 1 Schematic diagrams of SHS reaction initiated by electrifying tungsten wire (a) and SHS reaction (b)
3 Results
In the sample without Al+TiO2+B2O3, the Ni+Al reaction wave propagated steadily, and the speed was about 25 mm/s. When adding Al+TiO2+B2O3 into Ni+Al, the igniting delay time prolonged. Once the reaction was initiated, the reaction wave spread rapidly. When the content of Al+TiO2+B2O3 increased to 15%, the spreading speed reached up to 40 mm/s. Thus, the whole reaction in the compacts almost took place at the same time, similar to thermal explosion. After the reactions finishing, the samples were slowly cooled down.
3.1 Phase analysis
Figure 2 shows the XRD pattern of the raw materials. It illustrates that the powder mixture is composed of Ni, Al, TiO2 and B2O3. Figure 3 shows the XRD patterns of products obtained by adding different contents of Al+TiO2+B2O3.
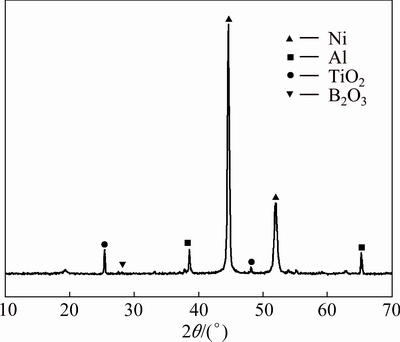
Fig. 2 XRD pattern of reactants
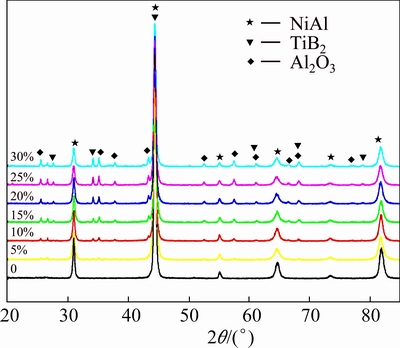
Fig. 3 XRD patterns of NiAl products with different contents of Al+TiO2+B2O3
The reaction products simply consist of NiAl when the mixture contains only Ni and Al, which is in accordance with Reaction (1). After adding Al+TiO2+ B2O3, TiB2 and Al2O3 ceramic phases appear besides NiAl phase. The higher the reactant content of Al+TiO2+B2O3 is, the more the TiB2 and Al2O3 ceramic phases can be generated. Moreover, there are hardly any unreacted raw materials. Thus, it can be deduced that the reactions were completely carried out, and the phases which are expected according to Reaction (2) are obtained.
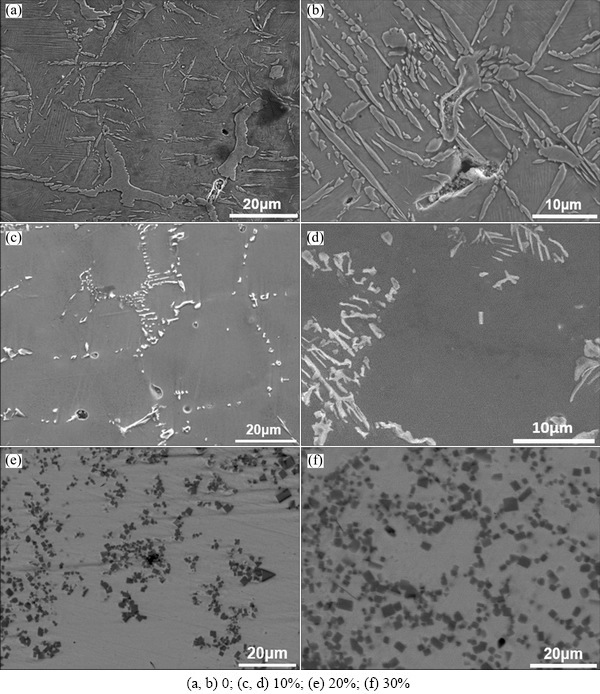
Fig. 4 SEM images of NiAl products with different contents of Al+TiO2+B2O3
3.2 Microstructure and hardness
The microstructures on polished surface of samples with different contents of ceramics are shown in Fig. 4. In the samples only containing Ni+Al, the NiAl grains are coarse with a lot of needles and parallel plates, as shown in Figs. 4(a) and (b), and this is in accordance with Ref. [20]. After adding Al+TiO2+B2O3, some white bars and plates (~5 μm) distributed along the boundary of the matrix appear, and the grains of NiAl matrix are refined remarkably, as shown in Figs. 4(c) and (d). Furthermore, some plates (10-15 μm) are alternatively distributed with a kind of grey phase, and their distributional patterns are very similar to herringbone structure. When the content of Al+TiO2+B2O3 increases to 20% , the white phase turns to be fine regular particles (smaller than 5 μm), mainly in the shape of cubes or cuboids gathering together as clusters on an irregular dark grey conglomerations, as shown in Fig. 4(e). Also the aggregation (60-70 μm) of white particle clusters on the irregular conglomerations and some white particles still distribute along the grains boundary of the matrix. The white particles seem to be inlaid in the dark grey conglomerations. With further increasing the content of Al+TiO2+B2O3, the amount of white particles increases significantly. But the size of aggregations of white particles, becomes smaller. In addition, some white particles disappear in the matrix, as shown in Fig. 4(f).
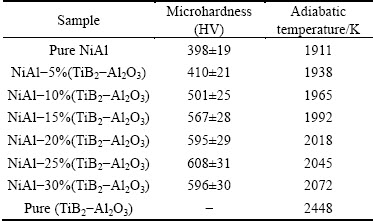
The microhardness test results are listed in Table 1. The average microhardnesses of the matrix in NiAl products without and with 10% (Al+TiO2+B2O3) are HV100 398 and HV100 501, respectively, which are close to the hardness of pure NiAl [20]. The microhardness of herringbone structure in the product with 10% (Al+ TiO2+B2O3) is HV100 (1160-1730), which fluctuates remarkably. Compared with the product with 10% (Al+TiO2+B2O3), the hardness of white cube or cuboids gathering clusters in the products with 20% and 30% (Al+TiO2+B2O3) is higher, and the highest hardness is up to HV100 2700. The hardness of dark grey irregular conglomerations which look like being pinned under the cube or cuboid particles is HV100 (1260-1480). The hardness of the products with 20% and 30% (Al+TiO2+ B2O3) also varies from HV100 570 to HV100 1420, and the highest hardness is close to that of dark grey conglomerations.
Table 1 Microhardness and adiabatic temperature of samples with different contents of Al+TiO2+B2O3
3.3 Composition
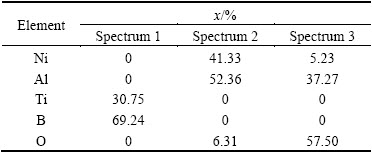
The SEM images of typical samples and the results of EDS composition analysis are shown in Fig. 5 and Tables 2 and 3, respectively. Combining the results of XRD analysis, microhardness test and EDS composition analysis, we can deduce that the white bars and plates which distribute along the boundary of the matrix or in herringbone structure at Spectrum 1 in Fig. 5(a) and the white particles in regular cube or cuboids shapes shown in Figs. 4(e) and (f) and Spectrum 3 in Fig. 5(b) are all TiB2 grains. The gray matrix shown in Figs. 4(a) and (c) and Spectrum 2 in Figs. 5(a) and (b) is NiAl intermetallic compound. The irregular dark grey clusters which are around or under the cube or cuboids particles in Figs. 4(e) and (f) and Spectrum 3 in Figs. 5(a) and (b) are Al2O3.

Fig. 5 SEM images of products with different contents of Al+TiO2+B2O3
Table 2 EDS compositions of composite with 5% (Al+ TiO2+B2O3)
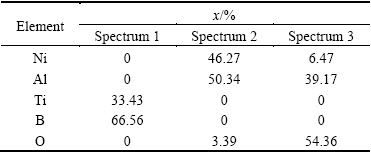
Table 3 EDS compositions of composite with 15% (Al+ TiO2+B2O3)
4 Discussion
The mixture of reactants could be considered as two parts: one is Ni+Al which follows Reaction (1), the other is Al+TiO2+B2O3 which follows Reaction (2). If the reaction systems are initiated at room temperature, the adiabatic temperature of Reaction (1) would be Tad=Tm,NiAl=1911 K [21], while the adiabatic temperature of Reaction (2) would be Tad=2448 K [22]. When preheating the samples at 300 °C, the adiabatic temperatures of Reactions (1) and (2) at 300 °C are much higher than their Tad at room temperature. The Tad of Ni+Al+TiO2+B2O3 hybrid system can be calculated according to the thermodynamic data provided in Ref. [23]. With the increase of Al+TiO2+B2O3 content, the Tad of the mixtures rises to above 1911 K. The adiabatic temperatures of samples with different mass fractions of Al+TiO2+B2O3 are listed in Table 1.
The reaction processes and microstructure evolution mechanisms of the system are shown in Fig. 6. As the melting points of Al and B2O3 are 660 and 445 °C, respectively, Al and B2O3 in the green powder (Fig. 6(a)) melt firstly during the reaction proces. The melted Al contacts with liquid B2O3 and infiltrates into the surface of TiO2 particles, as shown in Fig. 6(b). Thus, B and Ti elements are displaced from B2O3 and TiO2, respectively [24,25]. At the same time, Al2O3 and TiB2 are produced rapidly through Reaction (2). Moreover, the surplus molten Al and solid Ni react to from liquid NiAl, as the melting point of NiAl is lower than the adiabatic temperature of reaction system, as shown in Fig. 6(c). The sequences of the whole reaction are as follows:
Ni+Al→NiAl (3)
2Al+B2O3→Al2O3+2B (4)
4Al+3TiO2→2Al2O3+3Ti (5)
Ti+2B→TiB2 (6)
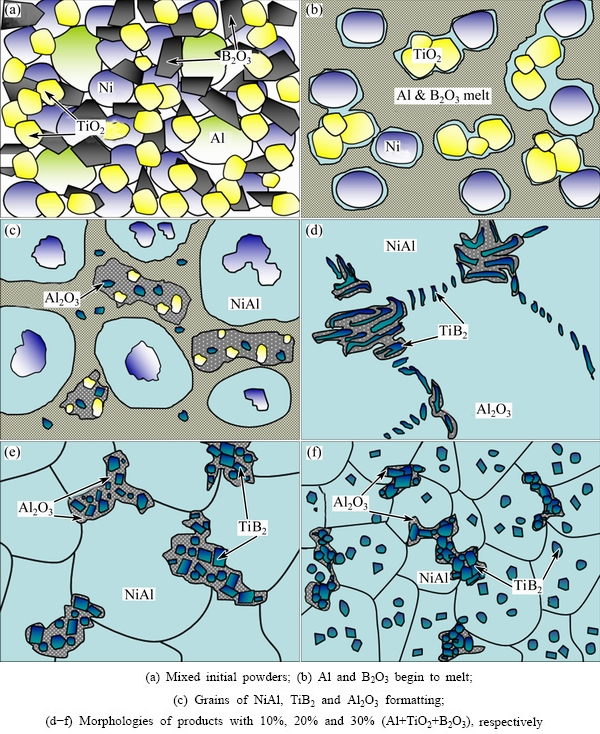
Fig. 6 Sketch of reaction processes and structure evolution modes
When the content of Al+TiO2+B2O3 is less than 10%, both the amounts of TiB2 and Al2O3, and the temperature of the reaction system are not sufficient. Therefore, the TiB2 grains grow as irregular bars and plates [26,27] (Figs. 4(d) and 6(d)). TiB2 and Al2O3 grow into herringbone alternative structure through “self- assembly” mode. When the reaction system cools down below the NiAl meting point, NiAl grains begin to nuclear and grow to form the matrix of composite gradually.
With increasing the content of Al+TiO2+B2O3, the Tad of reaction system increases and a great amount of TiB2 and Al2O3 is produced. The abundant TiB2 resources and higher temperature are better for the regularization of TiB2 [26]. Thus, TiB2 particles grow freely, develop fully and take shapes of cube or cuboids finally. As Al2O3 grains form around the TiB2 grains almost simultaneously, it is possible for them to attach together to form large irregular conglomerations, as shown in Figs. 4(e) and (f) and 6(e) and (f). During the grow process of NiAl grains, the conglomerations are pushed to the corner of grains or distribute along the boundaries. Also, this kind of distribution impedes the boundary’s movement and restricts the grain’s growth of NiAl matrix.
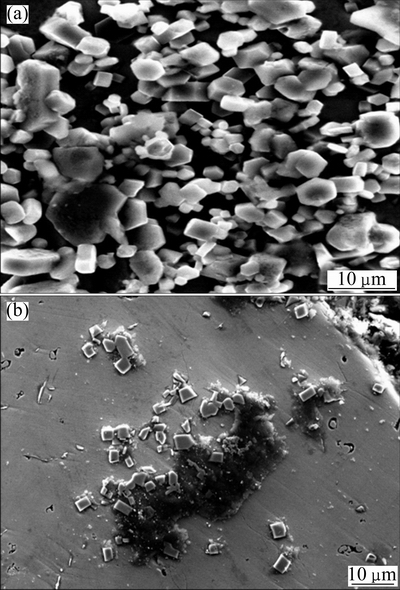
Fig. 7 SEM images of regular TiB2 particles (a) and Al2O3 conglomerations (b)
When the Al+TiO2+B2O3 content increases to 30%, much higher Tad can be reached and more TiB2 and Al2O3 are produced. All the above contribute a lot to TiB2 grains to grow as regular cubes cuboids or hexagons, as shown in Figs. 4(e) and (f) and 7(a). In addition, a large amount of Al2O3 adheres to TiB2 grains to nuclear, as shown in Fig. 7(b), and grows around them. Because there are no plates to subdivide the spaces between TiB2 grains, as shown in Fig. 4(b), the Al2O3 grains develop more freely until touching each other to form conglomerations, as shown in Figs. 4(c) and (d). In the meantime, there are also some single TiB2 cubes or cuboids dispersed in the NiAl liquid and some Al2O3 grains adhere to the impurities in NiAl liquid to nuclear. Finally, when NiAl begins to solidify, almost all the above solid phases that form before NiAl will be pushed to the grain boundaries in the similar way shown in Figs. 4(b) and 6(e), which makes contribution to the formation of refine NiAl grains. What is more, the Al2O3 grains which nucleate on the impurities may remain in the NiAl matrix to help to improve the hardness. These Al2O3 grains may flock together and become big conglomerations because of the self-diffusion of Al2O3. Therefore, the hardness of matrix in Fig. 4(c) varies greatly, and the highest hardness is close to that of pure conglomerations.
5 Conclusions
1) (TiB2+Al2O3)/NiAl composites were synthesized by combustion synthesis using Ni, Al, TiO2 and B2O3 mixed powders. With increasing the content of Al+TiO2+B2O3, more TiB2 and Al2O3 ceramic phases are generated in the NiAl matrix. When the reactant content of Al+TiO2+B2O3 is less than 10%, TiB2 and Al2O3 grow into herringbone alternative structure through self-assembly mode.
2) When more TiB2+Al2O3 form, TiB2 particles would grow freely and develop fully and take shapes of cube, cuboids or hexagons finally. At the same time, more Al2O3 which forms around TiB2 grains tends to attach together to form large irregular conglomerations.
3) The microhardness of NiAl matrix composite increases by 50% from HV (398±19) to HV (608±31), due to grain refining with increasing the content of TiB2+Al2O3 to 25%.
References
[1] OZDEMIR O, ZEYTIN S, BINDAL S. A study on NiAl produced by pressure-assisted combustion synthesis [J]. Vacuum, 2010, 84: 430-437.
[2] FANG H C, XIAO P, XIONG X, YU G J. Microstructures, mechanical and oxidation behaviors of C/C composites modified by NiAl alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 196-202.
[3] ADABI M, AMADEH A A. Formation mechanisms of Ni-Al intermetallics during heat treatment of Ni coating on 6061 Al substrate [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 3959-3966.
[4] RAY P K, AKINC M, KRAMER M J. Formation of multilayered scale during the oxidation of NiAl-Mo alloy [J]. Applied Surface Science, 2014, 301: 107-111.
[5] RIYADI T W B, ZHANG T, MARCHANT D, ZHU X M. Synthesis and fabrication of NiAl coatings with Ti under layer using induction heating [J]. Surface and Coatings Technology, 2014, 258(6): 154-159.
[6] TANAK Y, OIKAW K, SUTOU Y, OMORI T, KAINUMA R, ISHIDA K. Martensitic transition and super elasticity of Co-Ni-Al ferromagnetic shape memory alloys with two-phase structure [J]. Materials Science and Engineering A, 2006, 438: 1054-1060.
[7] GUO J T, YUAN C, HOU J S. Effects of rare earth elements on NiAl-based alloys [J]. Acta Metallurgica Sinica, 2008, 44(5): 513-520. (in Chinese)
[8] SHENG L Y, YANG F, XI T F, GUO J T, YE H Q. Microstructure evolution and mechanical properties of Ni3Al/Al2O3 composite during self-propagation high-temperature synthesis and hot extrusion [J]. Materials Science and Engineering A, 2012, 555(41): 131-138.
[9] YANG W, DONG S J, LUO P, YANGLI A Z, LIU Q, XIE Z X. Effect of Ni addition on the preparation of Al2O3–TiB2 composites using high-energy ball milling [J]. Journal of Asian Ceramic Societies, 2004, 2(4): 399-402.
[10] CUI H Z, MA L, CAO L L, TENG F L, CUI N. Effect of NiAl content on phases and microstructures of TiC-TiB2-NiAl composites fabricated by reaction synthesis [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 346-353.
[11] SHOKATI A A, PARVIN N, SABZIANPOUR N, SHOKATI M, HEMMATI A. In situ synthesis of NiAl-NbB2 composite powder through combustion synthesis [J]. Journal of Alloys and Compounds, 2013, 549(5): 141-146.
[12] SHENG L Y, YANG F, GUO J T, XI T F, YE H Q. Investigation on NiAl-TiC-Al2O3 composite prepared by self-propagation high temperature synthesis with hot extrusion[J]. Composites Part B: Engineering, 2013, 45(1): 785-791.
[13] MOHAMMAD M, SHAMANIAN M, ENAYATI M H, SALEHI M, HOSEYNIAN A. Microstructures and properties of NiAl-TiC nanocomposite coatings on carbon steel surfaces produced by mechanical alloying technique [J]. Surface and Coatings Technology, 2014, 238: 180-187.
[14] MICHALSKI A, JAROSZEWICZ J,  M, SIEMIASZKO D. NiAl-Al2O3 composites produced by pulse plasma sintering with the participation of the SHS reaction [J]. Intermetallics, 2006, 14(6): 603-606.
M, SIEMIASZKO D. NiAl-Al2O3 composites produced by pulse plasma sintering with the participation of the SHS reaction [J]. Intermetallics, 2006, 14(6): 603-606.
[15] CAMURLU H E, MAGLIA F. Self-propagating high-temperature synthesis of ZrB2 or TiB2 reinforced Ni-Al composite powder [J]. Journal of Alloys and Compounds, 2009, 478(1): 721-725.
[16] SHOKATI A A, PARVIN N, SHOKATI M. Combustion synthesis of NiAl matrix composite powder reinforced by TiB2 and TiN particulates from Ni-Al-Ti-BN reaction system[J]. Journal of Alloys and Compounds, 2014, 585(3): 637-643.
[17] LIU J, OWNBY P D. Enhanced mechanical properties of alumina by dispersed titanium diboride particulate inclusions [J]. Journal of the American Ceramic Society, 1991, 74(1): 241-243.
[18] GU M L, HUANG C Z, XIAO S R, LIU H L. Improvements in mechanical properties of TiB2 ceramics tool materials by the dispersion of Al2O3 particles [J]. Materials Science and Engineering A, 2008, 486(1-2): 167-170.
[19] DENG J X, CAO T K, LIU L L. Self-lubricating behaviors of Al2O3/TiB2 ceramic tools in dry high-speed machining of hardened steel [J]. Journal of the American Ceramic Society, 2005, 25(7): 1073-1079.
[20] CUI H Z, WEI N, ZENG L L, WANG X B, TANG H J. Microstructure and formation mechanism of Ni-Al intermetallic compounds fabricated by reaction synthesis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1639-1645.
[21] ZHU P, LI J C M, LIU C T Adiabatic temperature of combustion synthesis of Al-Ni systems [J]. Materials Science and Engineering A, 2003, 357(357): 248-257.
[22] MEYERS M A, OLEVSKY E A, MA J, JAMET M. Combustion synthesis/densification of an Al2O3-TiB2 composite [J]. Materials Science and Engineering A, 2001, 311(1-2): 83-99.
[23] MA Z, JI X W, LIN P, DONG S Z, LI Z C. Thermo dynamics and kinetics analysis of composite ceramic coatings Al-TiO2-B2O3 system [J]. Bulletin of the Chinese Ceramic Society, 2010, 29(3): 582-587. (in Chinese)
[24] ZHU H G, WANG H Z, GE L Q, CHEN S, WU S Q. Formation of composites fabricated by exothermic dispersion reaction in A1-TiO2-B2O3 system [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(3): 590-594.
[25] SHARIFI E M, KARIMZADEH F, ENAYATI M H. Synthesis of titanium diboride reinforced alumina matrix nanocomposite by mechanochemical reaction of Al-TiO2-B2O3 [J]. Journal of Alloys and Compounds, 2010, 502(2): 508-512.
[26] LI P T, WU Y Y, LIU X F. Controlled synthesis of different morphologies of TiB2 microcrystals by aluminum melt reaction method [J]. Materials Research Bulletin, 2013, 48(6): 2044-2048.
[27] CHENG E J, KATSUI H, TU R, GOTO T. Rod-like eutectic structure of arc-melted TiB2–TiCxN1-x composite [J]. Journal of the European Ceramic Society, 2014, 34(9): 2089-2094.
自蔓延高温合成(TiB2+Al2O3)/NiAl复合材料的显微组织及演化机制
宋晓杰1,崔洪芝1,曹丽丽1,P. Y. GULYAEV2
1. 山东科技大学 材料科学与工程学院,青岛 266590;
2. Polytechnic Institute, Yugra State University, Rhanty-Mansiysk 628012, Russian Federation
摘 要:采用自蔓延高温合成技术制备(TiB2+Al2O3)增强NiAl基复合材料,并研究其相组成、显微组织和演化机制。结果表明:复合材料的组织及陶瓷相的形貌随添加(TiB2+Al2O3)含量的不同而变化,TiB2在短时间内以自组装的模式形成长条状、片状、鱼骨状以及规则的四方体和六棱柱等不同形态。TiB2的尺寸在2~5 μm之间,与不规则的Al2O3伴生存在,少量TiB2分布于NiAl基体中。随着陶瓷相含量的增多,TiB2的形状趋于规则,且在NiAl基体中的分布更加均匀。
关键词:(TiB2+Al2O3)/NiAl复合材料;自蔓延高温合成;显微组织;演化机制
(Edited by Mu-lan QIN)
Foundation item: Project (51272141) supported by the National Natural Science Foundation of China; Project (ts20110828) supported by the Taishan Scholars Project of Shandong Province, China; Project (2015AA034404) supported by the Ministry of Science and Technology of China
Corresponding author: Hong-zhi CUI; Tel: +86-532-86057929; E-mail: hzhcui@163.com
DOI: 10.1016/S1003-6326(16)64265-6
Abstract: (TiB2+Al2O3)/NiAl composites were synthesized by self-propagation high-temperature synthesis, and their phase compositions, microstructures and evolution modes were studied. The microstructures and shapes vary with the TiB2+Al2O3 content in the NiAl matrix. TiB2 particles take a great variety of elementary shapes such as white bars, plates, herringbones, regular cubes and cuboids. These results outline a strategy of self-assembly processes in real time to build diversified microstructures. Some TiB2 grains in sizes of 2-5 μm are embeded in Al2O3 clusters, while a small number of TiB2 particles disperse in the NiAl matrix. It is believed that the higher the TiB2+Al2O3 content is, the more the regular shapes and homogeneous distributions of TiB2 and Al2O3 will be present in the NiAl matrix.


