Trans. Nonferrous Met. Soc. China 23(2013) 1191-1198
Electrosynthesis and physicochemical properties of α-PbO2-CeO2-TiO2 composite electrodes
Bu-ming CHEN1, Zhong-cheng GUO1,2, Rui-dong XU1
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Kunming Hengdera Science and Technology, Kunming 650105, China
Received 14 March 2012; accepted 11 July 2012
Abstract:
In order to investigate the effect of solid particles dopants on physicochemical properties of α-PbO2 electrodes, α-PbO2 composite electrodes doped with nano-TiO2 and nano-CeO2particles were respectively prepared on Al/conductive coating electrodes in 4 mol/L NaOH solution with addition of PbO until saturation by anodic codeposition. The electrodeposition mechanism, morphology, composition and structure of the composite electrodes were characterized by cyclic voltammogram (CV), SEM, EDAX and XRD. Results show that the doping solid particles can not change reaction mechanism of α-PbO2 electrode in alkaline or acid plating bath, but can improve deposition rate and reduce oxygen evolution potential. The doping solid particles can inhibit the growth of α-PbO2 unit cell and improve specific surface area. The diffraction peak intensity of α-PbO2-CeO2-TiO2 composite electrode is lower than that of pure α-PbO2 electrode. The electrocatalytic activity of α-PbO2-2.12%CeO2-3.71%TiO2 composite electrode is the best. The Guglielmi model for CeO2 and TiO2 codeposition with α-PbO2 is also proposed.
Key words:
Al; lead dioxide; composite electrodes; electrocatalysis; physicochemical property;
1 Introduction
Composite coating is a special coating formed in the process of metal or metal oxide layer with inclusion of one or several insoluble solid particles by metal or metal oxide electrodeposition method [1]. YEO et al suggested that mixed oxide doped with some ions could evidently reduce the oxygen evolution overpotential and pointed out that the overpotential oxygen-transfer reactions and oxygen evolution were correlated because the two processes were both discharged of water anode [2,3]. Since 1996, some composite electrodes have been prepared by composite electrodeposition of PbO2, Ti2O3 as matrix metal oxides and RuO2, Co3O4 catalytic particles as disperse phases [4], and applied to the oxygen evolution of electro-catalysis materials. The incorporation of solid particles into PbO2 led to significant change of the electrocrystallization morphology. Furthermore, the contents of dispersed phases in the composite electrodes depend on electrolyte compositions and electrodeposition conditions [5].
Nano-composite coating is mixed with nano- particles in the size range of 0.1-100 nm, which have higher hardness, wear resistance, friction reduction and corrosion resistance owing to the small size effect, surface effect, quantum size effect and quantum tunnel effect of nano-particles. Nano-TiO2 particle as a photocatalyst is widely used in air purification, cleaning, and sterilization because of its good chemical stability, insolubility, non-toxicity and low cost. Rare earth has a wide range of catalysis and co-catalysis because of its unique 4f electronic structure, physical and chemical properties. The course of electrocrystallization will be changed when cerium dioxide particles are embedded in the coating, which promotes the crystal plane preferring to orientation growth. The microstructures for coatings are more uniform and compact, so the corrosion resistance is improved [6].
In order to get a kind of composite material with high electrochemical activity, Al/conductive coating/ α-PbO2-CeO2-TiO2 composite electrodes were prepared by anodic codeposition in an alkaline bath. The effects of solid particles dopants on physicochemical properties of α-PbO2 electrodes were investigated. The electro- deposition mechanism, morphology, composition and structure of the composite electrodes were characterized.
2 Experimental
2.1 Preparation of composite electrodes
Aluminum (Al) in the dimensions of 50 mm×25 mm×2 mm was used as the substrate, which was first roughened by sand-blasting, degreased and chemically etched, and then coated by a conductive coating. The procedure was described as follows: 1) the conductive solution was applied to the substrate by brushing; 2) the substrate was surface dried under ultraviolet lamp; 3) the substrate was dried in an electricity box at 423 K for 2 h. The undercoating produced in this research was 20-30 mm thick, and the details were presented in Ref. [7]. Thereafter, the composition and process conditions of the PbO2 plating bath were as follows: 4 mol/L NaOH solution saturated with litharge PbO(s) (the soluble PbO species were  ), pH>14, anode current density of 0.3-1.0 A/dm2, mildly stirring using a magnetic stirrer, bath temperature of 20-50 °C, 0-20 g/L TiO2 grain (rutile, the average particle size of 30 nm), 0-20 g/L CeO2 grain (the average particle size of 50 nm), and electroplating time of 4 h. The bath was dispersed for 30 min by the ultrasonic device before electrodeposition in order to assure that TiO2 and CeO2 particles were dispersed in oxide substrate, Finally, Al/conductive coating/α-PbO2-CeO2-TiO2 composite electrode was obtained.
), pH>14, anode current density of 0.3-1.0 A/dm2, mildly stirring using a magnetic stirrer, bath temperature of 20-50 °C, 0-20 g/L TiO2 grain (rutile, the average particle size of 30 nm), 0-20 g/L CeO2 grain (the average particle size of 50 nm), and electroplating time of 4 h. The bath was dispersed for 30 min by the ultrasonic device before electrodeposition in order to assure that TiO2 and CeO2 particles were dispersed in oxide substrate, Finally, Al/conductive coating/α-PbO2-CeO2-TiO2 composite electrode was obtained.
2.2 Characterization of composite electrodes
The electrochemical performance of Al/conductive coating/α-PbO2-CeO2-TiO2 composite electrode was measured with the three-electrode cell. The composite electrode was used as the working electrode, Hg/Hg2Cl2 (KC1, saturated) was used as the reference electrode, and graphite was used as the auxiliary electrode. Cyclic voltammetric curves were measured in an alkaline bath. The alkaline bath, called S1, contained 4 mol/L NaOH solution saturated with litharge PbO(s) at 40 °C in the potential range of 0-1.4 V. The acid bath, called S2, contained 30% Pb(NO3)2 (pH=1.5). The bath temperature was kept at 60 °C in the potential range of 0-2.1 V. The surface morphology of the composite electrodes was characterized by SEM (XL30 ESEM, Philip, Holland). The composition of the composite electrode was obtained by EDS (PHOENIX, EDAI, USA). The phase structure of the composite electrode was studied by X-ray diffraction (using Co Ka radiation, X’pert Highscore produced by Netherlands Philips Instrument Company).
3 Results and discussion
3.1 Cyclic voltammetry curves
3.1.1 Cyclic voltammetry curves of composite electrode in alkaline solution
The cyclic voltammetry curves in 4 mol/L NaOH solution and in S1 solution at 40 °C are shown in Fig. 1. Figure 1(a) shows that an oxidation peak appears at 0.2 V vs SCE, which may be a result of the redox transition PbO/PbO2 (PbO+2OH--2e=PbO2+H2O), and an oxygen evolution when the potential is over 0.7 V vs SCE. As shown in Fig. 1(b), the oxidation peak intensity does not change when the oxygen evolution potential changes. It can be seen from Fig. 1(c) and Fig. 1(d) that the original potential of α-PbO2 is 0.20 V vs SCE [8]. Two anodic current density peaks at φP1=0.20 V and φP2=0.30 V are observed on the positive branch of the cyclic voltammetry curve. These peaks may be related to the formation of Pb3O4 and PbO2 on the surface of the composite electrodes. The cathodic current density peak is observed at approximately φP3=0.01 V on the negative potential scan due to the cathodic dissolution of PbO2 deposited during the previous positive scan [9]. It can be also seen that in Fig. 1(c) and Fig. 1(d) the oxidation peak intensities in different electrodes do not change evidently in the S1 solution. The oxygen evolution potential changes, only showing that doping solid particles cannot change reaction mechanism of α-PbO2 deposition [10].
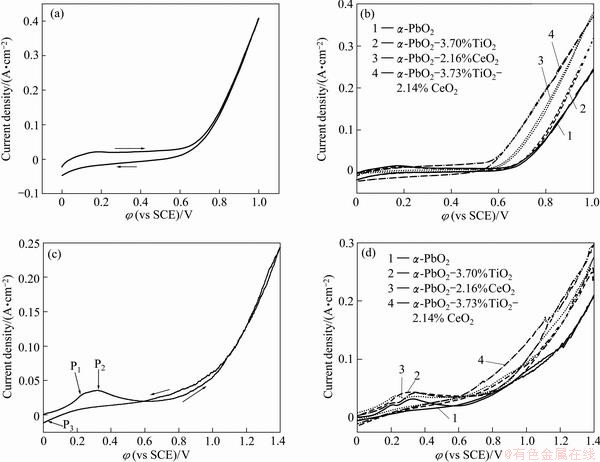
Fig. 1 Cyclic voltammetry curves in 4 mol/L NaOH solution (a, b) and in S1 solution (c, d) with scan rate of 5 mV/s
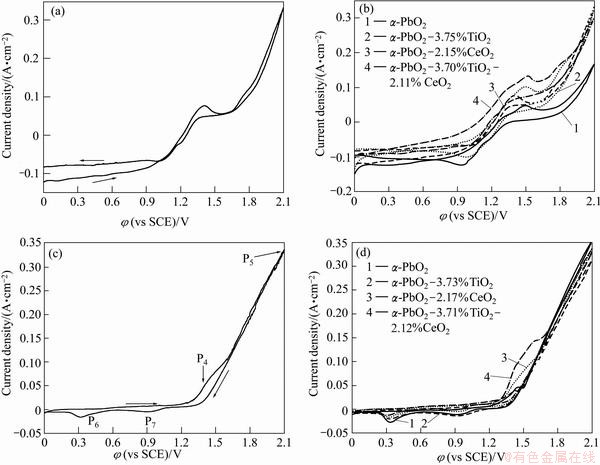
Fig. 2 Cyclic voltammetry curves in 2 mol/L KNO3 solution (pH=1.5) (a, b) and in S2 solution (c, d) with scan rate of 5 mV/s
3.1.2 Cyclic voltammetry curves of composite electrodes in acid solution
The cyclic voltammetry curves in 2 mol/L KNO3 solution (pH=1.5) and in S2 solution at 60 °C are shown in Fig. 2. Figure 2(a) shows that an oxidation peak appears at 1.45 V vs SCE. This may be attributed to the solid-state surface redox transition of Pb/PbO2. Another possible result is that the Pb2+ oxidizes to PbO2 (Pb2++2H2O-2e= PbO2+4H+) because it is found that the current density is rather negative at a low potential. The exponential growth of the anodic current density at potential over 1.9 V is due to oxygen evolution. The cathodic current density peak is observed at approximately 1.0 V on the negative potential scan due to the cathodic dissolution of PbO2 deposited during the previous positive scan [11]. As shown in Fig. 2(b), the number of oxidation peaks with different electrodes does not change when the potential of oxygen evolution changes. It is shown that the doping solid particles cannot change the depositing mechanism of α-PbO2electrode, while the anodic current density peak at potential over 1.9 V of α-PbO2-3.71%TiO2-2.12%CeO2 composite coating is higher than that of α-PbO2. Figure 2(c) shows that the potential of the beginning deposition of β-PbO2 is higher than 1.30 V vs SCE. Two anodic current density peaks at φP4=1.4 V and φP5=2.1 V are observed on the positive branch of the cyclic voltammetry curve. These peaks probably correspond to the formation of surface PbO2 and O2, respectively. The cathodic current density peaks at φP6=0.30 V and φP7=0.91 V are observed on the negative branch, and these cathodic peaks are probably due to the formation of the soluble Pb(II) and Pb(III) species. The cathodic peak at φP3=0.33 V does not appear in Ref. [12]. It is possible that the lower bath temperature restrains the formation of the soluble Pb(II) species. It can be also seen from Fig. 2(c) and Fig. 2(d) that the intensities of oxidize peaks of different electrodes in S2 solution do not change evidently, and only the oxygen evolution potential changes, indicating that the doped solid particles cannot change the electrodepositing mechanism of α-PbO2. However, the cathodic current density peaks and the potential of the formation of the soluble Pb(III) species respectively decrease toward more negative with the addition of doped particles, showing that the quasi-reversible extent of electrode reaction process is large, especially the α-PbO2-3.71%TiO2-2.12%CeO2 composite electrode.
The electrocatalytic activity of the electrode is determined mainly by the phase and chemical composition of the active mass. Therefore, the electrochemical deposition of lead dioxide is of great interest to achieve a desired physical and chemical property. The elucidation of the PbO2 electrodepositing mechanism is hence very important. These data are interpreted with the following mechanism [13] (originally proposed by BECK [14]). The first step is an equilibrium hydration in aqueous solution (Eq. (1)) followed by adsorption (Eq. (2)). It follows the formation of  and
and 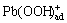 intermediate on the substrate. The final reaction ends with the production of PbO2. These data are interpreted on the electrode surface according to the following schemes:
intermediate on the substrate. The final reaction ends with the production of PbO2. These data are interpreted on the electrode surface according to the following schemes:
 (1)
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)
 (5)
(5)
It can be seen from the CV curves in alkaline and acid solutions that two anodic peaks (except oxygen evolution peak) are observed in the alkaline plating solution, and only one anodic peak (except oxygen evolution peak) is seen in the acid plating solution, which shows that in the acid plating solution Pb2+ or PbO may oxide directly to PbO2 while in the alkaline plating solution Pb2+ or PbO may oxide to Pb3O4 first, then gradually oxide to PbO2. This implies that in the alkaline plating solution, the first anodic peak corresponds to the formation of Pb3O4 and the second one corresponds to PbO2. In other words, 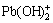 and
and 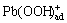 truly exist on the surface and the further study is to make sure whether they are the soluble substance or not.
truly exist on the surface and the further study is to make sure whether they are the soluble substance or not.
3.2 Guglielmi model of α-PbO2 composite codeposition
The α-PbO2 composite coatings were obtained in 4 mol/L NaOH solution saturated with litharge PbO(s) by adding nano-TiO2 and nano-CeO2 particles.
The main chemical reactions of electro-deposition are as follows:
on the anode:
 +OH-→PbO2+H2O+2e (6)
+OH-→PbO2+H2O+2e (6)
4OH-→O2↑+2H2O+4e (7)
on the cathode:
 +H2O+2e→Pb+3OH- (8)
+H2O+2e→Pb+3OH- (8)
2H2O+2e→H2↑+2OH- (9)
TiO2 and CeO2 co-deposition can be described as
 +OH-+TiO2→PbO2-TiO2+H2O+2e (10)
+OH-+TiO2→PbO2-TiO2+H2O+2e (10)
 +OH-+CeO2→PbO2-CeO2+H2O+2e (11)
+OH-+CeO2→PbO2-CeO2+H2O+2e (11)
 +OH-+CeO2+TiO2→PbO2- CeO2- TiO2+H2O+2e (12)
+OH-+CeO2+TiO2→PbO2- CeO2- TiO2+H2O+2e (12)
The co-deposition mechanism can be described by Guglielmi model shown in Fig. 3. Bilumbite ion  and other ions including solvent are adsorbed on TiO2 and CeO2 particles, which are loosely adsorbed on α-PbO2 anode. Under the influence of an electric field, TiO2 and CeO2 particles enter the α-PbO2 compact layer and the oxidation of
and other ions including solvent are adsorbed on TiO2 and CeO2 particles, which are loosely adsorbed on α-PbO2 anode. Under the influence of an electric field, TiO2 and CeO2 particles enter the α-PbO2 compact layer and the oxidation of  leads to a strong irreversible adsorption, which consequently makes TiO2 and CeO2 particles deposit along with α-PbO2 crystals.
leads to a strong irreversible adsorption, which consequently makes TiO2 and CeO2 particles deposit along with α-PbO2 crystals.
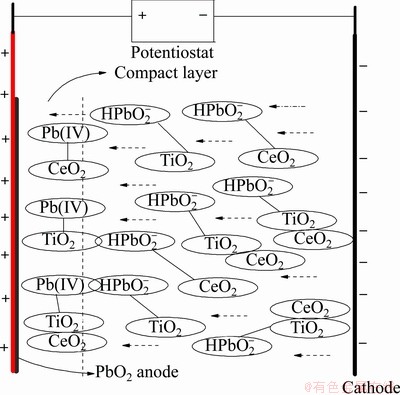
Fig. 3 Schematic diagram of Guglielmi model for TiO2 and CeO2codeposition with α-PbO2
3.3 Analysis of organization structure on α-PbO2composite coating
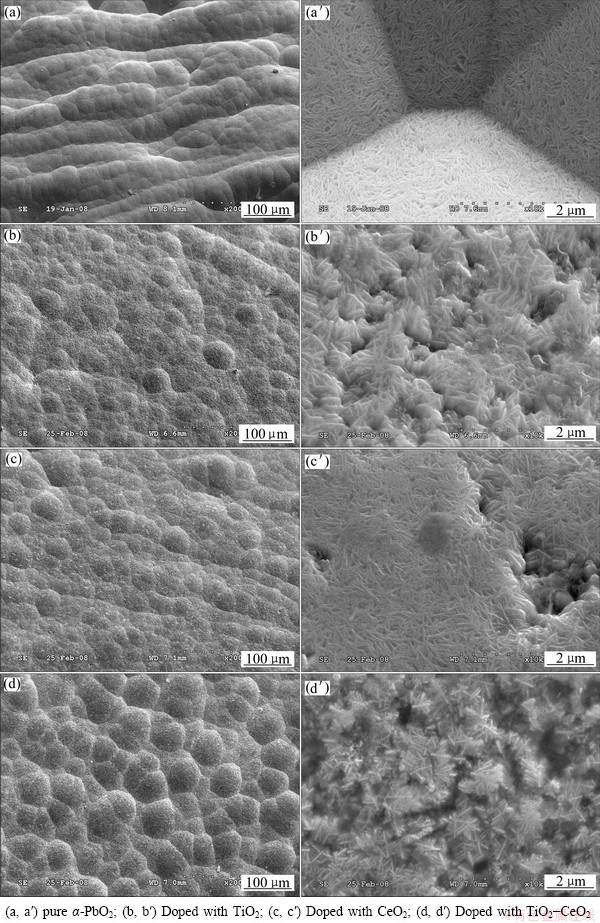
Fig. 4 SEM images of PbO2 coatings
Figure 4 shows the SEM images of α-PbO2 and α-PbO2 composite coating prepared on Al/conductive coating electrodes in 4 mol/L NaOH solution with addition of yellow PbO until saturation with different solid particles. Figures 4(a), (b), (c), and (d) respectively correspond to the morphologies of pure α-PbO2 coating, doped with TiO2 (15 g/L), doped with CeO2 (10 g/L) and doped with TiO2(15 g/L)-CeO2(10 g/L) composite coatings. And Figs. 4(a′), (b′), (c′) and (d′) show respectively the higher magnification images of Figs. 4(a), (b), (c), and (d). As shown in Fig. 4(a), there are cracks in the pure α-PbO2 coating surface, and those cylindrical unit cells are sticked out of the surface with uneven shapes. There are no cracks in the α-PbO2 doped with solid particles and even the surface is closely linked, especially α-PbO2-CeO2composite coating has the closest and the most compact structure. The one with higher magnification reveals that the deposits are much rougher than the pure α-PbO2, which indicates that the addition of solid particles can inhibit the growth of α-PbO2 unit cells, and improve the specific surface area of coating surface. The increased effective area is a favourable feature in the view of a possible application as electrode materials [15]. The energy spectra of the PbO2 coatings (from Fig. 4) are shown in Fig. 5.
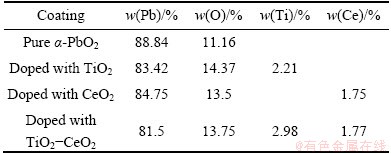
Composition of coating was tested by EDAX and the different α-PbO2coating components are shown in Table 1. From the table we can calculate that TiO2mass content of TiO2-α-PbO2 is 3.68 %, CeO2 mass content of CeO2-α-PbO2composite coating is 2.15 % and TiO2 and CeO2 mass contents of α-PbO2-TiO2-CeO2 composite coating are respectively 3.73 % and 2.17 %.
Table 1 Compositional analysis of PbO2 coatings
To avoid the thickness of PbO2coating affecting the phase composition, the coating thickness is controlled to be about 100 μm by XRD using Co target and Kα radiation. The phase compositions of α-PbO2 coating doped with different particles are shown in Fig. 6 and the corresponding data are obtained with the JCPDS card. From Fig. 6, we can see that pure α-PbO2 coating and α-PbO2 composite coating doped with particles contain not only α-PbO2but also a little PbO impurity. The impurity may be obtained in the electro-deposition due to the co-deposition between insoluble Pb(OH)2 or PbO and α-PbO2. It is identical with the reported ones [8,16] that the strength of crystal face (200) in α-PbO2, and the incorporation of either TiO2 or CeO2 particles do not affect the orienting growth of PbO2. It can also be seen from Fig. 6 that the diffraction peak intensity reduces by the addition of particles [10]. Especially, the lowest intensity of diffraction peak of coating is obtained by the addition of TiO2 and CeO2solid particles together, which indicates that the addition of solid particles decreases the crystallinity of composite coating. This phenomenon can also be seen in the surface morphology shown in Fig. 4. As shown in Fig. 6, two low diffractive peaks at 42.1° and 99.7° and three low diffractive peaks at 38.6°, 69.8° and 92.2° are observed, indicating the formation of TiO2(PDF#034-0180) and CeO2(PDF#043-1002) on Al/conductive coating during the electrodeposition. But the contents of TiO2 and CeO2 are small comparable with PbO2 film.
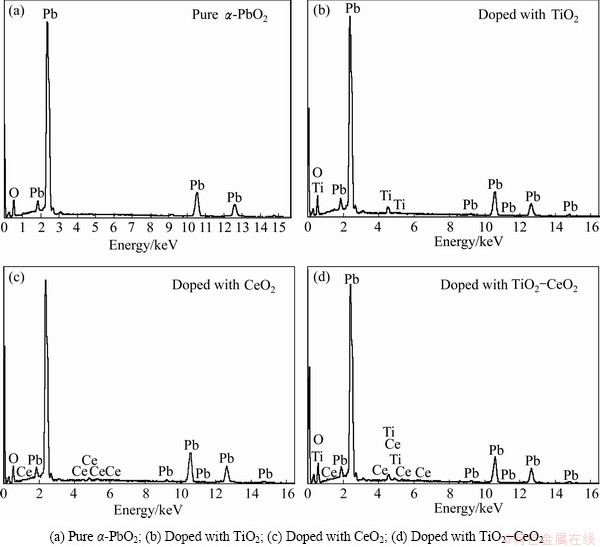
Fig. 5 EDAX spectra for different coatings
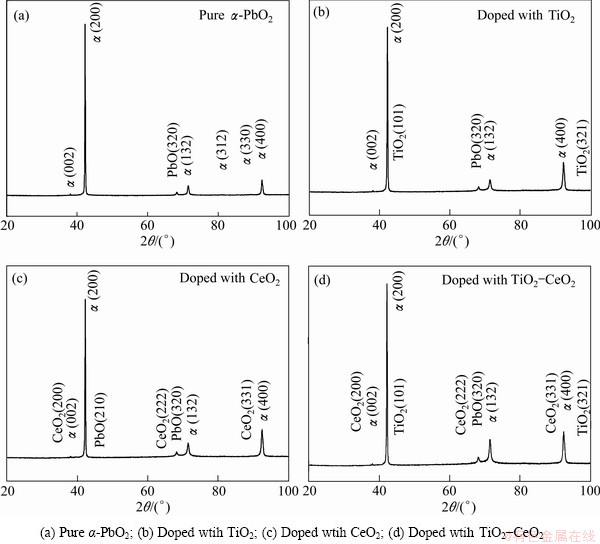
Fig. 6 X-ray diffraction patterns of different coatings
3.4 Anodic polarization curves
Figure 7 shows the anodic polarization curves of Al/conductive coating/α-PbO2, Al/conductive coating/ α-PbO2-3.73%TiO2, Al/conductive coating/α-PbO2- 2.17%CeO2 and Al/conductive coating/α-PbO2- 3.71%TiO2-2.12%CeO2 electrodes in 50 g/L Zn2+, 150 g/L H2SO4 solution at 25 °C. The anodic polarization curve of Pb electrode is also shown for comparison. From Fig. 7, it is apparent that the electrocatalytic activity of the Al/conductive coating/α-PbO2 is much superior to that of Pb for the anodic evolution of oxygen. It can also be seen that the potential of A1/conductive coating/α-PbO2-3.71%TiO2-2.12%CeO2 anode is the lowest at a constant current density, which shows that the electro-catalytic activity of the anode is the best. The potential of A1/conductive coating/α-PbO2-2.17%CeO2 anode is lower than that of A1/conductive coating/ α-PbO2-3.73%TiO2, which indicates that the electrocatalytic activity of electrode doped CeO2 is better than that of electrode doped with TiO2. The potential of A1/conductive coating/α-PbO2 anode is the highest, which indicates that the electrocatalytic activity of electrode is improved evidently with addition of particles.
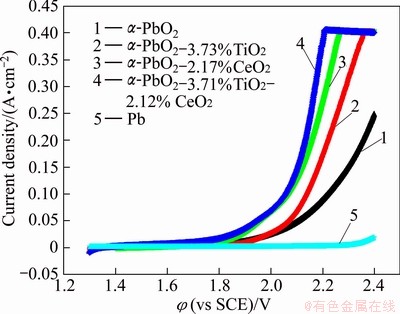
Fig. 7 Polarization curves of different electrodes in Zn2+50 g/L, 150 g/L H2SO4at 25 °C
4 Conclusions
1) CV curves measurement shows that doping solid particles cannot change the reaction mechanism of coating in acid or alkaline plating solution but they can improve the deposition rate and reduce the reaction potential. Based on the mechanism of electro-deposition, Pb3O4 oxidative peak appears in alkaline bath but in acid.
2) The physicochemical properties of the composites significantly differ from those of lead dioxide and are determined by the compositions of these materials. SEM and XRD analyses show that doping solid particles can inhibit the growth of α-PbO2 unit cell, and improve specific surface area of coating surface. The surface roughness of α-PbO2-TiO2-CeO2composite coating is the highest, and the diffraction peak intensity of the coating with doped solid particles is lower than that of pure α-PbO2 coating. Especially, the lowest of the diffraction peak intensity of the coating is obtained with addition of TiO2 and CeO2.
3) The α-PbO2-3.72%TiO2-2.12%CeO2 electrode exhibits the best electrocatalytic activity for O2 evolution, and the Gugliemi model for CeO2 and TiO2 codeposition with α-PbO2 is also proposed.
References
[1] GUO He-tong, ZHANG San-yuan. Composite coating [M]. Tianjin: Tianjin University Press, 1991. (in Chinese)
[2] YEO I H, JOHNSON D C. Electrocatalysis of anodic oxygen-transfer reactions: Effect of groups III A and VA metal oxides in electrodeposited β-lead dioxide electrodes in acidic media [J]. Journal of Electrochemical Society, 1987, 134(8): 1973-1977.
[3] YEO I H, KIM S, JACOBSON R, JOHNSON D C. Electrocatalysis of anodic oxygen transfer reactions: Comparison of structural data with electrocatalytic phenomena for bismuth-doped lead dioxide [J]. Journal of Electrochemical Society, 1989, 136(5): 1395-1401.
[4] BERTONCELLO R, FURLANETTO F, GUERRIERO P, MUSIANI M. Electrodeposited composite electrode materials: Effect of the concentration of the electrocatalytic dispersed phase on the electrode activity [J]. Electrochimica Acta, 1999, 44(23): 4061-4068.
[5] VELICHENKO A B, KNYSH V A, LUK’YANENKO T V, VELICHENKO Y A, DEVILLIERS D. Electrodeposition PbO2-TiO2 and PbO2-ZrO2 and its physicochemical properties [J]. Materials Chemistry and Physics, 2012, 131(3): 686-693.
[6] ZHANG Xin, SUN Cen-sheng, LONG Min, ZHAO Bao-dong. Application of rare earth in plating of Cr(Ш), Cu and composite plating of Ni-P-SiC [J]. Journal of Rare Earth, 1996, 17(1): 42-45. (in Chinese)
[7] GUO Zhong-cheng. Preparation method of energy saving and inertanodic material for non-ferrous metals electrowinning: China, 200810058194. 5 [P]. 2008-03-15. (in Chinese)
[8] DEVILLIERS D, DINH-THI M T, MAHE E, XUAN Q L. Cr(III) oxidation with lead dioxide-based anodes [J]. Electrochimica Acta, 2003, 48(28): 4301-4309.
[9] FENG J, JOHNSON D C. Electrocatalysis of anodic oxygen-transfer reactions: Alpha-lead dioxide electrodeposited on stainless steel substrates [J]. Journal of Applied Electrochemistry, 1990, 20(1): 116-124.
[10] VELICHENKO A B, DEVILLIERS D. Electrodeposition of fluorine-doped lead dioxide [J]. Journal of Fluorine Chemistry, 2007, 128(4): 269-276.
[11] HYDE M E, JACOBS R M J, COMPTON R G. An AFM study of the correlation of lead dioxide electrocatalytic activity with observed morphology [J]. Journal of Physical Chemistry B, 2004, 108(20): 6381-6390.
[12] CHEN Bu-ming, GUO Zhong-cheng, YANG Xian-wan. Morphology of alpha-lead dioxide electrodeposited on aluminum substrate electrode [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 97-103.
[13] SURYANARAYANA V, NAKAZAWA I, YOSHIHARA S, SHIRAKASHI T. The influence of electrolyte media on the deposition/dissolution of lead dioxide on boron-doped diamond electrode—A surface morphologic study [J]. Journal of Electroanalytical Chemistry, 2006, 592(2): 175-182.
[14] BECK F. Cyclic behaviour of lead dioxide electrodes in tetrafluorborate solutions [J]. Journal of Electroanalytical Chemistry, 1975, 65(1): 231-243.
[15] MUSIANI M, FURLANETTO F, BERTONCELLO R. Electrodeposited PbO2+RuO2: A composite anode for oxygen evolution from sulphuric acid solution [J]. Journal of Electroanalytical Chemistry, 1999, 465(2): 160-167.
[16] CASELLATO U, CATTARIN S, GUERRIERO P, MUSIANI M. Anodic synthesis of oxide–matrix composites. Composition, morphology and structure of PbO2-matrix composites [J]. Chemistry of Materials, 1997, 9: 960-966.
α-PbO2-CeO2-TiO2复合电极材料的电合成及物化性能
陈步明1,郭忠诚1,2,徐瑞东1
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 昆明理工恒达科技有限公司,昆明 650105
摘 要:采用电化学阳极复合电沉积技术,将纳米TiO2和CeO2 颗粒加入氧化铅中并溶入4 mol/L NaOH溶液中至饱和,在铝基体上制备α-PbO2-CeO2-TiO2 复合镀层。利用循环伏安(CV)、扫描电镜(SEM)、能谱(EDAX)和X射线衍射(XRD)分析复合电极的沉积机理、表面形貌、成分及结构。结果表明:在酸、碱性镀液中,掺杂固体颗粒不会改变α-PbO2电极的反应机理,但能提高沉积速率和降低析氧电势。掺杂固体颗粒能抑制α-PbO2 晶胞的长大,增大镀层的比表面积,且掺杂固体颗粒的复合镀层的衍射峰强度比未掺杂的α-PbO2镀层的低很多。α-PbO2-2.12%CeO2-3.71%TiO2 复合电极材料的析氧催化活性最好。提出了两种颗粒的复合共沉积模型。
关键词:铝;二氧化铅;复合电极;电催化;物化性能
(Edited by Hua YANG)
Foundation item: Project (51004056) supported by the National Natural Science Foundation of China; Project (KKZ6201152009) supported by the Opening Foundation of Key Laboratory of Inorganic Coating Materials, Chinese Academy of Sciences; Project (2010ZC052) supported by the Applied Basic Research Foundation of Yunnan Province, China; Project (20125314110011) supported by the Specialized Research Fund for the Doctoral Program of Higher Education; Project (2010247) supported by Analysis & Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: Zhong-cheng GUO; Tel: +86-871-8352598; E-mail: guozhch@vip.163.com
DOI: 10.1016/S1003-6326(13)62583-2
Abstract: In order to investigate the effect of solid particles dopants on physicochemical properties of α-PbO2 electrodes, α-PbO2 composite electrodes doped with nano-TiO2 and nano-CeO2particles were respectively prepared on Al/conductive coating electrodes in 4 mol/L NaOH solution with addition of PbO until saturation by anodic codeposition. The electrodeposition mechanism, morphology, composition and structure of the composite electrodes were characterized by cyclic voltammogram (CV), SEM, EDAX and XRD. Results show that the doping solid particles can not change reaction mechanism of α-PbO2 electrode in alkaline or acid plating bath, but can improve deposition rate and reduce oxygen evolution potential. The doping solid particles can inhibit the growth of α-PbO2 unit cell and improve specific surface area. The diffraction peak intensity of α-PbO2-CeO2-TiO2 composite electrode is lower than that of pure α-PbO2 electrode. The electrocatalytic activity of α-PbO2-2.12%CeO2-3.71%TiO2 composite electrode is the best. The Guglielmi model for CeO2 and TiO2 codeposition with α-PbO2 is also proposed.


