文章编号:1004-0609(2013)S1-s0639-06
Ti3Zr2Sn3Mo25Nb医用钛合金表面载银复合抗菌涂层的制备及其抗菌性能
余 森,于振涛,韩建业,张 强,牛金龙,刘春潮
(西北有色金属研究院, 西安710016)
摘 要:
采用阳极氧化在医用β钛合金Ti3Zr2Sn3Mo25Nb表面制备TiO2纳米管预涂层,然后通过AgNO3溶液浸泡和紫外光照射处理实现银颗粒在预涂层上的固定,再在含银电解液中经过微弧氧化处理制备出载银多孔涂层。利用SEM、EDS对涂层表面形貌、元素构成和载银量进行表征,并通过金黄葡萄球菌评价涂层的抗菌性能。结果表明:通过阳极氧化和微弧氧化两步电化学处理可以在Ti3Zr2Sn3Mo25Nb合金表面制备出含银量较高的多孔涂层,且银多为小于100nm的熔融状颗粒。当载银涂层与金黄葡萄球菌接触1 d后,平均杀菌率达到96.94%,7 d后抑菌率仍可达71.27%。
关键词:
Ti3Zr2Sn3Mo25Nb合金;载银抗菌涂层;阳极氧化;微弧氧化;
中图分类号:TG146.4 文献标志码:A
Preparation and antibacterial properties of Ag-loaded antimicrobial coatings on Ti3Zr2Sn3Mo25Nb alloy
YU Sen, YU Zhen-tao, HAN Jian-ye, ZHANG Qiang, NIU Jin-long, LIU Chun-chao
(Northwest Institute for Nonferrous Metal Research, Xi’an 710016, China)
Abstract: The pre-coatings were prepared on Ti3Zr2Sn3Mo25Nb alloy by anodic oxidation, and then soaked in the AgNO3 solution followed by UV irradiation treatment, after micro-arc oxidation treatment the Ag-loaded composite coatings were prepared finally. The surface topography of the Ag-loaded coatings, the element characteristics and the amount of Ag loading in the coatings were characterized by SEM and EDS. And the Staphylococcus aureus was used to evaluate the antibacterial activity of the coatings. The results show that the coatings prepared by two- step electrochemical method contain a high amount of silver, and most of silver is present as molten particles with the size less than 100 nm. In vitro antibacterial activity tests indicate that the composite coating incorporated with silver has efficient antibiotic ability. 96.94% of the Saphylococcus aureus are killed in the first day and the antibacterial ratio is as high as 71.27% after 7 d-incubation.
Key words: Ti3Zr2Sn3Mo25Nb alloy; silver-loaded antimicrobial coatings; anodization; micro-arc oxidation
钛合金凭借其优良的生物相容性、耐腐蚀性和卓越的综合生物力学性能,逐渐成为牙种植体、骨创伤产品以及人工关节等人体硬组织替代物和修复物的首选材料[1-3]。然而,钛合金等人工假体植入后,其周围组织有伴生感染的危险。研究表明全髋关节置换后,感染率为0.1%~1%,全肘关节感染率为1%~4%,且金属与金属连接的膝关节假体的感染率是金属与塑料连接膝关节的20倍[4-7]。一旦感染发生,不仅会增加病人住院治疗费用,而且有时需要取出内植物重新手术,甚至面临截肢、死亡等危险,给患者带来极大的痛苦,而常规的抗生素疗法很难奏效。由于人工假体通过表面与人体组织相接触,因此,生物材料表面抗菌性能的研究已经成为当前研究的热点,而以载银涂层为代表的无机抗菌涂层因其广谱杀菌、无耐药性、低毒副作用等优势越来越受到人们的关注[8-10]。就安全性和抗菌性综合考虑,在目前发现的各种具有抗菌功能的金属离子中,银离子是最佳的抗菌金属离子。银离子和微生物体内含—SH 基酶的亲和力较强,在很低浓度下就能与其形成不可逆的硫银化合物,破坏微生物细胞的活性,并导致其死亡,同时,银离子还能破坏微生物的DNA分子[9-10]。
但目前载银涂层中有效含银量较低[7],且表面物理附着的银元素在植入人体后会迅速释放,造成早期抗菌银浓度过高,存在毒副作用;同时抗菌作用时间太短,临床治疗效果差[9-10]。为赋予β钛合金Ti3Zr2Sn3Mo25Nb良好的抗菌性能,本研究在微弧氧化之前采用阳极氧化法在Ti3Zr2Sn3Mo25Nb合金表面制备TiO2纳米管作为预涂层,并通过物理吸附将银附着在预涂层表面,再通过在含银电解液中微弧氧化法对样品进行处理,即通过两步电化学法制备载银多孔抗菌涂层,为制备载银抗菌涂层提供一种新思路。
1 实验
1.1 实验材料
实验样品均为直径10 mm × 2 mm的Ti3Zr2Sn3Mo25Nb钛合金圆片,依次经300、800、1000号砂纸初步打磨平整,再在金相抛光机上抛光至镜面,最后依次在丙酮、去离子水、无水乙醇中超声波清洗30 min后,真空干燥备用。
1.2 预涂层制备和载银处理
以Ti3Zr2Sn3Mo25Nb钛合金圆片为阳极,铜片为阴极,60 V恒压,阳极氧化处理4h。阳极氧化电解液组成为0.5%NH4F,2%H2O,97.5%乙二醇。
阳极氧化处理后将样品经去离子水超声波清洗30min,并室温真空干燥后,再将样品浸入到1mol/L的AgNO3溶液浸泡2 h,然后经紫外光照射5 h,紫外光中心波长为253.7 nm。
1.3 微弧氧化处理
将载银处理后的阳极氧化预涂层样品(NT-Ag)进行微弧氧化处理(NT-Ag-MAO)。电解液组成如下:β-甘油磷酸钠浓度0.02 mol/L,乙酸钙0.16 mol/L,银0.2 mol/L,电源为恒压模氏,电压350 V,频率100 Hz,占空比40%,处理时间30 min。
1.4 涂层的表征
将制得的样品通过扫描电镜(SEM; JEOL, JSM- 6700, JEOL Ltd, Tokyo, 日本)观察形貌,并采用扫描电镜附带的EDS附件检测膜层成分。
1.5 涂层抗菌性能检测
为验证涂层的抗菌能力,选用最常见的金黄色葡萄球菌菌株(Staphylococcus aureus,ATCC6538,第6代培养物)验证其抗菌能力,实验在符合GB—19489规定的实验室设施和安全管理规定条件下进行。实验分为两组,一组为载银微弧氧化涂层(NT-Ag-MAO),另一组为无银普通微弧氧化涂层对照组(MAO)。采用平板涂布法测定其抗菌能力,具体操作流程参考GB/T 21866—2008及GB 4789.2—2010进行。首先将菌液滴加在载银多孔涂层和无银对照样表面,对照样采用不含银的纯钛微弧氧化涂层,之后将培养液稀释1000倍,加入吐温80表面活性剂分散均匀后在37 ℃、相对湿度95 %、避光条件下继续培养,分别培养1、4和7 d后分别对其进行菌落计数,为获得稳定准确数据,每组数据设置3个平行样品,分别标为样品1、2和3,菌落计数后取其平均值,并依以下公式计算抑菌率(η):
 (1)
(1)
式中:η为抑菌率;De为对照样菌落树数;Dn为试验样菌落数。
为直观验证涂层的抗菌效果,细菌接种培养1、4和7 d后在实验组样品表面滴加一定量L7012 LIVE/DEAD BacLight Bacterial Viability Kit荧光染色液,其包含有两种染液SYTO9和PI,其中SYTO9使活细菌发出绿色荧光,而PI则使死细菌发出红色荧光,从而可以在区分活细菌和死细菌。染色固定后将样品置于FV1000型激光共聚焦显微镜下观察,观察条件为:Ex488 nm, 543nm, Exm500~530 BP, 560 LP, 物镜,20倍率。
2 结果与讨论
2.1 涂层表面特征
Ti3Zr2Sn3Mo25Nb钛合金样品表面阳极氧化预涂层在1 mol/L的AgNO3溶液中浸泡2 h并紫外辐射相同时间5 h后,样品表面宏观颜色逐渐变深并最终显黑色,其表面微观形貌的SEM像如图1(a)所示,可以看出,Ti3Zr2Sn3Mo25Nb钛合金样品经阳极氧化后表面生成大量密集的TiO2纳米管阵列,通过Image J进行处理并计算得出平均管径为123 nm。经AgNO3浸泡和紫外光照后,纳米管层表面及内部吸附了大量细小的颗粒,颗粒尺寸为100~500 nm,分析认为这些颗粒是银,这也是导致预涂层表面变黑的主要原因。
多孔阳极氧化预涂层由密集排列的纳米管构成,形成大量暴露表面,导致纳米管涂层具有更大的比表面积和更高的表面能,更容易使银盐溶液附着在其表面以降低系统能量。经过银盐溶液浸泡后,甚至有部分银盐溶液进入纳米管内,最终在管内分解,如图1(b)中A和B和C所示,NT-Ag涂层表面附着数量较多的含银颗粒,这些粒状物的存在位置和聚集状态主要有以下3种情况:1) 以颗粒状附着在纳米管口;2) 团聚在一起封堵纳米管阵列表面,甚至在局部出现较大的片状含银物质封堵在纳米管口;3) 附着在管内壁甚至深入到纳米管口内一定距离。
由此可见,借助阳极氧化预涂层粗糙多孔的高比面积结构可获得较好的银吸附能力,这有利于通过进一步的微弧氧化而制备较大载银量的涂层。
进一步微弧氧化处理后的样品表面与普通微弧氧化的多孔、孔洞呈火山口状微观形貌有较大不同,呈现出沟壑状(见图1(b)),表面支离,沟壑形态复杂多样,沟壑突起部分呈现熔融后液态喷溅急冷状,表面较为粗糙,并有大量的微米以下的小孔,推测可能是由之前的纳米管被熔融后的留下的痕迹,该种形貌说明在微弧氧化处理过程中,先前的预涂层被电弧击穿,TiO2纳米管在微弧氧化的高温下熔融[10-16]。有研究结果表明,一定范围内较大的粗糙度将有利于成骨细胞在种植体表面附着、增殖及骨组织的长入[17-19],因此,这种形貌有可能加速植入材料的骨整合进程。同时可见有大量银颗粒均匀地分布在涂层表面和孔洞内壁,大部分颗粒直径在100 nm以下,少数尺寸达到几百纳米,也呈熔融的液滴状(见图1(c)中箭头)。物理吸附的银在术后初期会在体液中过快释放导致银离子浓度过高可能带来细胞毒性[20],并导致后期抗菌能力过快衰减,难以实现抗菌剂的平稳缓释和长期发挥抗菌作用,在本研究中,通过微弧氧化过程中的剧烈物理化学反应[21],在涂层表面和微孔内壁、涂层内部的镶嵌状态的银将有可能减缓这一现象。
2.2 涂层组成及元素特征
图2所示为载银多孔涂层微区EDS谱。可以发现,涂层表面含有较多的银元素和钙、磷、钛、氧元素,钛和氧元素主要来源于在涂层表面阳极氧化和微弧氧化所生成的的氧化钛。
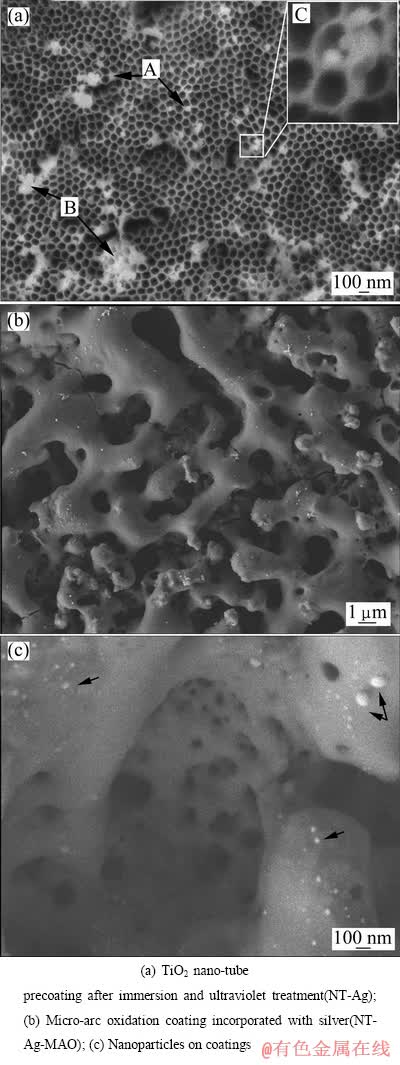
图1 涂层表面微观形貌的SEM像
Fig. 1 SEM images of coatings surface

图2 NT-Ag-MAO涂层和涂层表面颗粒的EDS谱
Fig. 2 EDS spectra of NT-Ag-MAO coatings (a) and nanoparticles on coatings (b)
结果表明涂层中主要含有钛和氧元素,质量分数分别为44.20%和36.85%。同时含有质量分数为7.64%的钙和3.14%的磷,而银元素的平均质量分数为8.16%,分析认为,预涂层和二次微弧氧化膜提供的微孔洞 为银在涂层表面和内部的大量固定提供了条件,而在含银电解液中微弧氧化处理也可将银元素进一步引入涂层表面或内壁,进而得到银含量较高的载银涂层[14, 21]。
对图1(c)中箭头所示颗粒进行EDS元素分析,结果证实涂层表面的亮色颗粒为银,其中银、钛、氧、钙和磷的质量分数分别为84.01%、5.24%、8.09%、2.10%和0.56%。
2.3 涂层抗菌性能
对抗菌涂层实验组和对照组采用平板涂布法测定其抗菌能力,不同培养时间并稀释后菌落生长情况见图3所示。
通过Image J图像识别软件辅助计数,根据式(1)计算NT-Ag-MAO载银微弧氧化涂层的抗菌率,结果见图4。可见3组样品数据较为稳定,未出现较大偏差,数据可靠性高,对其取均值后确定NT-Ag-MAO载银涂层在1、4和7 d的抗菌率分别达到96.94%、81.35%和71.27%。这说明NT-Ag-MAO载银涂层与无银对照样相比有显著高效的抑菌能力,其抗菌能力随时间的延长而减弱,这与银离子释放速率测试结果基本对应一致,但在7 d后,仍可杀灭70%细菌量,并且有较好的长期抗菌能力。
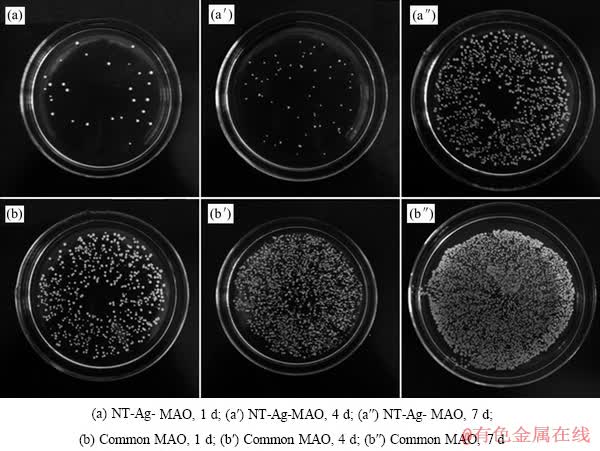
图3 实验组和对照组细菌培养1、4、7d后菌落聚集情况
Fig. 3 Amount of colony aggregated on NT-Ag-MAO coating and common MAO coating after 1, 4, 7-d incubation
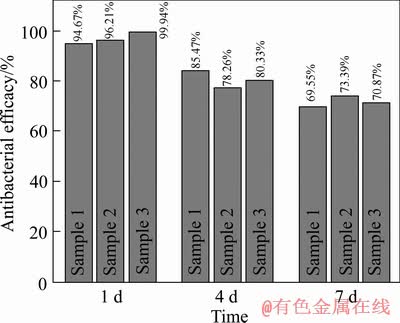
图4 将细菌培养1、4、7d后的抗菌率
Fig. 4 Antibacterial efficacy of NT-Ag-MAO coating tested after 1, 4 and 7 days incubation
宏观的细菌培养和计数测试抗菌率存在一定的偶然性和误差,为直观展示涂层对细菌的生长情况的影响,在此进一步采用激光共聚焦显微镜对其进行死菌和活菌染色观察。
图5所示为NT-Ag-MAO载银涂层和无银对照样的死菌活菌染色照片。图中活菌呈绿色,死菌呈红色,死菌/活菌荧光强度比显示实验组细菌培养在1、4、7 d后细菌数量均大于活菌数,且远大于同期对照组死菌/活菌荧光强度比,说明NT-Ag-MAO实验组在1d几乎抑制了所有细菌,培养4d和7d后,死菌数和活菌数均有所增加,说明载银涂层在7d时仍表现出明显的抗菌效果,但抗菌率较1d和4d有所下降,与平板涂布计数法结论一致。
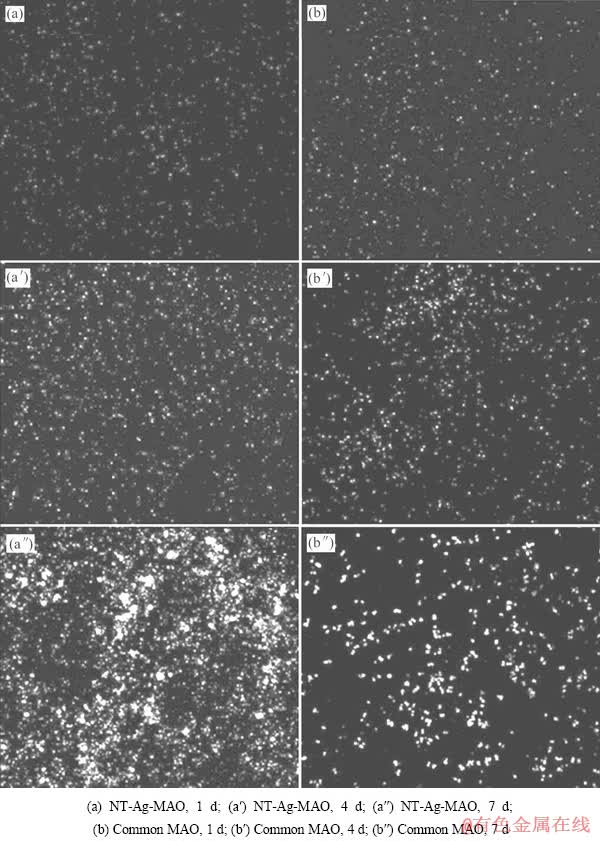
图5 实验组和对照组1、4、7d后死菌和活菌的染色照片
Fig. 5 Images showing viability of bacteria on samples after 1, 4 and 7 d of incubation displayed by fluorescence staining (live bacteria appear green while dead ones are orange)
3 结论
1) 采用阳极氧化制备多孔预涂层并载银处理后再进行微弧氧化的两步电化学处理是制备含银量较高载银多孔涂层的有效途径。
2) 涂层中的银分布在涂层表面和微孔内壁,呈熔融的颗粒状,颗粒尺寸大部分小于100 nm。
3) 载银复合涂层在1、4和7 d对金黄葡萄球菌的抑菌率分别达到96.94%、81.35%和71.27%,相比不含银微弧氧化涂层,具有良好的抗菌性能。
REFERENCES
[1] 于振涛, 余 森, 张明华, 韩建业, 麻西群. 外科植入物用新型医用钛合金材料设计、开发与应用现状及进展[J]. 中国材料进展,2010,29(12): 35-51.
YU Zhen-tao, YU Sen, ZHANG Ming-hua, HAN Jian-ye, MA Xi-qun. Design, development and application of novel biomedical Ti alloys materials applied in surgical implants[J]. Materials China, 2010,29(12): 35-51.
[2] GEETHA M, SINGH A K, ASOKAMANI R, GOGIA A K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review[J]. Progress in Materials Science 2009, 54: 397-425.
[3] 麻西群, 于振涛, 牛金龙, 余 森. Ti3Zr2Sn3Mo25Nb医用钛合金相变与生物力学性能[J]. 中国有色金属学报,2010,20 (S1): s410-s413.
MA Xi-qun, YU Zhen-tao, NIU Jin-long, YU Sen. Phase transition andbiomechanical properties of Ti3Zr2Sn3Mo25Nb for biomedical application[J]. The Chinese Journal of Nonferrous Metals, 2010, 20 (S1): s410-s413.
[4] LI Kai,XIE You-tao,AO Hai-yong,HUANG Li-ping,JI Heng, ZHENG Xue-bin. The enhanced bactericidal effect of plasma sprayed zinc-modified calcium silicate coating by the addition of silver[J]. Ceramics International, 2013, 39(7): 7895-7902.
[5] HUANG H L,CHANG Y Y,LAI M C,LIN C R,LAI C H,SHIEH T M. Antibacterial TaN-Ag coatings on titanium dental implants[J]. Surface and Coatings Technology, 2005(5): 1636-1641.
[6] ZHAO G L,LI X,XIA L,WEN G,SONG L,WANG X Y,WU K. Structure and apatite induction of a microarc-oxidized coating on a biomedical titanium alloy[J]. Applied Surface Science, 2010, 257(5): 1762-1768
[7] SONG W H, RYU H S, HONG S H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation[J]. Biomed Mater Res A, 2009, 88(1): 246-254
[8] CHEN W, OH S, ONG A P, et al. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process[J]. Biomed Mater Res A, 2007, 82(4): 899-906
[9] KELLY P J,LI H,WHITEHEAD K A,VERRAN J,ARNELL R D,IORDANOVA I. A study of the antimicrobial and tribological properties of TiN/Ag nanocomposite coatings[J]. Surface and Coatings Technology, 2009, 204(6/7): 1137-1140.
[10] KAKOLI D, SUSMITA B. Amit bandyopadhyay, surface modifications and cell–materials interactions with anodized Ti[J]. Acta Biomaterialia, 2007, 3(4): 573-85.
[11] LI Y, LEE I S, CUI F Z, CHOI S H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium[J]. Biomaterials, 2008, 29(13): 2025-32.
[12] KIM D Y, KIM M Y, KIM H E, et al. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition[J]. Acta Biomaterialia, 2009, 5(6): 2196-205.
[13] HUANG Y, WANG Y J, NING C Y, et al. Hydroxyapatite coatings produced on commercially pure titanium by micro-arc oxidation[J]. Biomed Mater, 2007, 3(2): 196-201.
[14] YU S, YU Z T, HAN J Y, et al. Biocompatibility and osteoconduction of active porous calcium-phosphate films on a novel Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy[J]. Colloids and Surfaces B: Biointerfaces, 2011, 85(2): 83-115.
[15] HUANG H H, PAN SZU JUNG, LAI Y L, et al. Osteoblast-like cell initial adhesion onto a network-structured titanium oxide layer [J]. Scripta Materiali, 2004, 51(11): 1017-21.
[16] LI L H, KONG Y M, KIM H W, et al. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation[J]. Biomaterials 2004, 25(14): 2867-75.
[17] ROLANDO A. GITTENS,TAYLOR MCLACHLAN,RENE OLIVARES-NAVARRETE, et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation[J]. Biomaterials, 2011, 32(13): 3395-340.
[18] 余 森, 于振涛, 韩建业, 张明华, 牛金龙. 多孔Ti3Zr2Sn3Mo25Nb钛合金表面活性次级微孔涂层的制备及其成骨性能研究[J]. 中国表面工程,2012, 25(6): 101-106.
YU Sen, YU Zhen-tao, HAN Jian-ye, ZHANG Ming-hua, NIU Jin-long. Preparation and osteogenesis of active secondary microporous on the porous Ti3Zr2Sn3Mo25Nb titanium alloy[J].China surface engineering, 2012, 25(6): 101-106.
[19] 余 森, 于振涛, 韩建业, 等. 表面TiO2/HA涂层改性Ti-5Zr-6Mo-15Nb的组织相容性及成骨活性研究[J]. 金属热处理, 2010, 35(9): 31-36.
YU Sen, YU Zhen-tao, HAN Jian-ye, et al. Histocompatibility and osteogenic activity of Ti-5Zr-6Mo-15Nb alloy with surface modification by porous TiO2/HA coating[J].Heat Treatment of Metals, 2010, 35(9): 31-36.
[20] BELLANTONE M, WILLIAMS H D, HENCH L L. Broad-spectrum bact-ericidal activity of Ag2O-doped bioactive glass[J]. Antimicrob Agents Chemother, 2002, 46(6): 1940- 1945
[21] TAO X J,LI S J,ZHENG C Y,FU J,GUO Z,HAO Y L,YANG R,GUO Z X. Synthesis of a porous oxide layer on a multifunctional biomedical titanium by micro-arc oxidation[J]. Materials Science and Engineering C, 2009, 29(6): 1923-1934.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(31100693/C100302);国家“十二五”科技支撑计划资助项目(2012BAI18B02);国家重点基础研究发展计划资助项目(2012CB619102);国家高技术研究发展计划资助项目(2011AA030101);陕西省工业攻关资助项目(2012K07-03)
收稿日期:2013-07-28;修订日期:2013-10-10
通信作者:余 森,工程师;电话:029-86222297;E-mail: wtwang@swjtu.edu.cn
摘 要:采用阳极氧化在医用β钛合金Ti3Zr2Sn3Mo25Nb表面制备TiO2纳米管预涂层,然后通过AgNO3溶液浸泡和紫外光照射处理实现银颗粒在预涂层上的固定,再在含银电解液中经过微弧氧化处理制备出载银多孔涂层。利用SEM、EDS对涂层表面形貌、元素构成和载银量进行表征,并通过金黄葡萄球菌评价涂层的抗菌性能。结果表明:通过阳极氧化和微弧氧化两步电化学处理可以在Ti3Zr2Sn3Mo25Nb合金表面制备出含银量较高的多孔涂层,且银多为小于100nm的熔融状颗粒。当载银涂层与金黄葡萄球菌接触1 d后,平均杀菌率达到96.94%,7 d后抑菌率仍可达71.27%。


