Trans. Nonferrous Met. Soc. China 26(2016) 2495-2501
Reductive leaching of zinc and indium from industrial zinc ferrite particulates in sulphuric acid media
Fan ZHANG, Chang WEI, Zhi-gan DENG, Cun-xiong LI, Xing-bin LI, Min-ting LI
Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
Received 29 July 2015; accepted 2 May 2016
Abstract:
Zinc ferrite is the principal constituent in zinc neutral-leach residue (NLR) which is commonly treated by hot-acid leaching in electrolytic zinc plants. Reductive leaching of zinc ferrite with sphalerite concentrate as a reducing agent was performed. It was found that leaching of zinc ferrite in the presence of sphalerit concentrate was a viable process that effectively extracted zinc and indium and converted Fe3+ into Fe2+ at the same time. Reflux leaching tests by two stages were performed to achieve extractions of 98.1% for zinc and 97.5% for indium, and a Fe2+/Fe3+ molar ratio of 9.6 in leach solution was also obtained. The leaching behaviors of other elements, such as iron, copper and tin were also studied. The results showed that iron and copper were completely leached, whereas tin presented lower extraction values.
Key words:
reductive leaching; zinc ferrite; zinc; indium; sphalerite concentrate;
1 Introduction
Indium is an important metal used in electrical industries extensively [1,2]. It is mostly associated with zinc ores, and has therefore been recovered as a by-product of zinc metal processing operations. The modern extractive metallurgy of zinc is dominated by the roast-leach-electrowin (RLE) process. Sphalerite present in sulfide ores is the main mineral source for the zinc and indium production [3]. In sphalerite concentrates, iron is present as: 1) in iron sulphide minerals (pyrite FeS2, pyrrhotite FeS, chalcopyrite CuFeS2, etc.), and 2) a substitute of zinc in sphalerite (Zn, Fe)S. During oxidative roasting, nearly all the iron in the sphalerite concentrate are converted to zinc ferrite which is a stable composition [4,5]. Under the mild conditions of temperature and acidity employed in the neutral-leach stage, zinc ferrite remains almost inert. The neutral leaching residues (NLR), usually contain 25% of zinc, 30% of iron (mass fraction) as well as a few hundred parts per million of indium, which is an important resource for recovery of indium and zinc [6].
The Waelz kiln process is the traditional way to recover zinc, indium and lead from the NLR, and nearly 95% of zinc and 80% of indium can be recovered. It has obvious disadvantages: high consume of energy, causing air pollution, the resulting slag causing eco compatibility problems [7-9]. The hot acid leaching is another conventional method to recover valuable metals from the NLR [10]. By dissolution of the NLR, ferric ion is released. However, the high concentration of ferric ion decelerates the dissolution rate of zinc ferrite [11,12]. Also, it is difficult to separate and recover zinc and indium from acidic ferric sulfate solution.
Besides the two methods mentioned above, the reductive leaching is another effective method to extract zinc and indium from the NLR. Many researchers have noted that the reducing conditions in the leaching process could improve dissolution rates of zinc from zinc ferrite [13-15]. Various electrochemical studies also have confirmed it [16,17]. Furthermore, indium can be selectively extracted by direct solvent extraction after iron reduction [18,19]. The remove of iron from sulfuric solution by the reduction of ferric to ferrous, and then oxidation and precipitation of ferrous iron as hematite would solve the problems in jarosite process [20,21].
The leaching of sphalerite concentrate with ferric iron has been studied in acidic solution [22-25]. In the leaching process of sphalerite concentrate with ferric iron, zinc and iron are dissolved, and the ferric iron is simultaneously reduced to ferrous iron. The spalerite concentrate can be used as a reducing agent to reduce the ferric iron and then be leached at the same time. In this work, the reductive leaching behavior of zinc and indium from the NLR using sphalerite concentrate as a reducing agent has been investigated. The aim is to provide an effective method to recover zinc and indium from the NLR by reductive leaching.
2 Experimental
2.1 Materials
The NLR and sphalerite concentrate were received from Yunnan Province of China. The particle size analysis of the used NLR and sphalerite concentrate is given in Table 1. The chemical compositions of the two materials are listed in Table 2.
Table 1 Particle size distribution of NLR and sphalerite concentrate
X-ray diffraction of the NLR indentified zinc ferrite (ZnFe2O4) and zinc silicate (ZnSiO3) as the main mineral components in the residue (Fig. 1(a)), and sphalerite (ZnS), christophite ((Zn,Fe)S) and pyrrhotite (FeS) as the main mineral components in the sphalerite concentrate (Fig. 1(b)).
2.2 Methods
A five-necked, round-bottomed flask (2 L) was fitted with a mechanical stirrer, a sample collection, a pH/Eh meter and two condenser tubes were used as the leaching reactor. The flask was then immersed in a water bath and kept at the selected temperature with accuraay of ±1.0 °C. 1.14 L of leaching reactant was placed in the flask and heated to the desired temperature while being magnetically stirred (400 r/min). 100 g of the NLR and the required amount of sphalerite concentrate was then added to the reactor. In the preliminary investigations, 5 mL of the solution was withdrawn from the flask to determine the dissolved contents of zinc, indium, iron and the residual H2SO4 concentration. In other experiments, the dissolved contents were calculated from the solid chemical analysis.
Table 2 Chemical compositions of NLR and sphalerite concentrate
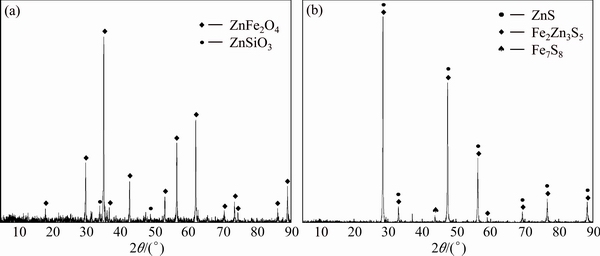
Fig. 1 XRD patterns of NLR (a) and sphalerite concentrate (b)
Zinc was analyzed by complex titration with EDTA. The concentrations of ferrous ion were analyzed by complex titration with potassium bichromate. The concentration of ferric ion was determined by finding the difference between overall iron and ferrous ion concentrations. Indium, copper and tin concentrations were determined by ICP with mass spectrometric detection (Agilent 7900, USA). X-ray powder diffraction was carried out using Rigaku D/MAX 2500v diffractometer (Japan). The redox potential of solution was measured by a platinum electrode and an Ag/AgCl electrode used as the reference electrode.
3 Results and discussion
3.1 Preliminary investigation and proposed mechanism
3.1.1 Leaching without reducing agent
Direct leaching of the NLR was performed at 160 g/L H2SO4, 90 °C and 5 h. As shown in Fig. 2, the extractions of zinc, iron and indium increased along with reaction time. The extractions were 88.8% for zinc, 78.6% for iron and 81.4% for indium after 5 h. It was also apparent from Fig. 2 that iron and indium displayed similar behavior under these conditions. That may be caused by indium present in the crystal lattice of zinc ferrite through substituted iron to form indium-bearing zinc ferrite during the roasting process [26].
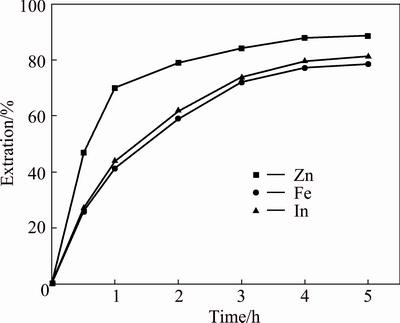
Fig. 2 Leaching of NLR in absence of sphalerite concentrate
The leach residue without a reducing agent was checked by XRD. As shown in Fig. 3, the main composition was zinc ferrite (ZnFe2O4). It was shown clearly that the NLR was only partially leached in the leach process without a reducing agent; this might be attributed to the high Fe3+ concentrations in the leach solution which decelerated the dissolution of zinc ferrite.
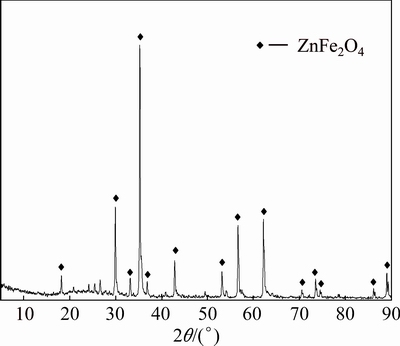
Fig. 3 XRD pattern of leach residue without reducing agent
3.1.2 Leaching with sphalerite concentrate as reducing agent
As the results mentioned above, the acid dissolution of zinc ferrite can be improved under reductive leaching conditions. Thus, reduction in solution is suggested here to promote the leaching of zinc ferrite. Sphalerite concentrate is considered the most suitable reducing agent for this purpose since no foreign ions will be introduced to the reaction system. Leaching experiments were carried out in the same conditions as the previous test (160 g/L H2SO4, 90 °C and 5 h) but with the addition of sphalerite concentrate, as 0.95 times of stoichiometric amount ( corresponding to the content of Fe in the NLR ). This was revealed by observing that no hydrogen sulphide was generated. In the presence of sphalerite concentrate, the extractions of zinc, iron and indium were shown in Fig. 4. It was clear that the extractions of zinc, iron and indium were markedly increased by adding sphalerite concentrate, compared with the extractions without sphalerite concentrate. The extractions were 95.4% for zinc, 93.8% for iron and 95.0% for indium after 5 h in the presence of sphalerite concentrate. Figure 5 presented the concentration curve for total iron and ferric ion in the presence of sphalerite concentrate. The concentration of total iron increased with leaching time. The concentration of ferric ion increased at first, reached a peak value of about 5.8 g/L after 1 h and then started to decrease to a small value of about 3.6 g/L. The variation of Fe3+ concentration with leaching time indicated that the kinetic of dissolution of the NLR was faster at the beginning and then slower than the kinetic of reduction of Fe3+.
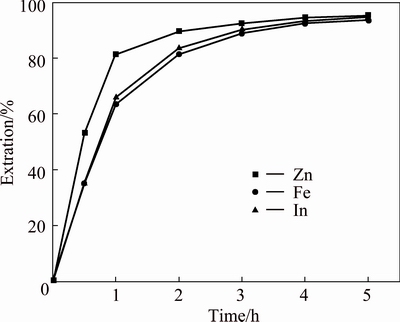
Fig. 4 Leaching of NLR in the presence of sphalerite concentrate
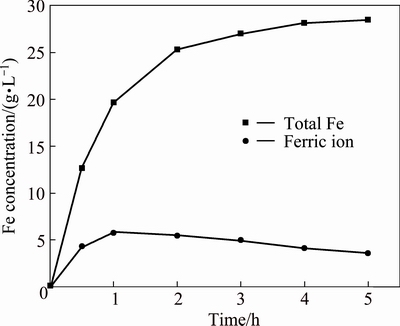
Fig. 5 Variation of Fe concentration during leaching of NLR at different time with addition of sphalerite concentrate
The leach residue in the presence of sphalerite concentrate was examined by XRD. As shown in Fig. 6, the main compositions of the leach residue were sulphur (S0), sphalerite (ZnS), christophite ((Zn,Fe)S) and pyrrhotite (FeS). Almost all the zinc ferrite was dissolved in the reductive leaching process.
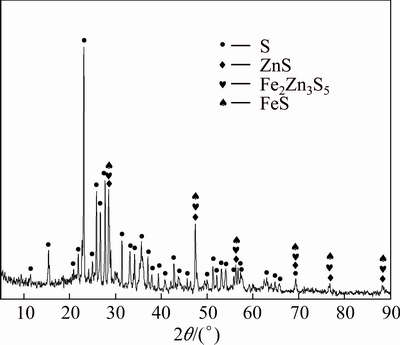
Fig. 6 XRD pattern of reductive leaching residue in the presence of sphalerite concentrate
Both residual H2SO4 concentrations were determined and shown in Fig. 7. In the absence of sphalerite concentrate after reaction for 5 h, the acid concentration continuously decreased from 160 g/L to 54.7 g/L. In the presence of sphalerite concentrate after 5 h, the acid concentration decreased from 160 to 39.7 g/L after 3 h and then slowly decreased to 37.4 g/L.
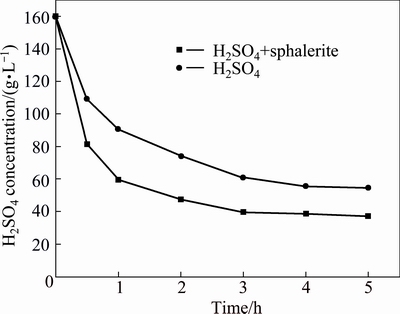
Fig. 7 Variation of residual H2SO4 concentration in leachate
The fall in acid concentration with the added sphalerite concentrate was more acute than that without sphalerite concentrate addition. That might be caused by faster reactions of acid with the NLR in the presence of sphalerite concentrate than in the absence of sphalerite concentrate.
The redox potentials (φ) of solution were detected during leaching process, and the results are shown in Fig. 8. In the absence of sphalerite concentrate, the φ of solution increased with increasing retention time. On the contrary, the φ of solution decreased with increasing retention time in the presence of sphalerite concentrate. The presence of Fe2+ and Fe3+ directly affect the φ of solution. In the absence of other reducing agents, Fe2+ becomes the ion determining the φ of solution. The lower the solution potential is, due to converting Fe3+ into Fe2+ (see Fig. 8), the faster the dissolution kinetics of zinc ferrite is.
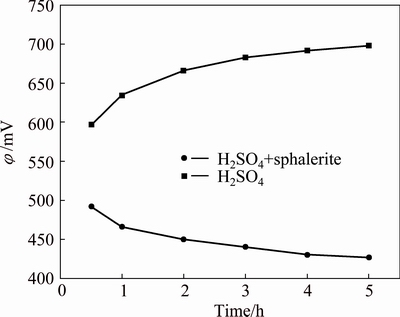
Fig. 8 Variation of φ of solution with time during leaching
3.2 Optimisation of leaching conditions
The leaching conditions, sphalerite concentrate stoichiometry, H2SO4 concentration and temperature and reaction time, were studied to obtain higher metal extractions from solid samples and lower ferric ion concentration in leach solution.
3.2.1 Effect of sphalerite concentrate stoichiometry
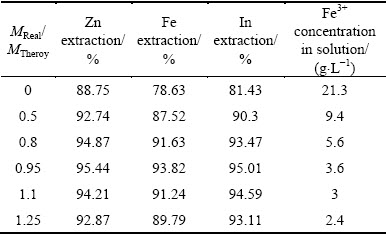
The effect of the stoichiometric amount of sphalerite concentrate added was studied in the range from 0.0 to 1.25 at 160 g/L H2SO4, 5 h at 90 °C. The results are shown in Table 3. It can be seen from Table 3 that the metal extractions increased at first and then decreased with the growing amount of sphalerite concentrate. That may be due to an increase in sulfide content, which is insoluble in acid solution without ferric ion. The concentration of Fe3+ decreased when increasing the amount of sphalerite concentrate, as shown in Table 3. The beneficial effect of increasing amount of sphalerite concentrate is related to the decreasing amount of Fe3+ in solution.
Table 3 Effect of amount of spahlerite concentrate on extraction of Zn, Fe, In and Fe3+ concentration in solution
3.2.2 Effect of H2SO4 concentration
A series of experiments were carried out with varying H2SO4 concentration from 100 to 180 g/L and other parameters were fixed such as the amount of sphalerite concentrate 0.95, the reaction time 5 h and temperature 90 °C. The results in Fig. 9 revealed that the leaching curve may be divided into two stages. In the first stage, when the acid concentration is between 100 to 160 g/L, the extractions of zinc, iron, and indium increased significantly with the increasing in sulfuric acid concentration. But the extractions of metal were only slightly improved when the sulfuric acid concentration was over 160 g/L.
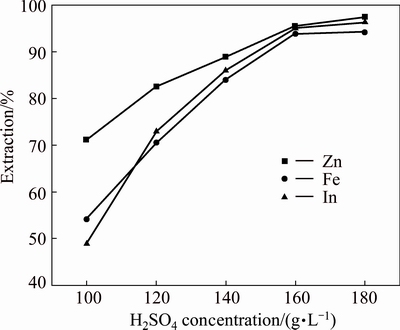
Fig. 9 Effect of H2SO4 concentration on extractions of Zn, Fe and In
3.2.3 Effect of temperature
Figure 10 presents the results of experiments carried out with varying the temperature from 60 to 90 °C while the other conditions were fixed, the amount of sphalerite concentrate 0.95, H2SO4 160 g/L and reaction time 5 h. The results indicated that the extractions of zinc, iron and indium increased when increasing the temperature. The temperatures have significant effects on the recovery of metals.
3.2.4 Effect of retention time
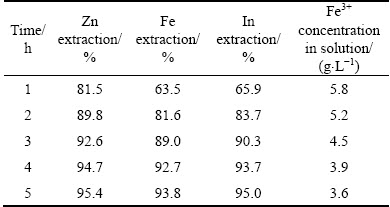
Leaching experiments were carried out for different retention time from 1 to 5 h at the amount of sphalerite concentrate 0.95, H2SO4 160 g/L and 90 °C. It can be seen from Table 4 that increasing retention time can increase the extractions of zinc, iron and indium efficiencies during the first 4 h and the extractions reached 94.7%, 92.7% and 93.7%, for zinc, iron and indium, respectively. But the leaching rate of metals increased slowly when the retention time was over 4 h. The concentration of Fe3+ decreased with increasing retention time. Thus, the selected optimum retention time was 4 h, corresponding to 3.9 g/L Fe3+ in solution.
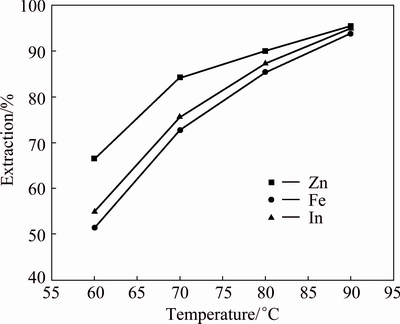
Fig. 10 Effect of temperature on extractions of Zn, Fe and In
Table 4 Effect of retention time on extractions of Zn, Fe, In and Fe3+ concentration in solution
3.3 Reflux leaching
The application of reflux leaching stages (Fig. 11), which is frequently used in the industry, allows obtaining good yields and decreasing the amount of acid. This strategy was studied in this work and Table 5 presented the experimental conditions used, whereas Fig. 12 showed some of the obtained results.
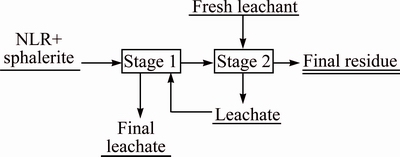
Fig. 11 Scheme of reflux leach process
Table 5 Experimental conditions for two stages leach tests

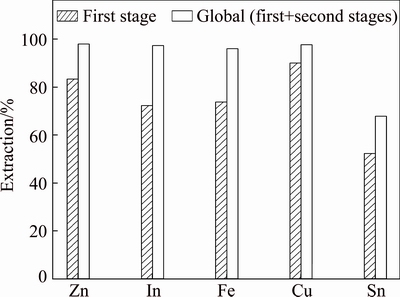
Fig. 12 Extractions of metal by reflux leach process
It can be seen that zinc, iron, indium and copper were almost leached, whereas tin presented lower extraction values. The total recoveries of zinc and indium after reflux leach process were 98.1% and 97.5%, respectively.
The final leach residue and leachate were chemically analyzed, and the results were presented in Tables 6 and 7. It can be seen in Table 6 that the final leach residue became a silver- and sulfur-rich material. The compositions of the final leachate presented in Table 7 shows that the concentration of Fe3+ was 2.8 g/L and the Fe2+/Fe3+ molar ratio was 9.6, which is suitable for further treatment. Indium in the leachate can be recovered efficiently by solvent extraction after Fe3+ reduction [19].
Table 6 Chemical compositions of final leaching residue (mass fraction, %)
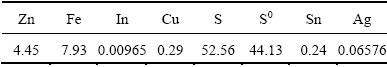
Table 7 Chemical compositions of final leachate (g/L)

4 Conclusions
1) The leaching of the NLR without and with addition of sphalerite concentrate as a reducing agent in sulfuric acid solution was investigated. The results obtained in this work showed that, with the addition of sphalerite concentrate, zinc and indium extractions were considerably enhanced. The XRD analyses showed that zinc ferrite was almost dissolved in reductive leaching.
2) Two stages reflux leaching tests were performed to achieve 98.1% of zinc and 97.5% of indium, respectively, and a Fe2+/Fe3+ molar ratio of 9.6 in the final leachate was also obtained. The reductive leaching of the NLR in the presence of sphalerite concentrate as a reducing agent was a viable process that effectively extracted zinc and indium and converted Fe3+ into Fe2+ at the same time.
References
[1] Hsieh S L, Chen C C, Say W C. Process for recovery of indium from ITO scraps and metallurgical microstructures [J]. Materials Science and Engineering B, 2009, 158(1-3): 82-87.
[2] Biswas N, Ghosh P, Sarkar S, Moitra D, Biswas P K, Jana S, Mukhopadhyay A K. Nanomechanical properties of dip coated indium tin oxide films on glass [J]. Tin Solid Films, 2015, 579: 21-29.
[3] Alfantazi A M, Moskalyk R R. Processing of indium: A review [J]. Minerals Engineering, 2003, 16(8): 687-694.
[4] Graydon J W, Kirk D W. The mechanism of ferrite formation from iron sulfides during zinc roasting [J]. Metallurgical Transaction B, 1988, 19(5): 777-785.
[5] Boyanov B S, Sandalski M P, Ivanov K I. Zinc sulfide concentrates and optimization of their roasting in fluidized bed reactor [J]. Word Academy of Science, Engineering and Technology, 2011, 5: 257-263.
[6] James S E, Watson J L, Peter J. Zinc production—A survey of existing smelters and refineries [C]//Lead-Zinc. Hoboken: John Wiley & Sons, 2000: 205-226.
[7] Menad N, Ayala J N, Garcia C F, Ruiz A E, Hernández A. Study of the presence of fluorine in the recycled fraction during carbothermal treatment of EAF dust [J]. Wast Management, 2003, 23(6): 483-491.
[8] HUANG Pao-chen, Chi K H, Chen M L, Chang M B. Characteristics of dioxin emissions from a Waelz plant with acid and basic kiln mode [J]. Journal of hazardous materials, 2012, 201-202: 229-235.
[9] Mombelli D, Mapelli C, Barella S, Gruttadauria A, Di L U. Laboratory investigation of Waelz slag stabilization [J]. Process Safety and Environmental Protection, 2015, 94: 227-238.
[10] Nii K, Hisamatsu Y. The dissolution mechanism of zinc ferrite [J]. Trans Nat Res Inst Metals, 1996, 8: 183-192.
[11] Filippou D, Demopoulos G P. A reaction kinetic model for the leaching of industrial zinc ferrite particulates in sulphuric acid media [J]. Canadian Metallurgical Quarterly, 1992, 31(1): 41-54.
[12] Ramachandra S V N, Kapil D, Biswas A K. Dissolution of zinc ferrite samples in acids [J]. Hydrometallurgy, 1976, 2(2): 171-184.
[13] Nii K, Hisamatsu Y. The promotion of acid dissolution of zinc ferrite [J]. Trans Nat Res Inst Metals, 1966, 8: 193-199.
[14] WU Xue-lan, WU Shun-ke, QIN Wen-qing, MA Xi-hong, NIU Yin-jian, LAI Shao-shi, YANG Cong-ren, JIAO Fen, REN Liu-yi. Reductive leaching of gallium from zinc residue [J]. Hydrometallurgy, 2012, 113-114: 195-199.
[15] ZHANG Chun, MIN Xiao-bo, ZHANG Jian-qiang, WANG Mi, ZHOU Bo-sheng, SHEN Chen. Reductive acid leaching of cadmium from zinc neutral leaching residue using hydrazine sulfate [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4175-4182.
[16] LU Zheng-ya, Muir D M. Dissolution of metal ferrites and iron oxides by HCl under oxidizing and reducing conditions [J]. Hydrometallurgy, 1988, 21(1): 9-21.
[17] Bhat K L. Natarajan K A, Ramachandran T. Electroleaching of zinc leach residues [J]. Hydrometallurgy, 1987(3), 18: 287-303.
[18] Fortes M C B, Benedetto J S. Separation of indium and iron by solvent extraction [J]. Minerals Engineering, 1998, 11(5): 447-451.
[19] LI Xing-bin, DENG Zhi-gan, LI Cun-xiong, WEI Chang, LI Min-ting, FAN Gang, RONG Hao. Direct solvent extraction of indium from a zinc residue reductive leach solution by D2EHPA [J]. Hydromatellurgy, 2015, 156: 1-5.
[20] Riveros P A, Dutrizac J E. The precipitation of hematite from ferric chloride media [J]. Hydrometallurgy, 1997, 46: 85-104.
[21] YANG Fan, DENG Zhi-gan, WEI Chang, LI Cun-xiong, LI Xing-bin. Iron-removal by hematite form leaching liquor of high iron sphalerite [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2392. (in Chinese)
[22] Dutrizac J E, MacDonald R J C. The dissolution of sphalerite in ferric chloride solutions [J]. Metallurgical Transaction B, 1978, 9(4): 543-551.
[23] Bobeck G E, Sue H. The kinetics of dissolution of sphalerite in ferric chloride solution [J]. Metallurgical Transaction B, 1985, 16(3): 413-424.
[24] Aydogan S, Aras A, Canbazoglu M. Dissolution kinetics of sphalerite in acidic ferric chloride leaching [J]. Chemical Engineering Journal, 2005, 114(1-3): 67-72.
[25] Heidi M, Sigmund F, Daniel V, Tapio S, Dmitry Y M, Marko L. Reduction of ferric to ferrous with sphalerite concentrate, kinetic modeling [J]. Hydrometallurgy, 2004, 73(3-4): 269-282.
[26] Rao B P, Rao K H. Distribution of In3+ ions in indium-substituted Ni-Zn-Ti ferrites [J]. Journal of Magnetism and Magnetic Materials, 2005, 292: 44-48.
湿法炼锌渣中锌和铟的还原浸出
张 帆,魏 昶,邓志敢,李存兄,李兴彬,李旻廷
昆明理工大学 冶金与能源工程学院,昆明 650093
摘 要:铁酸锌是锌中性浸出渣中的主要物相,热酸浸出是处理中性浸出渣的主要方法之一。研究了一种采用硫化锌精矿作为还原剂对锌中性浸出渣进行还原浸出的方法。研究发现,采用硫化锌精矿作为还原剂不仅能高效浸出锌中性浸出渣中的有价金属,而且同时实现溶液中Fe3+向Fe2+的还原。采用两段逆流浸出工艺,98.1%锌和97.5%铟被浸出,浸出液中Fe2+/Fe3+的摩尔比达到9.6。同时发现,浸出过程中铁和铜几乎完全浸出,而锡只有部分浸出。
关键词:还原浸出;铁酸锌;锌;铟;硫化锌精矿
(Edited by Yun-bin HE)
Foundation item: Project (2014CB643404) supported by the National Basic Research Program of China; Projects (51564030, 51474117, 51304093, 51364022) supported by the National Natural Science Foundation of China; Project (0120150070) supported by Yunnan Applied Basic Reach Project, China; Project (ZD2014003) supported by the Education Department of Yunnan Province, China
Corresponding author: Chang WEI; Tel/Fax: +86-871-65188819; E-mail: weichang502@sina.cn
DOI: 10.1016/S1003-6326(16)64342-X
Abstract: Zinc ferrite is the principal constituent in zinc neutral-leach residue (NLR) which is commonly treated by hot-acid leaching in electrolytic zinc plants. Reductive leaching of zinc ferrite with sphalerite concentrate as a reducing agent was performed. It was found that leaching of zinc ferrite in the presence of sphalerit concentrate was a viable process that effectively extracted zinc and indium and converted Fe3+ into Fe2+ at the same time. Reflux leaching tests by two stages were performed to achieve extractions of 98.1% for zinc and 97.5% for indium, and a Fe2+/Fe3+ molar ratio of 9.6 in leach solution was also obtained. The leaching behaviors of other elements, such as iron, copper and tin were also studied. The results showed that iron and copper were completely leached, whereas tin presented lower extraction values.


