
J. Cent. South Univ. (2021) 28: 72-88
DOI: https://doi.org/10.1007/s11771-021-4587-z
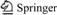
Separation and purification of tantalum from plumbomicrolite of amazonite deposit in Kola Peninsula by acid leaching and solvent extraction
S M MASLOBOEVA, L G ARUTYUNYAN, M N PALATNIKOV, D V MANUKOVSKAYA
Tananaev Institute of Chemistry – Subdivision of the Federal Research Centre,
Kola Science Centre of the Russian Academy of Sciences, Apatity 184209, Russia
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
A plumbomicrolite concentrate (PMC) was leached with the mixture of HF and H2SO4, HF and HNO3 acids, respectively. Optimal conditions ensuring high recovery of tantalum and niobium (up to 99%) into solution, and radionuclides into insoluble residue were determined. Fluoride-sulfuric acid and fluoride-nitric acid schemes were proposed for PMC leaching by an extractive separation of tantalum form niobium, lead and impurities, and production of high-purity tantalum compounds. Octanol-1 was used as an extractant. Optimal conditions for production of high-purity tantalum strip solutions were defined for all stages (extraction-scrubbing-stripping). Produced tantalum compounds, such as tantalum pentoxide and potassium heptafluotanthalate, comply with the norms for high-purity substances in terms of impurities content. Final choice of the PMC processing scheme is determined by its profitability.
Key words:
mineral plumbomicrolite; tantalum; niobium; acid leaching; radioactivity; solvent extraction;
Cite this article as:
S M MASLOBOEVA, L G ARUTYUNYAN, M N PALATNIKOV, D V MANUKOVSKAYA. Separation and purification of tantalum from plumbomicrolite of amazonite deposit in Kola Peninsula by acid leaching and solvent extraction [J]. Journal of Central South University, 2021, 28(1): 72-88.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4587-z1 Introduction
Tantalo-niobium ores are raw materials for production of tantalum and niobium, which are widely used as valuable components in many alloys, metallurgy, nuclear power, chemical industry, and also as components of functional materials in optics, electronics and electrical engineering. With development of high-tech industries, nanoindustry, future energy projects are impossible without rare metals. It is a well-known fact that the world reserves of tantalum-niobium ores are concentrated, mainly, in Africa, South and North Americas [1].
Tantalum belongs to the category of strategic materials. It is one of the less common elements, and its percentage abundance in the upper continental crust is 2.2×10-6 [2]. Today, we know about 100 tantalum and tantalum-containing minerals, most of which are attributed to a subclass of complex oxides, and only 20 minerals are used for commercial production of tantalum [1,3].
Explored and estimated tantalum deposits in Russia vary considerably in terms of scale, tantalum content in ores, and component composition. Balance deposits are traditionally divided into three groups by their quantitative ratio of tantalum and niobium, and specific fraction of tantalum in component composition of ores: 1) tantalum ores with the ratio of Nb2O5/Ta2O5≤4; 2) niobium-tantalum ores with the ratio of Nb2O5/Ta2O5 from 5 to 20; 3) niobium, titanium and other deposits containing tantalum as an associated component with the ratio of Nb2O5/Ta2O5>20.
World prices for tantalum remain high. Therefore, development of even small deposits with sufficiently high tantalum content can be profitable.
Kola Peninsula has the following tantalum and niobium deposits, and prospective areas for their development: 1) Lovozero (a unique complex niobium-tantalum and lithium-tantalum deposit); 2) Voronya tundra and Kolmozero (perspective for development); 3) Neske-Vara, Vuariyarvi and Polmos tundra deposits (complex niobium-tantalum and lithium-tantalum deposits with inactive reserves).
The amazonite deposit Ploskaya Gora is located in the central part of Kola Peninsula. Amazonite-free blocks of the deposit contain a number of rare metals tantalum and niobium minerals: plumbomicrolite Pb1.8(Ta,Nb)2O6(F,OH), plumbopyrochlore Pb1.8(Nb,Ta)2O6(F,OH), and manganocolumbite MnNb2O6. These minerals can be recovered by beneficiation methods during the deposit development. The results on the amazonite deposit mineralization studies on the Ploskaya Gora are presented in Refs. [4-6]. All minerals usually contain elevated concentrations of natural radionuclides: U-238 and Th-232 series. Scientists worldwide are interested in Ta and Nb minerals, and new minerals are still discovered in the deposit Ploskaya Gora [7] and other deposits [8]. Some recent works also demonstrate interest in Ta- and Nb- containing ores of Kola Peninsula [9].
The rare metal mineral plumbomicrolite Pb1.8(Ta,Nb)2O6(F,OH) (PMC) contains high amount of lead and low amount of calcium along with the target components tantalum and niobium. General formula of PMC is А2В2О7. The A group obviously corresponds to lead; the B group is apart from tantalum, niobium and titanium, also contains tin, iron and antimony because crystal-chemical properties of these elements are close to tantalum ones. According to the literature data, different PMC samples contain 47.4wt%- 55.2wt% PbO, 27.0wt%-31.3wt% Ta2O5, 10.9wt%- 14.2wt% Nb2O5, 2.6wt%-3.5wt% SnO2, 1.2wt%- 1.7wt% Fe2O3, 0.3wt%-2.1wt% TiO2, 0- 1.5wt% CaO. This mineral has a cubic syngony of the pyrochlore structure under a highly distorting influence of lead. Samples density is 6700- 8200 kg/m3, and micro-hardness at the load of 40 g is 5.98-6.57 GPa [4, 5]. There is no known processing experience worldwide concerning the raw materials described above.
The most perspective way to process rare metal raw material in complex is hydrometallurgy [10-13]. This method allows us to achieve high recovery of elements, select metals with close properties (for example, Nb and Ta), reduce energy consumption and environmental pollution by hazardous waste. The chemical behavior of tantalum and niobium is characterized by an extreme tendency to hydrolysis, hydrolytic polymerization and complexation. This is why their aqueous solutions, especially nitric acid ones, are very unstable. The latter complicates the possibility of applying of extraction methods for separation of niobium and tantalum. Columbite-tantalite concentrates are known to be decomposed with 30%-70% hydrofluoric acid and its mixture with sulfuric acid [14, 15]. However, recovery of tantalum and niobium into the solution was relatively low (66%-81%) and achieves higher values (96.3%-99.9%) only when the mixture of acids contains up to 400 g/L H2SO4 [13, 14]. During acid leaching of columbite concentrate with 50% HF, the degree of tantalum and niobium recovery to the solution is 68.6% and 55.8%, respectively [16]. At the same time, acid leaching with a mixture of acids HF and HNO3 leads to almost complete recovery (98%) with a simultaneous separation of uranium and thorium to insoluble precipitate. Thus, it is appropriate to decompose tantalum-niobium raw material, for example, PMC, with a mixture of sulfuric or nitric acid with hydrofluoric acid.
The extraction is one of the most optimal techniques for production of high-purity tantalum and niobium compounds. It is flexible (almost all elements could be extracted in almost all concentrations), effective (in terms of extraction power ability and the degree of multicomponent mixtures separation) and simple (experiments can be easily conducted).
It is widely known that a choice of an industrial extractant is determined by a compromise between numerous, often contradictory requirements [12]. The most important things among them are extraction power ability, reactivity, selectivity, interfacial tension, density, toxicity and cost. The aggregates of these requirements are largely satisfied in methyl isobutyl ketone (MIBK), tributyl phosphate (TBP), triisoamyl phosphate (TIAP), cyclohexanone (CHN) and octanols. These extractants are widely used in the industry during producing of tantalum and niobium [12, 17, 18]. Though, it should be noted that MIBK and CHN are explosive and well soluble in aqueous solutions. TBP is hydrolyzed in acidic solutions to form mono- and dibutyl phosphoric acids and pollutes products with phosphorus. TIAP [19, 20] has a number of advantages compared to TBP, such as low solubility in water, better compatibility with aromatic hydrocarbons, low rate of hydrolysis and radiolysis. Owing to these, TIAP demonstrates high extraction ability to rare metals. As a general drawback of CHN, TBP and TIAP, it is the fact that stripping is only effective with ammonium fluoride (60%-80% of tantalum during one stage).
Today octanols (OСL) of various structures are widely used for extraction of tantalum and niobium [16-18, 21-28]. OCL ultimate capacity is found to be lower in comparison with other extractants (CHN, TBP, TIAP), but OCL has a higher resistivity, and stripping with water should be more effective. Moreover, OCL is the cheapest extractant, and it has a low solubility in water and even lower in salts solutions. Thus, we believe that use of OCL is the most perspective extractant for treatment of PMC leaching solutions. With development of processing, determination and extraction method for Nb- and Ta-containing minerals is still a relevant task [29-31]. Extraction of radioactive and rare-earth elements from Nb- and Ta-containing raw material is also a problem being solved worldwide [31-33]. Earlier we have outlined ways to solve a problem of PMC leaching and have shown a possibility to obtain products based on tantalum, niobium and lead from PMC [34]. Besides, it should be noted that processing profitability of PMC from the amazonite deposit Ploskaya Gora can increase significantly due to parallel development of a valuable ornamental stone inclusions, i.e., amazonite.
This work is devoted to the development and analysis of various technological schemes for the integrated processing of PMC and choice of the optimal way for obtaining commercial products. For this purpose, it is necessary to solve the following tasks:
1) Study conditions of PMC leaching with mixtures of acids HF and H2SO4, HF and HNO3.
2) Study conditions for extraction of tantalum compounds with octanol from solutions obtained during the leaching of PMC. Obtain tantalum- containing products as tantalum hydroxide (pentoxide) and potassium heptafluorotantalate from strip solutions and evaluate its purity.
3) Study distribution of radionuclides in processing schemes.
2 Materials and methods
Samples used in this work were selected during manual separation of large pockety segregations in the open pit during amazonite mining. The samples consisted of a lump material (100-300 g) from gray to black. Despite external differences, content of Nb(V) and Ta(V) and ratio between them were approximately the same. Table 1 demonstrates composition of the PMC samples determined by X-ray fluorescence. The analysis was carried out on Spektroskan (NPO Spektrotron, Russia). The measurement error is ±10%. Specific radioactivity of natural radionuclides of uranium series (U-238 or Ra-226), thorium series (Th-232) and K-40 in PMC was determined by gamma-spectrometry on gamma- spectrometer “Progress” (SPC Doza, Russia) with a scintillation sensor NaJ/Tl of 63 mm×63 mm.
Most probes were samples of 0.5-15 g. This is why they were analyzed in the geometry “Petri dish” with a diameter of 30 and 80 mm, respectively. The volume of liquid phase (filtrates and scrubbing solutions) was 50-900 mL. Petri dishes and Marinelli vessels 1 L were used for preparation of liquid samples.
Table 1 Composition of PMC samples
Effective specific activity (Аeff, Bq/kg) was calculated as follows:
Аeff=ARa+1.3ATh+0.09AK
where ARa and ATh are the specific activities of Ra-226 and Th-232 that are equilibrated with other member of uranium and thorium series, respectively; AK is the specific activity of K-40, Bq/kg.
Average values of measurement uncertainty were detected to depend on radionuclides concentration, measurement geometry and exposition during gamma-spectrometry. They are 12%-15% for 40K, 14%-25% for 226Ra, 12%-17% for 232Th at a confidence interval of 0.95. The error at determination of specific activity was not more than 10% at a confidence interval of 0.95, which meets the requirements of radiation monitoring.
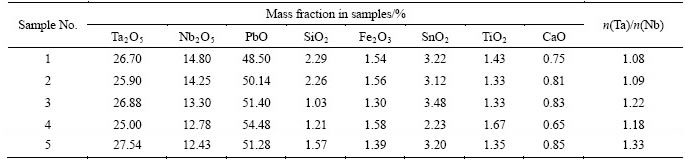
A gamma-spectrometric analysis revealed that the samples contain natural radionuclides of U-238 (0.25 wt%-0.60 wt%) and Th-232 (0.04 wt%- 0.07 wt%) series depending on the mineral and grain-size distribution with the general Th/U ratio of 0.2. Contribution of Ra-226 and U-238 to the effective specific activity is more than 90%; contribution of Th-232 is about 10%. Thus, the PMC radioactivity is mostly of U-Ra character. It is apparently associated with accessory uraninite and thorite [4]. Ratio between α-activity and β-activity is 3.6-6.4 depending on concentrate fraction. The research has revealed that average values of specific activity (Aeff) of initial PMC are in the range of 20000-70000 Bq/kg, and it depends on granular and mineral composition of the exact sample. Therefore, PMC is a material with increased content of natural radionuclides. The analyses were carried out on concentrated samples produced by elutriation after milling of the ore in a ball mill. Table 2 shows grain-size distribution of samples.
PMC was leached using acids: НF (40%-45.0%), H2SO4 (92.0%-95.6%) (“Ekos-1”, Russia); HNO3 (63%-65%) (Vekton, Russia). Hydrofluoric acid was added in sufficient amount for formation of fluoride complexes of niobium (V), tantalum (V) and titanium (IV), and transfer of impurities to insoluble fluorides. Stoichiometric calculations (the smallest necessary amount of moles) for acid consumption were carried out according to chemical equations, which take into account the numerical ratio between the amounts of reactants in accordance with the stoichiometry laws. We have used excess of acid towards minimal stoichiometry (120%-200% of stoichiometry), and the exact excess was established empirically. Sulfuric or nitric acids were added for binding of lead (II) by anions SO42-, NO3-. The analyses were carried out by varying acid consumption, the S:VL (mass of solid to volume of liquid) ratio and temperature.
Table 2 PMC grain-size distribution
Mixture of concentrated acids (HF and H2SO4 or HF and HNO3) was poured into a fluoroplastic beaker with lid and stirrer, and heated in a water bath to 40 °C. After that a ground concentrate (50, 100, 200 g) was added in small portions (5g). The resulting suspension was heated to a temperature predetermined in the researched range and soaked for 2.5 to 6 h at constant mixing.
Research of extraction of tantalum and its separation from niobium and other impurities was carried out in a PTFE beaker with a stirrer. Organic and aqueous phases were separated in a polypropylene separating funnel. Extractant was OCL-1 (≥99%, Panreac Sintesis). For extraction of tantalum compounds were used: HF (45%), HNO3 (70%) (Vekton, Russia), H2SO4 (93%), aqueous solution of a 25% NH4OH (EKOS-1, Russia), KCl (Komponent-Reaktiv, Russia).
Ta, Nb, Pb, Fe, Ti and other elements contents were determined using emission spectrometer ICPS-9000 Shimadzu (Japan) with a preliminary distillation of fluorine by evaporation of an aliquot of the sample from H2SO4 to thick vapors. The measurement error of this method is ±10%. Elements content was also determined by ICP-MS equipped with a dynamic reaction cell Elan 9000 KRC-e (PerkinElmer, USA) and a system of a laser sampling UR 266 MACRk (New Wave Research, UK) without a preliminary distillation of fluorine. Due to particularities of sample preparation an error of this method also was ±10%. Fluorine in solutions was determined by potentiometry on the ionomer EV-74 (Gomel Plant of Measuring Devices, Belarus) with an F-selective electrode due to a method based on measurement of fluoride ions against the background of citrate buffer solution with рН=6±0.5, and by pyrohydrolysis in a solid phase. Purity of final products of schemes (tantalum pentoxide and potassium heptafluorotantalate) was determined using atomic emission spectrographic analysis provided by a spectrometer DFS-13 (Leningrad enterprise LOMO, USSR). IR spectra were obtained by a FTIR spectrometer Nicolet 6700 (Thermo Scientific, USA).
3 Results
3.1 PMC leaching
3.1.1 Leaching with fluoride-sulfuric acids
Figures 1 and 2 demonstrate the influence of sulfuric and hydrofluoric acids consumption, grinding degree and processing time on PMC leaching. Sample 2 (fraction of <0.1 mm, processing time of 3 h at 95 °C) was used as an example. Fractions were separated by a sieve analysis for results in Figure 2. Decomposition degree was determined according to the tantalum content in a washed insoluble residue. Kinetic characteristics of PMC fractions are provided for leaching by sulfuric and hydrofluoric acids with consumption of 120% of a stoichiometry. The study revealed that if we increase the consumption of sulfuric acid above a stoichiometry to bind Pb(II) and Sn (IV), it will have little effect on the PMC decomposition degree. At the same time, the stoichiometric amount of hydrofluoric acid is not enough because accessory impurities contain strong fluorine acceptors, such as Si (IV), Al (III) that bind fluoride ion.
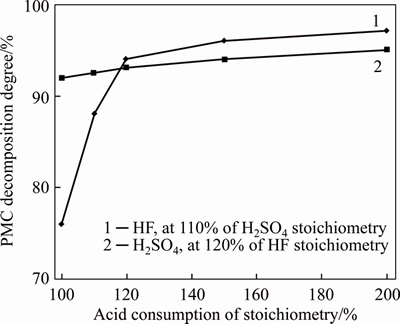
Figure 1 Influence of sulfuric and hydrofluoric acids consumption on PMC decomposition degree
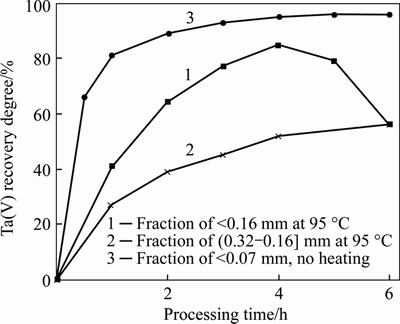
Figure 2 Influence of processing time and PMC grinding on Ta (V) recovery into solution
Material grain size considerably influences PMC decomposition degree. Plumbomicrolite mainly reports to slurry fraction during grinding. The fraction decomposes without heating, producing a strong exothermic effect. Temperature of the reaction mass reaches 85 °С after addition of acids and 10 min mixing. Decomposition degree rises up to 63% for coarse fractions after 6 h of processing. In this case, apart from natural decrease in decomposition speed with the surface decrease, lead sulfate blocks the surface and a high specific gravity of PMC complicates mixing. Another reason of a low decomposition degree in coarse fractions is its high chemical resistance because a metamictic part of the mineral is transferred to slurry during grinding. During decomposition of a <0.16 mm fraction, recovery of Та(V) to solution reaches its maximum, and drops to 64% after 6 h of processing. This could be explained by the fact that a fine PMC fraction decomposes first, then accessory impurities decompose, and these impurities bind fluoride-ions. Lack of the latter leads to hydrolysis and precipitation of Та(V) at high temperature. Decomposition of a slurry fraction without heating does not lead to this effect: in 2 h, temperature of the reaction mass decreases, and processes of impurities decomposition and Та(V) hydrolysis slow down. Lead (II) fluorosulfates formation can also result in binding of fluoride-ions, but this fact needs further clarification.
Thus, a necessary condition for PMC leaching with a mixture of sulfuric and hydrofluoric acids is grinding to size of <0.07 mm and addition of excess fluoride-ion. The excess should be calculated considering not only the main solutes, but also Si(IV) and Al(III) from impurity minerals. Pb(II) almost completely stays in precipitate as PbSO4; Si(IV) reports to solution. The degree of Та(V) and Nb(V) report to a solution reaches 96%-97%. An increase in extraction (up to 99%) can be achieved by returning of undecomposed PMC to reprocessing. Undecomposed PMC can be separated by gravitational methods, because density and size of PMC particles are much higher than that of lead sulfate.
We have determined optimal conditions for PMC leaching due to scheme (Figure 3): fraction of <0.07 mm, S:VL=1:3, T=90-95 °С, consumption 120% HF and 150% H2SO4 of stoichiometry, processing time of 3 h. Gamma spectrometry studies revealed that at these conditions uranium-238 reports to a filtrate, thorium, radium and lead are at 86.5%-95.4% precipitated in a fluoride sulphate media and remain in the precipitate in the form of fluorides and sulfates. Effective specific activity of precipitate was with an average of 97000 Bq/kg.
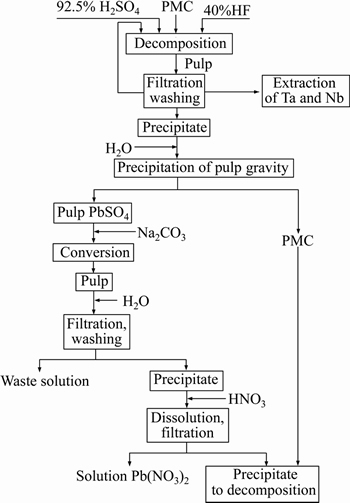
Figure 3 Scheme of PMC leaching with fluoride-sulfuric acid
In order to study complex processing of PMC and to obtain Pb(NO3)2, we have researched lead sulphate to carbonate conversion with dissolving the precipitate in nitric acid and evaporating the lead nitrate solution. Experiments show that conversion of lead sulfates to carbonates followed by dissolution of the precipitate in nitric acid enables us to recover lead and return mineral fraction back to decomposition. Conversion of lead sulfate from precipitate after PMC leaching to carbonates was found to be almost complete under the following conditions: S:VL=1:4, T=60-70 °С, consumption of Na2CO3-120% of a stoichiometry, processing time of 3 h. Radionuclides were not detected in carbonate solution after conversion. In lead nitrate, radionuclides are mainly represented by unstable lead isotopes. The fluoride-sulfuric acid scheme for complex processing of PMC is demonstrated in Figure 3.
The resulting solutions contain high amount of tantalum (112.4-138 g/L Ta2O5) and niobium (65.2-72.5 g/L Nb2O5), which facilitates their recovery and treatment with standard methods.
Table 3 demonstrates experimental data of one of the experiments (mass balance). 100 g of PMC with a fraction of <0.06 was processed with a mixture of 40 g 93% H2SO4, 75 mL 40% HF, 100 mL Н2О during thorough mixing. Processing time was 2 h at 75 °С. The residue was filtered and washed at a filter with water; filtrate and washing water were united.
3.1.2 Leaching with fluoride-nitric acids
PMC was leached at temperatures 40, 70, 100 and 120 °С, varying S:VL ratio from 2 to 5, HF consumption from 120% to 150% and HNO3 consumption from 100% to 200% of a stoichiometry.
In the first experiment series carried out on sample 4 (Table 1), hot pulp was filtered at the temperature of 95-98 °С. We chose sample 4 because it has a middle n(Ta)/n(Nb) value. Fraction composition of the sample is demonstrated in Table 2. The resulting precipitate was washed with water in a filter at S:VL ratio of 1:10 to 1:20. The washing water contained tantalum, niobium and lead, and their content depended on PMC leaching conditions (Table 4). Filters were cooled, and at the same time,lead nitrate crystalized from supersaturated solutions. After that lead nitrate was separated and washed with a saturated solution of Pb(NO3)2.
Table 3 Distribution of basic components at PMC decomposition by HF and H2SO4 mixture
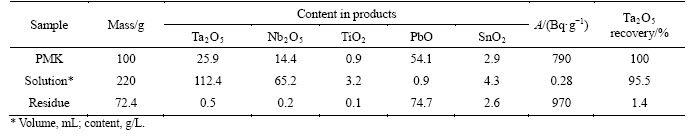
Table 4 Result of PMC leaching under conditions of concentrate mass 50 g, HF 120% of a stoichiometry, temperature 95-98 °С, decomposition time 3 h, hot pulp filtered in all experiments (the beginning)
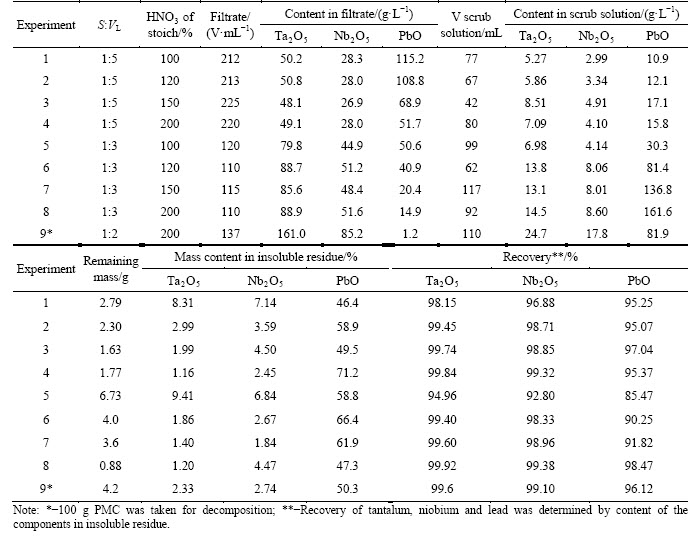
Analysis of the experimental data (Table 4) showed that with an increase in HNO3 consumption, a degree of niobium and tantalum extraction into solution increases. At the consumption 200% of a stoichiometry and S:VL ratio of 1:2 to 1:5, the extraction reaches 99%. Under the same conditions, 95%-98% of lead is extracted, and Pb(NO3)2 crystals distribute between insoluble residue, filtrate and scrubbing solution. A decrease in S:VL ratio and an increase in HNO3 consumption reduce the amount of lead extracted to filtrate (Table 4). This is explained by Pb(NO3)2 solubility decreasing with HNO3 concentration growth.
Studies of PMC leaching kinetics showed that the process can take 3 h with nitric acid consumption 150%-200% of a stoichiometry. Under these conditions, tantalum and niobium extraction to solution reaches a high level (Figure 4).
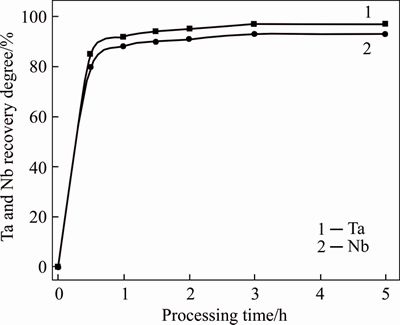
Figure 4 Influence of PMC treatment time on tantalum and niobium recovery to solution
Based on the analyses of all PMC samples (Table 1), temperature is an important factor that influences leaching. Decomposition degree is 80%-85% at HNO3 consumption 200% of a stoichiometry, and temperature ranges between 40-50 °С. The temperature increase to 100 °С results in 99% extraction of Ta and Nb to solution from samples 2 and 4 at the given S:VL ratios (Table 1).
Other samples required a higher temperature (up to 120 °С). This is explained by the fact that samples 1, 3 and 5 were taken during secondary grinding of the mineral coarse fraction. The fraction is less metamictural, and therefore, it has higher mechanical and chemical resistance.
The processing scheme (including hot pulp filtration) described above provides the following products of PMC leaching: raw residue, filtrate, scrubbing solutions and lead nitrate.
In order to decrease a number of filtrations, report almost all lead to a lead nitrate solution and obtain less radioactive tantalum-niobium concentrate. We have carried out experiments where pulp after PMC leaching was cooled to a room temperature and filtered. Besides, we have checked lead nitrate solubility in nitric acid of various concentrations (from 2 to 12 mol/L) at T=18 °C. Figure 5 shows that with an increase in HNO3 concentration, Pb(NO3)2 solubility in HNO3 sharply decreases. That is why we proposed to wash the leaching residue with 35%-45% HNO3 at S:VL=1:1.5-2, and return washing solution to the PMC decomposition process. This enables to avoid tantalum and niobium losses with mother liquor. Table 5 demonstrates results of one of the experiments, and Figure 6 shows PMC leaching with fluoride-nitric acid.
A high extraction rate of tantalum and niobium to solution (over 99%) with simultaneous separation of lead (98%) as lead nitrate was achieved under optimal conditions (<0.07 mm fraction in PMC concentrate from 78% to 90%, S:VL=1:3, T=100-120 °С, consumption 120% HF and 200% HNO3 of a stoichiometry, processing time 3 h) according to the scheme (Figure 6).
Solutions used for extraction of tantalum and niobium are stable for a long period of time and contain: 85.1-89.8 g/L Ta2O5 and 46.0-53.2 g/L Nb2O5.
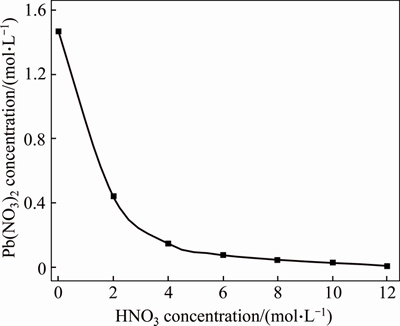
Figure 5 Lead nitrate solubility vs nitric acid concentration in the solution
Table 5 Distribution of the main solutes during PMC leaching and washing of undecomposed residue with HNO3 solution
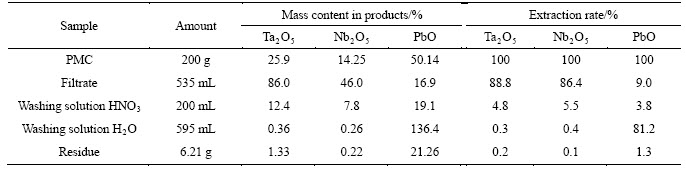
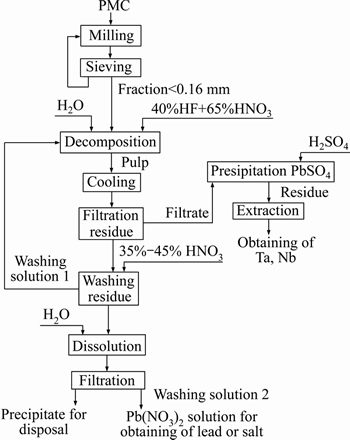
Figure 6 PMC leaching with fluoride-nitric acid
3.2 Extraction processing of filtrates after PMC leaching by OCL-1
Despite the fact that the extraction method for extraction, concentration and separation of tantalum and niobium from fluoride media is well studied, during development of the PMC extraction technology, it is necessary to consider characteristics of initial solutions and optimize conditions of tantalum and niobium compounds extraction for different extraction, scrubbing and stripping stages.
Due to gamma spectrometry analysis, solutions obtained during PMC leaching in optimal conditions by fluoride-sulfuric acid and fluoride- nitric acid schemes are radiation safe and can be used for extractive separation of tantalum from niobium and impurities. We have chosen selective extraction of tantalum by octanol-1 with obtaining of pure strip solutions containing tantalum.
3.2.1 Processing of fluoride-sulfuric acid solutions
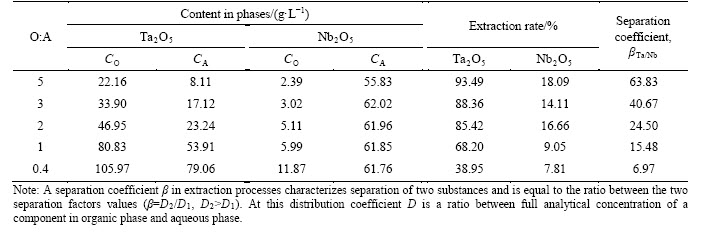
We have analyzed extraction of tantalum, niobium and impurities from fluoride-sulfuric acid solutions by OCL-1. Initial solution had composition: 185.5 g/L H2SO4, 155 g/L HF, 119.7 g/L Ta2O5, 66.8 g/L Nb2O5, 16.6 g/L SnO2, 3.9 g/L SiO2, 5.8 g/L TiO2, 5.3 g/L Fe2О3, 0.005 g/L PbО. Table 6 demonstrates concentrations of tantalum and niobium in organic (СO) and aqueous (СA) phases, degree of report of tantalum and niobium to extract at one stage, separation coefficients βTa/Nb obtained at different O:A ratios.
Table 6 clearly shows that degree of tantalum extraction and separation coefficients βTa/Nb decrease at a decrease in O:A ratio. The maximal concentration of tantalum in the extract was detected to be about 120 g/L. We found out that O:A=0.4-5 is enough for complete extraction of tantalum into the solution.
We studied scrubbing of tantalum extract at different ratios O:A=5-10 with solutions containing: 100-200 g/L H2SO4, 0-20 g/L HF, 50 g/L (NH4)2SO4. The most effective scrubbing results were obtained with the scrubbing solution compound 100 g/L H2SO4 and 50 g/L (NH4)2SO4, and the ratio O:A=5. In this case stripping was carried out with water at the ratio O:A=1, and up to 75% Та2О5 was extracted during one stage.
Table 6 Distribution of components during extraction from fluoride-sulfuric acid solutions with OCL-1
As a result of ammonia precipitation from strip solutions of tantalum hydroxide with scrubbing from NH4+ and F- ions, drying and calcination at 1000 °С, we recovered tantalum pentoxide with the amount of admixtures corresponding to a high- purity grade. The content of cationic impurities in different Та2О5 samples was Nb<30×10-4 wt%; Ti, Zr<3×10-4 wt%; Ca<20×10-4 wt%; Fe≤ 3×10-4 wt%; Mn< 1×10-4 wt%; Mg≤3×10-4 wt%; Pb≤5×10-4 wt%; Cu, Sn, Ni, Cr, V<2×10-4 wt%; Co, Mo, Al<5×10-4 wt%; Si<10×10-4 wt%. A gamma-spectrometric analysis revealed that both tantalum hydroxide and tantalum pentoxide obtained from it by calcination contain no radioactivity, because OCL-1 fails to extract radionuclides from fluoride sulphate leaching solutions. Thus, OCL-1 can successfully separate tantalum from niobium and other admixtures in fluoride-sulfuric acid solutions of PMC leaching. Obtained results coincide well with data from works [17, 18, 23, 26-28] concerning efficiency of tantalum and niobium recovery from fluoride sulfate media by OCL-1.
The following products were obtained during enlarged experiments form 1 kg PMC:
1) Initial fluoride sulfate solution after PMC decomposition, 1.9 kg solution, d=1.29 g/cm3, light-yellow.
2) Residue, lead sulfate, 0.7 kg, white.
3) Raffinate-fluoride sulfate solution after tantalum extraction, 1.8 kg, d=1.203 g/cm3, light- yellow.
4) Tantalum hydroxide, 0.43 kg, white.
3.2.2 Processing of fluoride-nitric acid solutions
The initial solution contained 85.1 g/L Ta2O5, 49.2 g/L Nb2O5, 15.7 g/L Pb, 2.68 g/L Fe, 115 g/L F-, 197 g/L NO3. Extraction was carried out at different ratios O:A=0.4-5, the results are shown in Table 7. The maximal concentration of tantalum in the extract was detected to be about 90 g/L at extraction by OCL-1. We found out that recovery of tantalum into the extract at O:A=2 during one stage is slightly lower in this case than recovery from fluoride sulphate solutions. Based on calculations, complete recovery of tantalum at the stage of extraction with OCL-1 requires 5-6 stages.
Extract recovered at the ratio O:A=2 and containing tantalum and co-extracted impurities was scrubbed by 0.8 mol/L HNO3 at the ratio O:A=5. Stripping was carried out with water at the ratio O:A=1. Tantalum pentoxides obtained from purified strip solutions did not comply with a high-purity grade. They contained large amounts of lead (3×10-3-7×10-3) wt%, niobium (2×10-3-4× 10-2) wt%, iron (5×10-3-6×10-3) wt%, titanium 3×10-3 wt%, tin (1×10-3-3×10-3) wt%, silica 6×10-2 wt%. Radiological analysis showed that tantalum pentoxides contained traces of radionuclides.
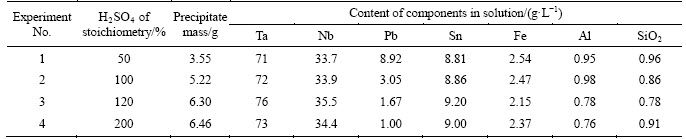
In order to clear fluoride-nitric acid solutions from impurities, including lead and radioactive elements, one more stage was added before extraction. The stage included precipitation of lead sulphate and other impurities with concentrated sulfuric acid. Table 8 shows results of experiments at sulfuric acid consumption varying from 50% to 200% of a stoichiometry. Maximal losses of tantalum and niobium with precipitate were 1% and 1.8%, respectively.
Tantalum was extracted with OCL-1 from the leaching solutions. The best ratio for extraction was O:A=2. Based on calculations, complete recovery of tantalum at the stage of extraction with OCL-1 requires 5-6 stages. Researches revealed that at the scrubbing stage and O:A=5 the best scrubbing solution is: 100 g/L HNO3, 50 g/L NH4NO3. Stripping of tantalum was carried out with water at O:A =1.
High-purity tantalum pentoxides recovered from strip solutions had the following composition,Nb<30×10-4 wt%; Ti, Zr<3×10-4 wt%; Ca<20× 10-4 wt%; Fe≤3×10-4 wt%; Mn<1×10-4 wt%; Mg≤ 3×10-4 wt%; Pb≤5×10-4 wt%; Cu, Sn, Ni, Cr, V<2×10-4 wt%; Co, Mo, Al<5×10-4 wt%; Si< 10×10-4 wt%. A gamma-spectrometric analysis demonstrates that tantalum oxides obtained as a result of PMC leaching in optimal conditions due to fluoride-nitric acid scheme (Figure 6) are free from Th-232, Ra-226 and Pb-214.
Table 7 Distribution of components during extraction from fluoride-nitric acid solutions with OCL-1
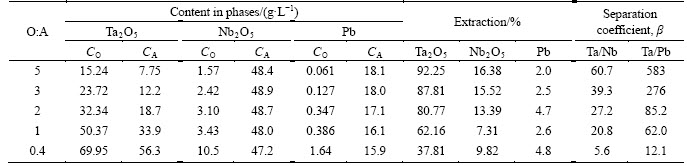
Table 8 Results of experiments of purification of fluoride-nitric acid solutions of PMC leaching from impurities (volume of the initial solution was 200 mL)
High-purity strip solutions were also used for precipitation of potassium heptafluotantalate К2ТаF7. The solution was corrected in terms of hydrofluoric acid content, after that it was heated to 80 °С, and potassium-containing reagent (KCl) heated to the same temperature was added. The produced pulp was cooled at the rate of 10-15 °C/h, and soaked for 12 h until crystals of К2ТаF7 appeared. The precipitate was nutsche filtered, washed 3 times with deionized water at S:VL=1:3, and dried with hot air at 60 °С for 8 h. The produced potassium heptafluotantalate contained the following impurities: Mn<3×10-4 wt %; Mg, Cr, V, Cu<5×10-4 wt%; Si, Fe Ni, Pb, Sn, Ti, Al, Mo, Sn<1×10-3 wt%; Co<3×10-3 wt%; Nb, Zr< 5×10-3 wt%; Ca<3×10-2 wt%. These amounts comply with the required standards specifying initial material for sodium reduction in tantalum powder for condensers. Similar results were obtained for the fluoride-sulfuric acid technology of PMC leaching.
During enlarged experiments the following products were obtained from 2 kg PMC:
1) Initial nitric fluoride solution after PMC decomposition, 5.2 L solution, d=1.313 g/cm3, light-yellow.
2) Decomposition residue, 68 g.
3) Residue after recovery of PbSO4 and radionuclides by concentrated sulfuric acid, about 100 g.
4) Washing solution 1, 2 L (35%-45% HNO3), d=1.230 g/cm3.
5) Solution of lead nitrate (washing solution 2), 4.85 L, d=1.218 g/cm3.
6) Raffinate-fluoride nitrate solution after tantalum extraction by OCL-1, 4.5 L, d= 1.161 g/cm3, light-yellow.
7) Tantalum hydroxide, 0.9 kg, white.
3.2.3 Resistivity OCL-1 in time
OCL-1 resistivity in time and solutions treatment intensity was tested in “extraction- scrubbing-stripping” cycles. Figure 7 demonstrates IR spectra of scrubbed OCL-1 extracts soaked for 1 year. Comparison of IR spectra of initial OCL and soaked for 1 year led to a conclusion that OCL structure remained the same in both fluoride- sulfuric acidic and fluoride-nitric acidic leaching schemes of PMC. The research demonstrated that during this period of time OCL-1 structure and its physical and chemical characteristics remained stable (i.e., capacity for metals, density, viscosity). Obtained results are important for economic efficiency of application of OCL-1 in studied technological schemes.
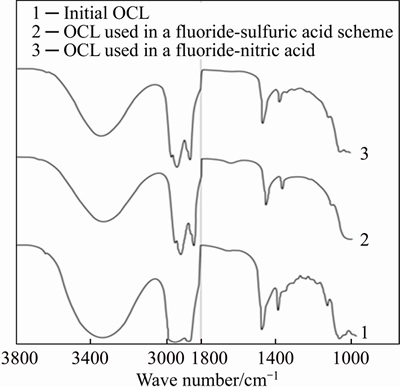
Figure 7 IR-spectra of OCL-1
3.3 Study of radionuclides behavior in hydrometallurgical technologies of PMC
3.3.1 Radionuclides distribution in fluoride-sulfuric acid PMC decomposition
In given conditions of PMC decomposition due to fluoride-sulfuric scheme uran-238 (63%-89%) and thorium-232 (75%-92%) proceed to an unsolvable residue, as shown in Tables 9-11. United filtrates (tantalo-niobium solutions) of different experiments contain very few radionuclides. The amount of radionuclides in residue increases with an increase in sulfuric acid consumption during PMC processing. During fluoride-sulfuric PMC decomposition an increase in sulfuric acid consumption leads to an increase in specific activity of residue with thorium-232, uranium-238 and radium-226 in 1.2-1.6 times. At the same time, specific activity of filtrates decreases. This favors obtaining more radiation-clean tantalum-niobium solutions.
Thus, at fluoride-sulfuric acid PMC decomposition the most thorium-232 can be removed from filtrate in dependence on decomposition conditions. At the conditions obtained in Table 11 the maximal movement of radionuclides to residue was achieved.
Results of gamma-spectrometric research (Table 12) have shown that in optimal conditions of PMC decomposition uranium-238 moves to filtrate, after extraction, to raffinate. 95.4% of thorium, radium and lead precipitate in fluoride-sulfuric acid media and stay in precipitate as fluorides and sulfates. Tantalum hydroxide is completely cleaned from radioactivity. The described conditions of PMC decomposition due to fluoride-sulfuric acid scheme can be considered as the most suitable for cleaning of tantalum-niobium solutions from radioactivity.
Table 9 Distribution of uranium-238 (radium-226) and thorium-232 at PMC decomposition with a mixture 60 mL HF+25 mL H2SO4
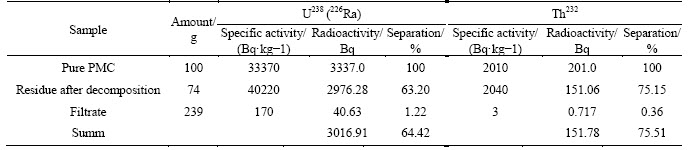
Table 10 Distribution of uranium-238 (radium-226) and thorium-232 at PMC decomposition with a mixture 60 mL HF+33 mL H2SO4
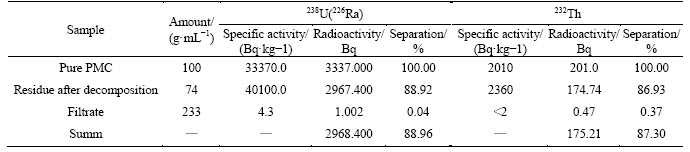
Table 11 Distribution of uranium-238 (radium-226) and thorium-232 at PMC decomposition with a mixture 60 mL HF+44 mL H2SO4
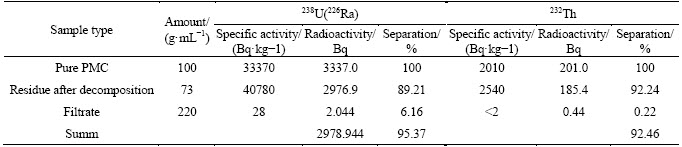
Table 12 Distribution of radionuclides in fluoride-sulfuric acid scheme of PMC decomposition (average values in 3-5 measurements)
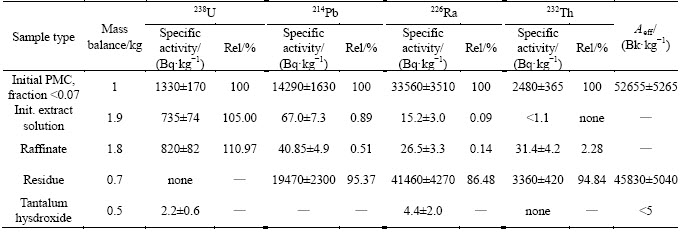
3.3.2 Radionuclides distribution in fluoride-nitric acid PMC decomposition
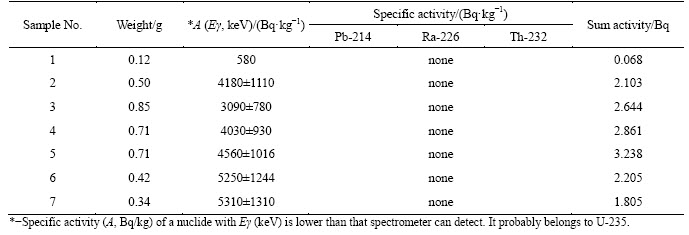
Analysis of non-decomposed residue revealed that it’s specific activity increases 4-10 times in dependence on decomposition conditions. Concentration of radioactivity in small amount of the residue is observed in all cases. Specific activity of thorium-232 was approximately 13-47 kBq/kg; specific activity of radium-226 changed in the range of 230-586 kBq/kg. In optimal PMC decomposition conditions activities of 232Th and 226Ra in the residue were also high. Table 13 demonstrates data on movement of radionuclides to the residue obtained at different conditions of PMC decomposition. The data show that optimal variant was achieved in experiment 6 (ratio S:VL=1:3, HF and HNO3 consumption 120% of stoichiometry).
At precipitation of lead nitrate from filtrates by sulfuric acid co-precipitation of radium was detected along with obtaining of PbSO4. Due to data in Table 14 the maximal movement of radium together with lead was achieved in experiment 1 (Table 8). In other cases, concentration of radium and lead were not detectable. Thorium-232 was absent from lead nitrate samples. Table 15 demonstrates radiation characteristics of technological products of fluoride-nitric acid PMC decomposition.
Thus, partial separation of radionuclides occurs in optimal conditions of PMC decomposition: thorium-232 stays in residue, uranium-238 moves to filtrate, up to 67% radium-226 and plumbum-214 move to washing solution 2. Two latter radionuclides can be separated as a technological product and processed on.
Uranium-238 concentrates in raffinates together with niobium and other impurities at tantalum extraction by OCL-1. Organic phase was determined to contain no uranium-238. Traces of lead and radium were detected in all products.
Table 16 demonstrates radiation characteristics of Та2О5 samples obtained from highly pure Ta-containing stripping solutions. Table 16 shows that tantalum oxides obtained due to fluoride-nitric acid scheme of PMC decomposition (Figure 6) are free of Th-232, Ra-226 and Pb-214. Fluoride-sulfuric acid scheme (Figure 5) shows analogues results.
Separation of niobium from radionuclides is a subject of future studies. We believe that niobium in this scheme can be separated as a commercial product.
4 Conclusions
1) A decomposition of plumbomicrolite concentrate by a mixture of hydrofluoric and sulfuric acids was studied. Sulfuric acid consumption above stoichiometry was determined to inactivate lead (II) and tin (IV). The excess acid consumption has a little influence on PMC decomposition degree. However, more radioactive nuclides move to insolvable residue. Kinetic characteristics were studied for different PMC fractions at HF consumption 120% of stoichiometry. Optimal conditions for PMC decomposition were determined.
A method for separation of lead was proposed. Also a method of mineral fraction returning to secondary decomposition was proposed that lead sulfates are converted to carbonates with solution in nitric acid. Rough mineral fractions were shown to be separated by gravitation methods from sulfate and carbonate pulps partially, and from nitric pulps completely.
Table 13 Distribution of radionuclides in non-solvable residue obtained in following conditions: PMC 50 g, HF-120% of stoichiometry, temperature 95-98 °С, decomposition time 3 h
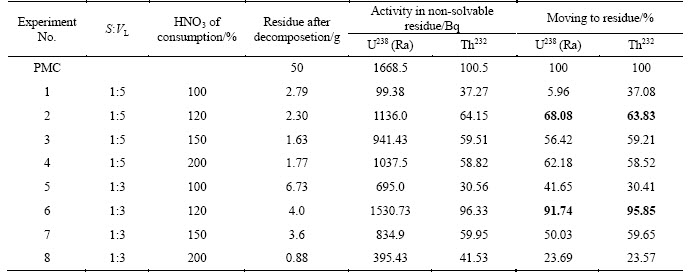
Table 14 Specific radioactivity of radionuclides in lead sulfate samples
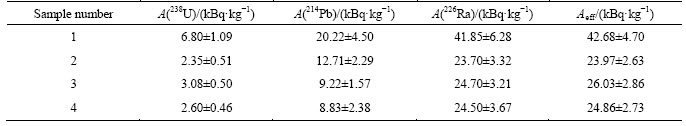
Table 15 Radiation characteristics of technological products of fluoride-nitric acid PMC decomposition (Average values of 3 experiments)
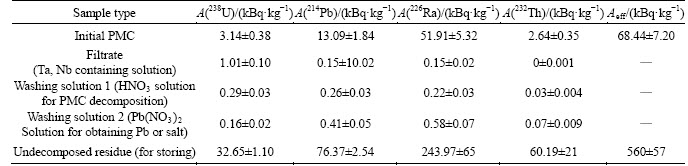
Table 16 Radiation characteristics of Та2О5 samples obtained from PMC (average values of 3-5 measurements in different conditions)
PMC decomposition by a mixture of hydrofluoric and nitric acids was studied. Optimal conditions for concentrate decomposition were determined. A high degree of tantalum and niobium (more than 99%) recovery to solution was achieved with a separation of lead (98%) as lead nitrate. A solubility of Pb(NO3)2 in nitric acid was researched at room temperature. On the basis of these data we recommended to wash residue obtained during PMC decomposition with 35%-45% HNO3 with addition of washing solution to PMC decomposition stage. Fluoride-sulfuric and fluoride- nitric acid schemes were developed for PMC processing. High-purity tantalum compounds can be obtained due to the schemes; lead can be transferred to solvable salt of lead nitrate.
2) Conditions of extraction of high-purity tantalum compounds by octanol-1 were researched for fluoride-sulfuric and fluoride-nitric acid solutions. Extraction of tantalum by OCL-1 was determined to be 83.7% from fluoride-sulfuric acid solutions and 80.8% from fluoride-nitric acid solutions for one stage at O:A=2.
In order to obtain high-purity tantalum compounds a cleaning of fluoride-nitric acid solutions from lead and radionuclides before extraction was studied. Optimal conditions were found for precipitation of lead sulfate and other impurities by concentrated sulfuric acid.
Washing of tantalum extracts from impurities was studied in researched systems. Optimal conditions providing high quality of final product were determined. Conditions for stripping were found.
3) It was determined that during leaching in all studied technologies the main part of radioactive substances concentrate in insoluble residue. Effective activity of the residue depends on decomposition conditions and is 500-700 kBq/kg. Residue and initial concentrate are radioactive, refering to radioactive unsafe substances. Liquid technological products (filtrates, washing solutions) obtained in different types of both studied technologies are radioactive safe.
Uranium-238 was determined to concentrate with niobium and other impurities in raffinates at extraction by OCL-1. Additional studies are necessary for separation of niobium in such conditions.
Tantalum strip solutions of high purity were obtained due to both studied technologies of PMC processing with extraction by OCL-1. Potassium heptafluorotantalate K2TaF7 and tantalum pentoxides were precipitated from the solutions. Content of impurities correspond to norms of high-purity chemicals. Radiological studies confirmed absences of radionuclides from Та2О5 and K2TaF7.
Note that high content of lead in PMC leads to unusual distribution of radionuclides during acid decomposition. This fact claim additional studies. Only after additional research one can determine area of application of lead nitrate obtained in both technological schemes of PMC processing.
From the radiation safety point of view two studied technologies are almost similar. The finel choice of the technology depends on the tantalum recovery percentage and cost-effectiveness of technology.
Conflicts of interest
The authors declare no conflict of interest.
Data availability statement
The raw data required to reproduce our findings are available in Ref. [35].
References
[1] ELENA E N, DMITRIY V D, ELENA N L. Niobium and tantalum: The state of the world market, applications, raw materials. Part II [J]. Universities’ Proceedings Non-Ferrous Metallurgy, 2014(1): 29-41. DOI: 10.17073/0021-3438- 2014-1-29-41. (in Russian)
[2] STUART R T, SCOTT M M. The continental crust: Its composition and evolution: An examination of the geochemical record preserved in sedimentary rocks [M]. Oxford: Blackwell Scientific Publications, 1985.
[3] ELENA E N , DMITRIY V D, ELENA N L. Niobium and tantalum: The state of the world market, applications, raw materials. Part I [J]. Universities’ Proceedings Non-Ferrous Metallurgy, 2013(5): 28-34. DOI: 10.17073/0021-3438- 2013-5-28-34. (in Russian)
[4] ANATOLYI М V, VLADIMIR V B, LIUDMILA I P. Plyumbomikrolit i plyumbopirohlor iz amazonitovyih pegmatitov Kolskogo poluostrova [J]. Mineralogicheskiy zhurnal, 1981(3): 20-34. (in Russian)
[5] ANATOLYI М V, YAKOV A P. Mineralyi i evolyutsiya mineraloobrazovaniya v amazonitovykh pegmatitah Kolskogo poluostrova [M]. Leningrad: Nauka, 1986. (in Russian)
[6] LUCA B, MATTEO Z, P B. Plumbomicrolite from the Ploskaya Mountain, Keivy Massif, Kola Peninsula, Russia: composition and crystal structure [J]. Periodico di Mineralogia, 2006, 75(2, 3): 51-58.
[7] DANIEL A, MARCELO B A, LUCA B, PAOLA B, MATTEO Z, CHRIS J S, ROY K. Kenoplumbomicrolite, (Pb,□)2Ta2O6[□,(OH),O], a new mineral from Ploskaya, Kola Peninsula, Rusia [J]. Mineralogical Magazine, 2018, 82(5): 1049-1055. DOI: 10.1180/minmag.2017.081.082.
[8] SERGEY I K, SERGEY A A, NIKITA V C, RAMIZA K R, SERGEY M A, ANNA A B, RAMIL R G, FARIT G V, OLEG N L, TATIANA S N. A new mineral species rossovskyite, (Fe3+,Ta)(Nb,Ti)O-4: Crystal chemistry and physical properties [J]. Physics and Chemistry of Minerals, 2015, 42(10): 825-833. DOI: 10.1007/s00269-015-0766-5.
[9] CHAKMOURADIAN A R, WILLIAMS C T. Mineralogy of high-field-strength elements (Ti, Nb, Zr, Ta, Hf) in phoscoritic and carbonatitic rocks of the Kola Peninsula, Russia [C]// Phoscorites and carbonates: From Mantle to Mine, the Key Example of the Kola Alkaline Province. London: Mineralogical Society of Great Britain & Ireland, 2004: 292-374.
[10] SONGINA O A. Rare metals [M]. Mettalurgiya, 1964. (in Russian)
[11] YAGODIN G A, SINEGRIBOVA O A, CHEKMAREV A M. Technology of rare metals in atomic technology [M]. Moscow: Atomizdat, 1974. (in Russian)
[12] BABKIN A G, MAYOROV V G, NIKOLAEV A I. Extraction of niobium and tantalum and other elements from fluoride solutions [M]. Leningrad: Nauka, 1988. (in Russian)
[13] VLADIMIR G M, VALERIY K K, IRINA V B, BALTSAT V I, MIMONOV A V, ANATOLYI I N. Technology for processing columbite concentrate of the Malyshevsky mine department [J]. Himicheskaya Technologiya, 2000, 1(7): 23-27. (in Russian)
[14] ANATOLYI I N, VLADIMIR G M, IRINA V B. Decrease of HF concentration in process solutions before extractive separation of tantalum(V) from niobium(V) [J]. Russian Journal of Applied Chemistry, 2002, 75(11): 1747-1752. DOI: 10.1023/A:1022233313994.
[15] MULUGETA S Cheru, ALBERTO Velázquez del Rosario, ABUBEKER Yimam, BOGALE Tadesse, GOITOM G. Berhe. Hydrometallurgical removal of uranium and thorium from Ethiopian tantalite ore [J]. Physicochemical Problems of Mineral Processing, 2019, 55(2): 448-457. DOI: 10.5277/ppmp18153.
[16] SOF’YA M M, YURIY I B, VLADIMIR A M, LARISA G A. Production of high-purity oxides of tantalum and niobium from unconventional raw materials [J]. Tsvetnyje Metally, 2004(3): 24-27. (in Russian)
[17] VLADIMIR G M, ANATOLYI I N, VALERIY K K. Extraction recovery of tantalum(V) and niobium(V) from hydrofluoric and hydrofluoric-sulfuric acid aqueous solutions with octanol [J]. Russian Journal of Applied Chemistry, 2001, 74(3): 363-367. DOI: 10.1023/A:101270 4502783.
[18] VLADIMIR G M, ANATOLYI I N. Extraction of tantalum and niobium by octanol to produce high-purity pentoxide [J]. Tsvetnyje Metally, 2002(7): 62-65. (in Russian)
[19] GALINA V K, OL’GA A K, ANNA M R. Extraction of scandium and concomitant elements with triisoamyl phosphate from aqueous solutions containing HNO3 and LiCl [J]. Russian Journal of Inorganic Chemistry, 2018, 63(2): 280-286. DOI: 10.1134/S0036023618020134.
[20] VENKATESAN K A, CHANDRAN K, RAMANATHAN N, ANTHONYSAMY S, GANESAN V, SRINIVASAN T G. Thermal decomposition characteristics of octyl (phenyl)-N, N-diisobutylcarbamoylmethylphosphineoxide-trin-butylphosphate-nitric acid systems [J]. Journal of Thermal Analysis and Calorimetry, 2014, 115(2): 1979-1988. DOI: 10.1007/ s10973-013-3504-6.
[21] VIKTOR F T, ANATOLYI I A, YURIY M G. Ekstraktsionnaya tehnologiya polucheniya tantala [J]. Tsvetnaya Metallurgiya, 1998, 8-9: 18-22. (in Russian)
[22] VLADIMIR G M, ANATOLYI I N, LEONID I S, IRINA V B. Extractive recovery of tantalum(V) and niobium(V) with octanol from hydrofluoric acid solutions containing large amounts of titanium(IV) [J]. Russian Journal of Applied Chemistry, 2001, 74(6): 945-949. DOI: 10.1023/A:101 3057221017.
[23] VLADIMIR G M, ANATOLYI I N, LUDMILA A S, Razdelenie tantala i niobiya ekstraktsiey oktanolom [J]. Tsvetnaya metallurgiya, 2002, 10: 39-43. (in Russian)
[24] MOTLALEPULA N, WALTER P, JOHANNES T. Nel Comparative study of tantalite dissolution using different fluoride salts as fluxes [J]. Journal of Fluorine Chemistry, 2014, 165: 20-26. DOI: 10.1016/j.jfluchem.2014.05.017.
[25] VLADIMIR G M, NATALIJA V K, IRINA R E, LUDMILA A S, ANATOLYI I N. Extraction of tantalum, niobium, and antimony fluorides [J]. Theoretical Foundations of Chemical Engineering, 2013, 47(4): 480-483. DOI: 10.1134/S0040 57951304012X.
[26] MONA N E, TARIK E A, EL-AZM M G A, RAAFAT M I, SALEH M E. Liquid-liquid extraction of tantalum and niobium by octanol from sulfate leach liquor [J]. Arabian Journal of Chemistry, 2012, 5(1): 31-39. DOI: 10.1016/ j.arabjc.2010.07.020.
[27] MPINGA J K, PHILIP L C. Separation of niobium and tantalum from Mozambican tantalite by ammonium bifluoride digestion and octanol solvent extraction [J]. Hydrometallurgy, 2012, 129-130: 151-155. DOI: 10.1016/ j.hydromet.2012.06.008.
[28] ZHU Zhao-wu, CHENG Chu-yong. Solvent extraction technology for the separation and purification of niobium and tantalum: A review [J]. Hydrometallurgy, 2011, 107(1, 2): 1-12. DOI: 10.1016/j.hydromet.2010.12.015.
[29] POLINA A D, ANDREY M Z, GALINA M K, LUBOV’ N O, ELENA V S, Prokudina N A. Adsorption ability of samples with nanoscale anatase to extract Nb(V) and Ta(V) ions from aqueous media [J]. Crystallography Reports, 2014, 59(3): 430-436. DOI: 10.1134/S1063774514030079.
[30] MARIA C R S, MARIO H R, ERNESTO P, ROBERTO A O. X-ray fluorescence analytical methodology for the determination of Nb, Ta, Fe and Mn extracted in hydrometallurgic processes [J]. Latin American Applied Research, 2004, 34(1): 23-27.
[31] KRISHNAKUMAR M, KANNAJI S, MUKKANTI K. Synergistic separation of rare earth elements (REEs, La-Lu), Y and Th from U-, Nb-, and Ta-rich refractory minerals for determination by ICP-AES [J]. Atomic Spectroscopy, 2015, 36(2): 74-81. DOI: 10.46770/AS.2015.02.003.
[32] SCOTT A M, RAHUL R, MARK I P, JAMES T, SURESH B. Characterisation and leaching studies on the uranium mineral betafite [(U,Ca)2(Nb,Ti,Ta)2O7] [J]. Minerals Engineering, 2015, 81: 58-70. DOI: 10.1016/j.mineng.2015.07.007.
[33] RETO G, BUCK E C, GUGGENHEIM R, MATHYS D, REUSSER E, MARQUES J. Alteration of uranium-rich microlite, MRS [J]. Online Proceedings Library (OPL), 2011, 663: 935. DOI: 10.1557/PROC-663-935.
[34] 34. MASLOBOEVA S M, KOLOSOV V N, ORLOV V M,PROKHPRPVA T Y, MIROSHNICHENKO M N. Sodium-reduced tantalum powders produced from plumbomicrolite raw materials [J].Russian Journal of Applied Chemistry, 2012, 85: 1025-1028. DOI: 10.1134/ S1070427212070051.
(Edited by ZHENG Yu-tong)
中文导读
酸浸法和溶剂萃取法分离提纯Kola Peninsula铅细晶矿中的钽
摘要:采用了氢氟酸和硫酸、氢氟酸和硝酸的混合酸浸出法,对铅细晶矿进行了浸取分离。在最佳条件下,钽和铌的收率达到99%,同时并确保放射性核素留在不溶性残渣中。提出了采用氟酸-硫酸和氟酸-硝酸萃取分离钽与铌、铅和杂质的浸出方案,并生产高纯钽化合物。确定了辛醇-1作为萃取剂,反萃生产高纯钽的各阶段萃取-洗涤-反萃的最佳条件。生产出的钽化合物,如五氧化二钽和七氟戊二酸钾,杂质含量符合高纯物质的标准。
关键词:铅细晶石;钽;铌;酸浸法;放射性;溶剂萃取
Foundation item: Project supported by the Federal Research Centre of Kola Science Centre of the Russian Academy of Sciences, Russian
Received date: 2019-10-11; Accepted date: 2020-08-14
Corresponding author: D V MANUKOVSKAYA, Junior Researcher; Tel: +7(81555)79-508; E-mail: deenka@yandex.ru; ORCID: https://orcid.org/0000-0002-9139-3502
Abstract: A plumbomicrolite concentrate (PMC) was leached with the mixture of HF and H2SO4, HF and HNO3 acids, respectively. Optimal conditions ensuring high recovery of tantalum and niobium (up to 99%) into solution, and radionuclides into insoluble residue were determined. Fluoride-sulfuric acid and fluoride-nitric acid schemes were proposed for PMC leaching by an extractive separation of tantalum form niobium, lead and impurities, and production of high-purity tantalum compounds. Octanol-1 was used as an extractant. Optimal conditions for production of high-purity tantalum strip solutions were defined for all stages (extraction-scrubbing-stripping). Produced tantalum compounds, such as tantalum pentoxide and potassium heptafluotanthalate, comply with the norms for high-purity substances in terms of impurities content. Final choice of the PMC processing scheme is determined by its profitability.


