DOI:10.19476/j.ysxb.1004.0609.2017.04.009
锂离子电池正极材料LiNi0.5Co0.2Mn0.3O2的热电化学
肖忠良,周清清,宋刘斌,胡超明,卢意鹏,曹 忠
(长沙理工大学 化学与生物工程学院 电力与交通材料保护湖南省重点实验室,长沙 410004)
摘 要:
采用电化学-量热法研究以LiNi0.5Co0.2Mn0.3O2为正极材料的锂离子电池在不同环境温度和充放电倍率下的热电化学性能。结果表明:环境温度和充放电倍率是影响电池比容量的重要因素,随着充放电倍率和环境温度的增加,电池比容量逐渐减小。在低倍率(0.2C)下,电池充放电初始阶段的热流缓慢增大,且出现多个放热峰;而在较高倍率(0.5、1.0、2.0C)下,电池充放电初始阶段的热流快速增长,且充电和放电过程分别仅出现一个明显的放热峰。通过热电化学研究,可获得电池充放电过程的产热量、化学反应焓变(ΔrHm)以及化学反应熵变(ΔrSm)等热力学参数。
关键词:
LiNi0.5Co0.2Mn0.3O2;电化学-量热法;热电化学;热力学参数;锂离子电池;
文章编号:1004-0609(2017)-04-0739-08 中图分类号:TM911 文献标志码:A
近年来,锂离子电池在军用及航空航天领域的应用逐渐增加,并逐步走向储能、电动汽车等领域,这对锂离子电池的发展提出了更高的要求[1-3]。动力型锂离子电池要求高比容量和高能量密度,镍钴锰氧化物复合材料(如LiNi0.5Co0.2Mn0.3O2、LiNi0.8Co0.1Mn0.1O2)因放电比容量高达200 mA·h/g,成为很有发展潜力的锂离子电池正极材料之一[4-8]。然而,电池在造福人类的同时,其安全性问题也逐渐暴露。电池在充放电过程中,进行着复杂的化学反应,这些反应都伴随着大量热量的释放,尤其在高倍率、高温工作环境中更明显。如果这些热不能及时散发,将可能引起电池热失控[9-12]。解决电池安全问题,对电池热效应的分析是必不可少的。
以前使用的量热工具主要为加速量热仪(ARC)[13]和差示扫描量热仪(DSC),这类仪器不能准确测试微弱的化学反应热过程,电池通常将电化学方法与各种量热技术相结合测定体系的电化学及化学反应热,称之为电化学-量热联用技术。与单一的电化学方法或者热分析法相比,这种方法能够更加客观地评估锂离子电池的电化学性能,并能获得电池在充放电过程中的热力学参数。SAITO等[14-15]、LU等[16]、KRAUSE等[17]和PING等[18]采用C80微量量热仪与电池充放电测试装置联用分别对不同种类锂离子电池在充放电过程中的热行为。EDDAHECH等[19]采用电化学-量热法和平衡电位法对高容量的商业化镍钴锰酸锂电池的热效应进行了研究,并获取相关热力学参数,验证这两种方法的可行性。
大多数研究者对锂离子电池电极反应的热效应关注很少,准确的热电化学参数计算也很少。本文作者采用TAM Air等温量热仪与LAND电池测试系统联用技术,以正极材料LiNi0.5Co0.2Mn0.3O2组成的锂离子电池为研究对象,测量在不同环境温度和倍率下电池充放电循环过程中的产热情况并计算热力学参数,揭示倍率对电池热、电参数的影响规律,为解决电池内部的散热问题和电池结构及热管理系统的优化设计提供理论依据。
1 实验
1.1 材料制备
将制备好的Ni0.5Mn0.2Co0.3(OH)2前驱体按照摩尔比(n(Li):n(Ni+Co+Mn)=1.05:1)配入Li2CO3,将配好的物质置于玛瑙研钵中研磨40 min,混合均匀的物质放入刚玉瓷舟中,于空气气氛下500 ℃预烧5 h,再升温到960 ℃焙烧10 h,升温速率为5 ℃/min,冷却,得到LiNi0.5Mn0.2Co0.3O2正极材料。
1.2 扣式电池的组装
LiNi0.5Mn0.2Co0.3O2活性物质、乙炔黑和PVDF按质量比为8:1:1混合并研磨均匀后,加入适量的有机溶剂NMP调成混合均匀的浆料,再均匀涂覆在0.02 mm铝箔上,放入真空干燥箱120℃下干燥制成14 mm厚的正极片,负极为金属锂片,隔膜为Celgard 2300微孔聚丙烯膜,电解液为1 mol/L LiPF6/EC+DMC+EMC (LiPF6/EC、DMC和EMC的体积比为1:1:1)混合溶液,在充满氩气的手套箱中组装成CR 2025型扣式锂离子电池。
1.3 热电化学性能测试
采用八通道微量热仪(3114/3236 TAM Air,瑞典)测量CR 2025型扣式锂离子电池在不同倍率下充放电时的产热量。在实验过程中,量热通道被维持在恒定的温度,电池被悬挂在量热通道中央,其正负极由铜导线和外部的电池测试系统(LAND CT2001A,武汉金诺)连接并分别以0.2、0.5、1.0、2.0C对电池进行充放电测试,电压范围为2.7~4.6 V,研究原理如图1所示。采用电化学工作站(CHI 760,上海辰华仪器公司)对电池进行循环伏安测试,扫描电压范围为2.7~4.8V,扫描速率为0.1 mV/s。
2 结果与讨论
2.1 电化学性能
LiNi0.5Co0.2Mn0.3O2电池的循环伏安曲线如图2所示。由图2可看出,在第一次循环中,有两个氧化峰和两个还原峰,氧化峰3.91 V,还原峰3.67 V对应Ni2+/Ni3+;氧化峰4.43 V,还原峰4.35 V对应Co3+/Co4+。在第二次循环中,氧化峰的电流密度减小,氧化峰转移到更低的电压(3.80V),然而还原峰的位置几乎不发生变化,但电流密度有所降低,4.35 V还原峰转移到了4.23 V,ZHU等[20]的研究也得到了类似的结果。锂离子脱嵌和嵌入的电势差越大,电极极化作用越大。在循环过程中电池氧化峰的转移导致电池极化作用增大,从而导致放电比容量逐渐减少。
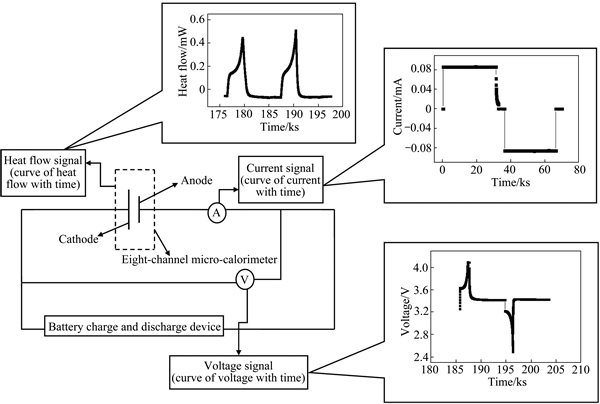
图1 LiNi0.5Co0.2Mn0.3O2/Li电池的热电化学研究示意图
Fig. 1 Schematic diagram of thermo-electrochemical study on LiNi0.5Co0.2Mn0.3O2/Li battery
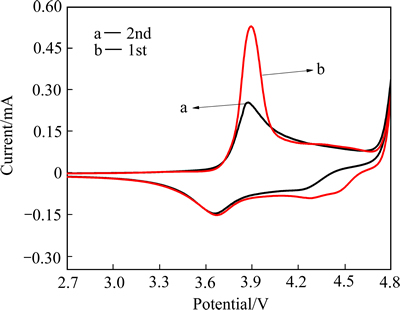
图2 2.7~4.8 V时LiNi0.5Co0.2Mn0.3O2/Li电池的循环伏安图
Fig. 2 Cyclic voltammetry of LiNi0.5Co0.2Mn0.3O2/Li battery at potential of 2.7-4.8 V
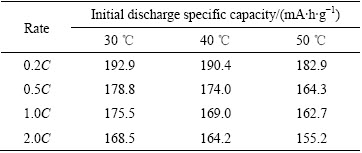
LiNi0.5Co0.2Mn0.3O2电池在不同温度和倍率下首次放电曲线以及首次放电比容量值分别如图3和表1所示。由图3和表1可知,电池放电比容量随温度的升高而减小,随充放电倍率的增加而减小,倍率从0.2C到0.5C、0.5C到1.0C、1.0C到2.0C,容量损失量逐渐增加,其值分别为24.4、26.2、27.7 mA·h/g。引起容量衰减的原因主要是由于放电倍率越大,电流越大,引起电极材料不可逆极化增大,导致电极材料结构和形貌部分受损。同时,伴随环境温度的升高,电池内部化学反应加剧,从而发生不可逆的副反应。因此,降低了电池电化学性能,导致电池容量的损失[21-22]。
2.2 热力学性能
本研究过程中,电池在工作条件下始终处于恒温状态,因此,可将电池自身温度变化产热近似为零。另外,在锂离子电池工作中,其因电极副反应产生的热比较小,也可不考虑。因此,电池在工作状态下主要产热QT来源于:Qr电池热效应可逆热;Qir电池热效应不可逆热(主要包括Qp电极极化热和Qj为电阻产生的焦耳热)。如式(1)所示:
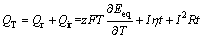 (1)
(1)
式中:z为电板反应中得失的电子数;F为法拉第常数;T为温度;Eeq为平衡状态下电池的开路电压;I为工作电流;t为工作时间;R为电池内阻;η为极化过电位。
图4~6所示分别为LiNi0.5Co0.2Mn0.3O2电池在30 ℃、40 ℃和50 ℃下首次充放电的电压、热流随时间变化曲线。从图4~6可以看出,所有曲线在充放电过程中均表现出明显的放热峰。在较高倍率(0.5C、1.0C、2.0C)下,充电过程和放电过程的热流曲线中均出现一个急剧增大的放热峰,在较低倍率(0.2C)下,充放电过程的热流曲线出现多个明显的放热峰,原因在于,在较低倍率下,电池充放电过程可近似为可逆过程,产生的热主要来自反应热,因此,在低倍率下出现的多个明显放热峰主要是电池电极反应的放热峰[23];而在较高倍率下,电池极化增大,反应热产生的峰逐渐被极化热产生的峰覆盖,因而在较高倍率下观察到的实际上是电极极化热及电池反应热的叠加,极化产热占主导地位。在不同工作温度下,电池充放电过程中热流均随倍率的升高而增大,这是由于电池不可逆热产热速率随充放电倍率升高而增大,结合上述循环伏安法所得结果,电池氧化过程电压范围为3.8~4.1 V和4.4 V附近,还原过程电压范围为3.5~3.8 V和4.2 V附近,分别与0.2C下电池充放电过程中热流曲线的放热峰位置接近。SHAJU等[24]研究得到3.6~3.8 V对应Ni2+/Ni4+的反应,4.55~4.65 V对应Co3+/Co4+的反应。
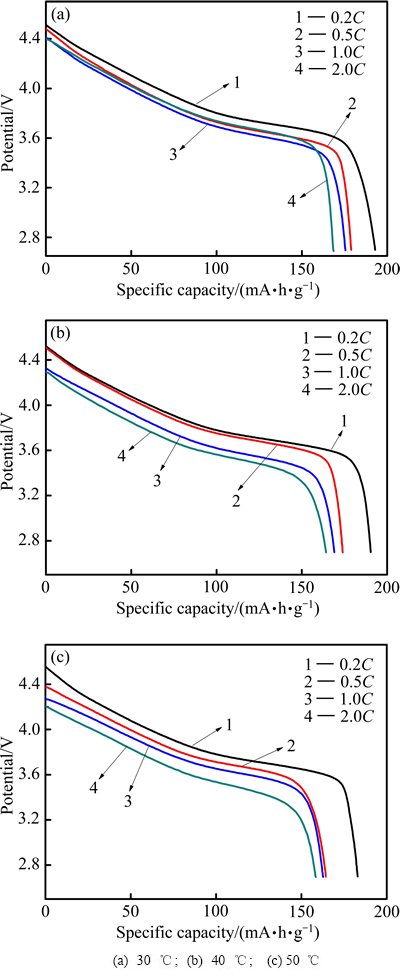
图3 LiNi0.5Co0.2Mn0.3O2/Li电池在不同温度下的首次放电曲线
Fig. 3 Initial discharge curves of LiNi0.5Co0.2Mn0.3O2/Li batteries at different temperatures
表1 LiNi0.5Co0.2Mn0.3O2/Li电池在不同温度下的首次放电比容量
Table 1 Initial discharge capacity of LiNi0.5Co0.2Mn0.3O2/Li batteries at different temperatures
2.3 热力学参数计算
通过对热流-时间曲线和电流-时间曲线进行积分,可得到电池充放电过程中的产热量q(mJ)和电量Q(C)[25-27],根据式(2)和(3)可计算得到充放电过程电极反应物质的量n(mol)和化学反应焓变△rHm(kJ/mol),F为法拉第常数,其值为96485 C/mol。
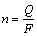 (2)
(2)
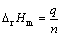 (3)
(3)
电池在不同环境温度和倍率下热力学参数见表2。由表2可知,电池在同一温度下,产热量随倍率的增加而增大。倍率越大,电池不可逆极化作用越大,导致产生更大的极化热。同一倍率下进行充放电时,随温度(30、40和50 ℃)的升高,电池产热量增加,原因在于温度越高,电池内部反应越快,伴随的不可逆副反应加剧,从而破坏电极结构,导致电池容量衰减。不同温度和倍率下电池放电过程中产热量变化如图7所示。由图7(a)可知,与较低温度相比,50 ℃产热量最大,且从0.2C增加到2.0C,电池产热量逐渐增大。由图7(b)可知,与较小放电倍率相比,电池在2.0C下的放电产热量最大,且从30 ℃增加到50 ℃,产热量逐渐增大。
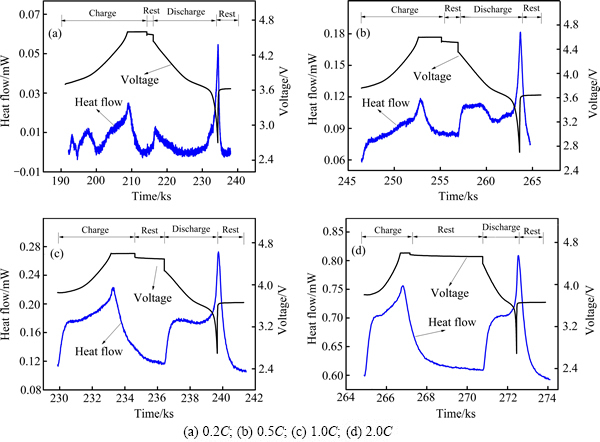
图4 LiNi0.5Co0.2Mn0.3O2/Li电池在30 ℃不同充放电倍率下热流和电压随时间的变化曲线
Fig. 4 Change curves of heat flow and voltage with time of LiNi0.5Co0.2Mn0.3O2/Li batteries at 30 ℃
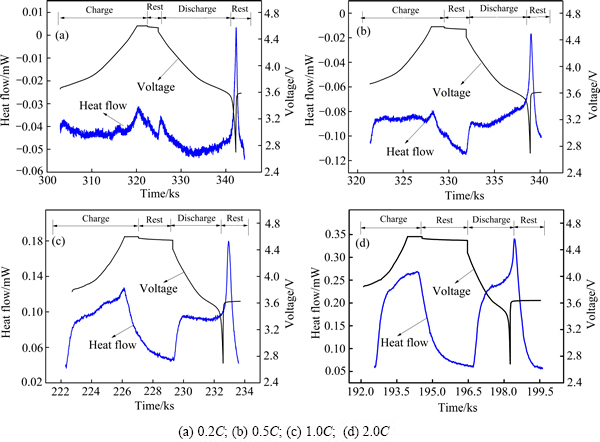
图5 LiNi0.5Co0.2Mn0.3O2/Li电池在40 ℃不同充放电倍率下热流和电压随时间的变化曲线
Fig. 5 Change curves of heat flow and voltage with time of LiNi0.5Co0.2Mn0.3O2/Li batteries at 40 ℃
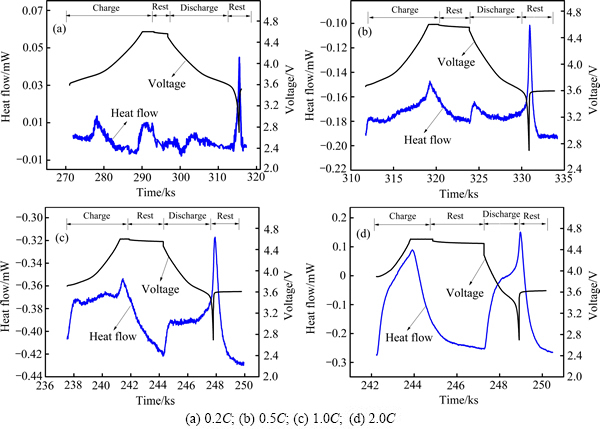
图6 LiNi0.5Co0.2Mn0.3O2/Li电池在50 ℃不同充放电倍率下热流和电压随时间的变化曲线
Fig. 6 Change curves of heat flow and voltage with time of LiNi0.5Co0.2Mn0.3O2/Li batteries at 50 ℃
表2 LiNi0.5Co0.2Mn0.3O2/Li电池在不同条件下热力学参数
Table 2 Thermodynamic parameters for LiNi0.5Co0.2Mn0.3O2/Li batteries under different conditions
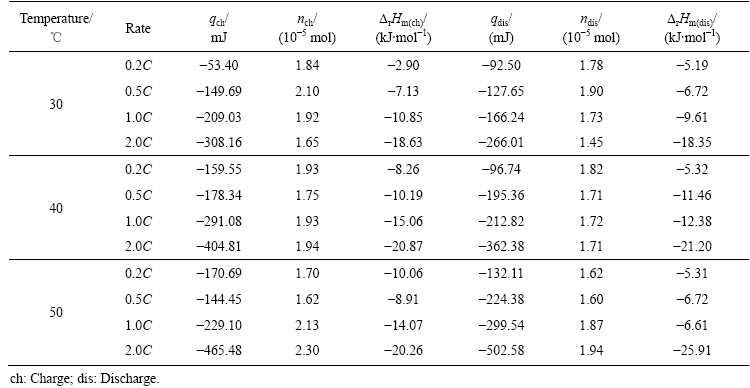
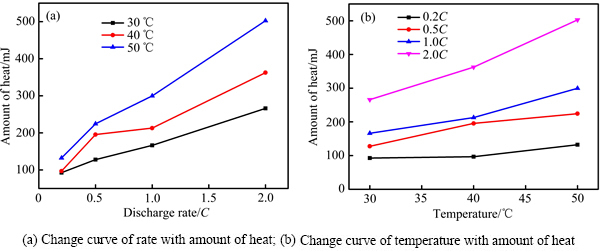
图7 LiNi0.5Co0.2Mn0.3O2/Li电池在不同条件下的产热量
Fig. 7 Amount of heat on LiNi0.5Co0.2Mn0.3O2/Li batteries at different conditions
电池充放电过程的热效应包括可逆热和不可逆热。在较低温度(30 ℃)及较小倍率(0.2C)下,电池充电和放电过程的不可逆热近似相等,可逆热的绝对值相等且互为相反数[25-27]。通过式(4)和(5)可计算得到近似电极充电过程可逆热(qre,ch)和放电过程可逆热(qre,dis)分别为19.55 mJ和-19.55 mJ,相应的化学反应熵变(ΔrSm)分别为-3.51 J/(mol·K)和3.62 J/(mol·K)。
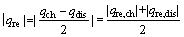 (4)
(4)
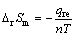 (5)
(5)
3 结论
1) 采用电化学-量热法研究了LiNi0.5Co0.2Mn0.3- O2/Li电池在不同温度和倍率下的产热情况。电池在较低倍率(0.2C)下,其产热主要来自电极反应热。
2) 随着倍率和环境温度的增加,产热量不断增大,在2.0C和50 ℃条件下电池的产热量为-968.06 mJ。电池在0.2C和30 ℃条件下的产热量为-145.90 mJ,且前者产热量是后者的5.7倍。
3) 近似计算得到电极充电过程可逆热(qre,ch)和放电过程可逆热(qre,dis)分别为19.55 mJ和-19.55 mJ,相应的化学反应熵变(ΔrSm)分别为-3.51 J/(mol·K)和3.62 J/(mol·K)。
4) 通过电化学-量热法得到电池充放电过程中的一系列热力学参数,可为解决电池热效应而引起的安全问题提供有力的理论指导。
REFERENCES
[1] AL-HALLAJ S, SELMAN J R. Thermal modeling of secondary lithium batteries for electric vehicle/hybrid electric vehicle applications[J]. Journal of Power Sources, 2002, 110: 341-438.
[2] HORIBA T, HIRONAKA K, MATSUMURA T, KAI T, KOSEKI M, MURANAKA Y. Manganese-based lithium batteries for hybrid electric vehicle applications[J]. Journal of Power Sources, 2003, 119-121: 893-896.
[3] TANG Wei, HOU Yu-yang, WANG Fa-xing, LIU Li-li, WU Yu-ping, ZHU Kai. LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries[J]. Nano Letters, 2013, 13: 2036-2040.
[4] LU Xi-bin, LI Xin-hai, WANG Zhixing, GUO Huajun, YAN Guochun, YIN Xing. A modified co-precipitation process to coat LiNi1/3Co1/3Mn1/3O2 onto LiNi0.8Co0.1Mn0.1O2 for improving the electrochemical performance[J]. Applied Surface Science, 2014, 279: 182-187.
[5] LI Ling-jun, LI Xin-hai, WANG Zhixing, LING Wu, ZHENG Jun-chao, LI Jin-hui. Synthesis of LiNi0.8Co0.1Mn0.1O2 cathode material by chloride co-precipitation method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 279-282.
[6] 曹景超, 胡国荣, 彭忠东, 杜 柯, 曹雁冰. 高倍率锂离子电池正极材料LiCo0.9Ni0.05Mn0.05O2的合成及电化学性能[J]. 中国有色金属学报, 2014 (11): 2813-2820.
CAO Jing-chao, HU Guo-rong, PENG Zhong-dong, DU Ke, CAOYan-bing. Synthesis and electrochemical properties of LiCo0.9Ni0.05Mn0.05O2 cathode material with high rate capability for lithium ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2014 (11): 2813-2820.
[7] 陈 巍, 李新海, 王志兴, 郭华军, 岳 鹏, 李灵均. 共沉淀法制备LiNi0.8Co0.1Mn0.1O2过程中加料速度对其性能的影响[J]. 中国有色金属学报, 2012, 22(7): 1956-1962.
CHEN Wei, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, YUE Peng, LI Ling-jun. Influence of feed rate on performance of LiNi0.8Co0.1Mn0.1O2 cathode materials prepared by co-precipitation[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(7): 1956-1962.
[8] 王接喜, 李新海, 王志兴, 李灵均, 郭华军, 岳 鹏, 伍 凌. 快速共沉淀过程pH值对LiNi0.8Co0.1Mn0.1(OH)2及LiNi0.8Co0.1Mn0.1O2性能的影响[J]. 中国有色金属学报, 2011, 21(9): 2175-2181.
WANG Jie-xi, LI Xin-hai, WANG Zhi-xing, LI Ling-jun, GUO Hua-jun, YUE Peng. Effect of pH value on performance of LiNi0.8Co0.1Mn0.1(OH)2 and LiNi0.8Co0.1Mn0.1O2 synthesized via fast co-precipitation process[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2175-2181.
[9] 杨乃兴, 张兄文, 李国君. 锂离子电池放电循环的发热特性研究[J]. 工程热物理学报, 2014(9): 1850-1854.
YANG nai-xin, ZHANG Xiong-wen, LI Guo-jun. Study of heat generation of a lithium-ion battery during discharge cycle[J]. Journal of Engineering Thermophyscis, 2014(9): 1850-1854.
[10] SHUKLA A K, PREM KUMAR T. Materials for next-generation lithium batteries[J]. Current Science, 2008, 94(3): 314-331.
[11] 王 浩, 杨聚平, 王 莉, 李建军, 何向明, 欧阳明高. 锂离子电池的安全性问题[J]. 新材料产业, 2012(9): 88-94.
WANG Hao, YANG Ju-ping, WANG Li, LI Jian-jun, HE Xiang-ming, OUYANG Ming-gao. Safety problem of lithium ion battery[J]. Advanced Materials Industry, 2012(9): 88-94.
[12] 吴 凯, 张 耀, 曾毓群, 杨 军. 锂离子电池安全性能研究[J]. 化学进展, 2011, 23(2/3): 401-409.
WU Kai, ZHANG Yao, ZENG Yu-qun, YANG Jun. Safety performance of lithium-ion battery[J]. Process in chemistry, 2011, 23(2/3): 401-409.
[13] RODER P, BABA N, WIEMHOFER H D. A detailed thermal study of a Li[Ni0.33Co0.33Mn0.33]O2/LiMn2O4-based lithium ion cell by accelerating rate and differential scanning calorimetry[J]. Journal of Power Sources, 2014, 248: 978-987.
[14] SAITO Y. Thermal behaviors of lithium-ion batteries during high-rate pulse cycling [J]. Journal of Power Sources, 2005, 146: 770-774.
[15] SAITO Y, SHIKANO M, KOBAYASHI H. Heat generation behavior during charging and discharging of lithium-ion batteries after long-time storage[J]. Journal of Power Sources, 2013, 244: 294-299.
[16] LU Wen-quan, YANG Hui, PRAKASH J. Determination of the reversible and irreversible heats of LiNi0.8Co0.2O2/mesocarbon microbead Li-ion cell reactions using isothermal microcalorimetery[J]. Electrochimica Acta, 2006, 51: 1322-1329.
[17] KRAUSE L J, JENSEN L D, DAHN J R. Measurement of parasitic reactions in Li ion cells by electrochemical calorimetry[J]. Journal of the Electrochemical Society, 2012, 159(7): 937-943.
[18] PING Ping, WANG Qing-song, HUANG Pei-feng, SUN Jin-hua, CHEN Chun-hua. Thermal behavior analysis of lithium-ion battery at elevated temperature using deconvolution method[J]. Applied Energy, 2014, 129: 261-273.
[19] EDDAHECH A, BRIAT O, VINASSA J M. Thermal characterization of a high-power lithium-ion battery: Potentiometric and calorimetric measurement of entropy changes[J]. Energy, 2013, 61: 432-439.
[20] ZHU Hua-li, XIE Tian, CHEN Zhao-yong, LI Ling-jun, XU Ming, WANG Wen-hua, LAI Yan-qing, LI Jie. The impact of vanadium substitution on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2[J]. Electrochimica Acta, 2014, 135: 77-85.
[21] SONG Liu-bin, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, XIAO Zhong-liang, ZHANG Feng, PENG San-jun. Thermal behaviors study of LiFePO4 cell by electrochemical-calorimetric method[J]. Electrochimica Acta, 2013, 90: 461-467.
[22] SONG Liu-bin, XIAO Zhong-liang, LI Ling-jun, ZHOU Qing-qing. Thermo-electrochemical study on cathode materials for lithium ion cells[J]. Journal of Solid State Electrochemistry, 2015, 19: 2167-2175.
[23] LU Wen-quan, YANG Hui, PRAKASH J. Determination of the reversible and irreversible heats of LiNi0.8Co0.2O2/mesocarbon microbead Li-ion cell reactions using isothermal microcalorimetery[J]. Electrochimica Acta, 2006, 51(7): 1322-1329.
[24] SHAJU K M, SUBBA RAO G V, CHOWDARI B V R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries[J]. Electrochimica Acta, 2002, 48(2): 145-151.
[25] 张 锋, 肖忠良, 宋刘斌, 谢峥璨, 曾巨澜. 磷酸铁锂电池充放电过程中的热性能[J]. 电源技术, 2013, 37(9): 1530-1532.
ZHANG Feng, XIAO Zhong-liang, SONG Liu-bin, XIE Zheng-can, ZENG Ju-lan. Thermal performance of LiFePO4 cell during charge-discharge process[J]. Journal Power Sources, 2013, 37(9): 1530-1532.
[26] SONG Liu-bin, XIAO Zhong-liang, ZHOU Ying. Thermo- electrochemical study on LiMn2O4 lithium-ion cells during charge-discharge process[J]. Electrochimica Acta, 2013, 114: 611-616.
[27] XIAO Zhong-liang, ZHOU Ying, SONG Liu-bin, ZHANG Feng, GAO Jie, ZENG Ju-lan, CAO Zhong. Thermal-electrochemical behaviors of LiMn2O4 lithium-ion cell studied by electrochemical-calorimetric method[J]. Journal of Alloys and Compounds, 2014, 592(12): 226-230.
Thermo-electrochemistry on LiNi0.5Co0.2Mn0.3O2 cathode material for lithium ion battery
XIAO Zhong-liang, ZHOU Qing-qing, SONG Liu-bin, HU Chao-ming, LU Yi-peng, CAO Zhong
(Changsha University of Science and Technology, School of Chemistry and Biological Engineering, Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha 410004, China)
Abstract: The electrochemical-calorimetric method was adopted to study the thermo-electrochemistry performance of lithium ion batteries in which LiNi0.5Co0.2Mn0.3O2 acted as cathode materials at different ambient temperatures and charge-discharge rates. The results show that the discharge specific capacity decreases with increasing the charge-discharge rates and ambient temperatures. At 0.2C, the heat flow of the batteries increase slowly and appear a plurality of exothermic peaks, but at higher charge-discharge rates (0.5, 1.0 and 2.0C), the heat flow increases rapidly and only an exothermic peak appears at the charge and discharge stage, respectively. Through investigating the thermo- electrochemistry, a series of thermodynamic parameters of lithium ion batteries during charge-discharge process, such as the amount of heat, enthalpy change of chemical reaction (ΔrHm), entropy change of chemical reaction (ΔrSm), are achieved.
Key words: LiNi0.5Co0.2Mn0.3O2; electrochemical-calorimetric method; thermo-electrochemistry; thermodynamic parameters; lithium ion battery
Foundation item: Projects(21501015, 31527803) supported by the National Natural Science Foundation of China
Received date: 2016-01-25; Accepted date: 2016-06-15
Corresponding author: SONG Liu-bin; Tel: +86-13875976497; E-mail: csustslb@csust.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(21501015,31527803)
收稿日期:2016-01-25;修订日期:2016-06-15
通信作者:宋刘斌,讲师,博士;电话:13875976497;E-mail: csustslb@csust.edu.cn
摘 要:采用电化学-量热法研究以LiNi0.5Co0.2Mn0.3O2为正极材料的锂离子电池在不同环境温度和充放电倍率下的热电化学性能。结果表明:环境温度和充放电倍率是影响电池比容量的重要因素,随着充放电倍率和环境温度的增加,电池比容量逐渐减小。在低倍率(0.2C)下,电池充放电初始阶段的热流缓慢增大,且出现多个放热峰;而在较高倍率(0.5、1.0、2.0C)下,电池充放电初始阶段的热流快速增长,且充电和放电过程分别仅出现一个明显的放热峰。通过热电化学研究,可获得电池充放电过程的产热量、化学反应焓变(ΔrHm)以及化学反应熵变(ΔrSm)等热力学参数。


