
In situ synthesis of nanostructured titania film on NiTi shape memory alloy by Fenton’s oxidation method
CHU Cheng-lin(储成林)1, HU Tao(胡 涛)1, S. L. Wu2, WANG Ru-meng(王如萌)1,
DONG Yin-sheng(董寅生)1, LIN Ping-hua(林萍华)1, C.Y. Chung2, P. K. Chu2
1. School of Materials Science and Engineering, Southeast University, Nanjing 211189, China;
2. Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, China
Received 27 February 2007; accepted 18 May 2007
Abstract:
Fenton’s oxidation method was successfully used to synthesize an ideal titania film in situ on NiTi shape memory alloy(SMA) for medical applications. Characterized with scanning electron microscopy, X-ray photoelectron spectroscopy, X-ray diffractometry, inductively coupled plasma mass spectrometry and electrochemical tests, it is found that the titania film produced by Fenton’s oxidation method on NiTi SMA is nanostructured and has a Ni-free zone near its top surface, which results in a notable improvement in corrosion resistance and a remarkable decrease in leaching of harmful Ni ions from NiTi SMA in simulated body fluids. The improvement of effectiveness to corrosion resistance and the reduction in Ni release of NiTi SMA by Fenton’s oxidation method are comparable to those by oxygen plasma immersion ion implantation reported earlier.
Key words:
NiTi shape memory alloy(SMA); Fenton’s oxidation; thin films; corrosion;
1 Introduction
NiTi shape memory alloy(SMA) has been considered to be a promising biomaterial because of its shape memory effect(SME) and super-elasticity(SE)[1]. But the high Ni content is of great concern with regard to its implantation, as the Ni release may cause toxic reactions[2]. Its biocompatibility could be improved by some surface modification such as high-temperature oxidation[3], plasma immersion ion implantation and deposition(PIII&D)[4], and biomimetic method with chemical pretreatment in alkali or H2O2 solutions[5] that was developed by KOKUBO et al[6] and HABIBOVIC et al[7].
As we know, there is a native thin titanium oxide surface layer on NiTi SMA that plays an important role on the corrosion resistance and biocompatibility[8]. However, nickel in the metallic or oxidized state is detected on the surface of NiTi SMA and its amount depends on the surface treatments[8]. Since NiTi SMA contains a large amount of Ti, it can be readily oxidized. Several high-temperature oxidation methods as well as different oxidation behaviors and surface properties of NiTi SMA have hitherto been reported[3, 9]. It has been found that NiTi SMAs after high-temperature oxidation are predominantly covered with titania. However, phase transformation between austenite (B2) and martensite (B19′) in NiTi SMA is very sensitive to the heat treatment conditions. e.g. temperature[1]. Thus, high-temperature oxidation will have an undesired effect on the shape memory properties of NiTi SMA.
It was found that titania film could be prepared on titanium by oxidation in H2O2 solution at relatively low temperatures and further studies showed improved in vitro and in vivo biocompatibility[10-11]. In our previous study, it was found that a titania film could also be formed on NiTi SMA by H2O2 oxidation, however, the remnant Ni and microcracks could be found in the titania film due to the insufficient oxidation reaction between the NiTi substrate and weak oxidant H2O2 solution[12-13].
Fenton’s oxidation is successful in removing various inorganic and organic pollutants from water by oxidation processes of hydroxyl radicals (·OH) produced by the catalysis decomposition of H2O2 with ferrous irons[14-15]. Hydroxyl radicals (oxidation potential 2.8 V) is a stronger oxidant than H2O2 (1.80 and 0.87 V at pH=0 and 14, respectively). In this work, the effectiveness of Fenton’s oxidation method on surface modification of NiTi SMA was evaluated by studying surface morphology, release of harmful Ni ions and corrosion resistance in simulated body fluids(SBF) with scanning electron microscopy(SEM), X-ray diffractometry(XRD), X-ray photoelectron spectroscopy (XPS), inductively coupled plasma mass spectrometry (ICPMS) and electrochemical tests.
2 Experimental
A NiTi (50.8% Ni, molar fraction) SMA plate was cut into rectangular blocks (10 mm×10 mm×1 mm). All samples were chemically polished with a solution containing H2O, HF and HNO3 in ratio of 5?1?4 for 5 min. They were divided into two groups. The first group was used as contrast. The second group was further treated by Fenton’s oxidation. H2O2 (30%) and FeSO4·7H2O were in AP reagent grade and used to prepare Fenton’s reagent, whose initial concentration of H2O2 and pH value were kept at 5% and 3.0 respectively, and H2O2 to Fe2+ ratio in molar concentration is 2 000?1. Fenton’s oxidation was carried out in 500 mL Fenton’s reagent in 600 mL flask open to atmosphere. Reaction temperature was controlled at 60 ℃ by water bath (±0.1 ℃). Mixing was achieved by magnetic stirring. After oxidation for 24 h, the samples were ultrasonically washed in acetone for 10 min and in deionized water for 10 min.
XRD patterns were acquired on an X-ray diffractometer (RAD IIA, Rigaku, Japan) using Cu Kα operated at 40 kV and 25 mA. The instrument was equipped with a thin-film attachment for a glancing angle of 1?. The surface morphology of the samples was observed by a Philips XL30 FEG SEM at 20 kV accelerating voltage after the surfaces were coated with gold films. The samples were analyzed by XPS on a VG Scientific ESCALAB 5 spectrometer with monochromatic Al Kα (1 486.6 eV) radiation. The base pressure in the analysis chamber was less than 10-8. High-resolution Ti 2p and Ni 2p spectra were acquired at a 20 eV pass energy to determine the chemical states and concentrations. The C 1s peak was used to identify any charging effects.
The electrochemical tests based on the ASTM G5—94 (1999) and G61—86 (1998) protocols[16] were performed using a potentiostat (VersaStat II EG&G, USA) in a standard simulated body fluid(SBF) at a pH of 7.42[17] and temperature of (37±0.5) ℃. The ionic concentrations in the SBF solution are (in mol/L): Na+ 142.0, K+ 5.0, Ca2+ 2.5, Mg2+ 1.5, ![]() 4.2, Cl- 148.5,
4.2, Cl- 148.5, ![]() 1.0 and
1.0 and ![]() 0.5. A cyclic potential spanning between -400 and 2 500 mV was applied at a scanning rate of 600 mV/h. Before the electrochemical tests, the medium was purged with nitrogen for 1 h to remove dissolved oxygen and nitrogen purging continued throughout the measurements.
0.5. A cyclic potential spanning between -400 and 2 500 mV was applied at a scanning rate of 600 mV/h. Before the electrochemical tests, the medium was purged with nitrogen for 1 h to remove dissolved oxygen and nitrogen purging continued throughout the measurements.
Every two samples of each group were immersed in 25 mL of the SBF solution in polypropylene (pp) bottles. The polypropylene bottles were closed tightly and incubated in a thermostatic chamber at (37±0.1) ℃ for two weeks, five weeks, and ten weeks, respectively. All the bottles were shaken gently for a few seconds every three days. After different immersion time, the SBFs were taken out and analyzed by inductively-coupled plasma mass spectrometry (ICPMS) to determine the concentrations of Ni leached from the specimens. All the results at each time point were averages of four samples.
3 Results and discussion
Fig.1 shows SEM photographs of the chemically- polished NiTi SMA and the oxidized NiTi SMA. The former is relatively smooth. In contrast, it can be obviously found that a nanostructured oxide film is formed on the NiTi SMA after Fenton’s oxidation treat- ment as shown by the high magnification photograph in the top right corner of Fig.1(b). Macro-scopically the new oxides formed on the modified surface make the NiTi sample appear golden.
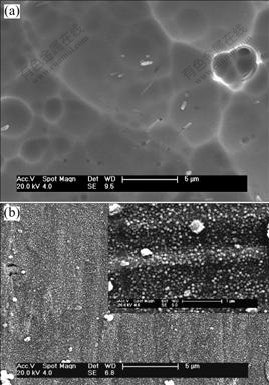
Fig.1 SEM photographs of surfaces of chemically-polished NiTi SMA (a) and NiTi SMA after Fenton’s oxidation treatment (b)
XRD patterns of the surfaces of the chemically-polished NiTi SMA and the oxidized NiTi SMA are presented in Fig.2. Except for the peak assigned to NiTi substrate, the weak peaks associated with anatase- and rutile-TiO2 are present only for the latter. Obviously it is a nanostructure titania film that is formed on the NiTi SMA by Fenton’s oxidation treatment. Both Ni oxide and free Ni could not be detected.
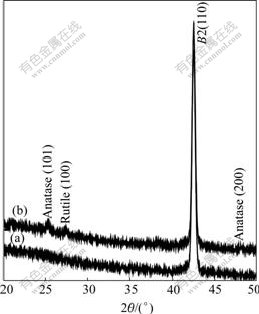
Fig.2 XRD patterns of surfaces of chemically-polished NiTi SMA (a) and NiTi SMA after Fenton’s oxidation treatment (b)
High resolution XPS collections of Ti and Ni binding energy regions for the oxidized NiTi SMA are recorded as shown in Fig.3. The Ti 2p XPS spectrum exhibits two dominant peaks, identified as Ti4+(TiO2) 2p3/2 at 459.3 eV and Ti4+(TiO2) 2p1/2 at 464.8 eV. No remnants of combined Ti species (TiNi-Ti) in intermetallic NiTi state could be found. In contrast, Ni element in any chemical states could not be detected from the Ni 2p XPS spectrum, which proves that there is a Ni-free zone near the top surface of the nanostructured titania film.

Fig.3 Ti 2p and Ni 2p XPS spectra of surface of NiTi SMA after Fenton’s oxidation treatment
Fig.4 shows the electrochemical testing results. The higher breakdown potential(φb) corresponding to initiation of corrosion pits suggests improvement in corrosion resistance. The breakdown occurs after 0.268 V and then corrosion current increases significantly to 2.58×10-2 A after 1.208 V for the chemically-polished NiTi SMA. In contrast, for the oxidized NiTi SMA, the breakdown occurs after 0.866 V and then the currents remain at relatively low levels only reaching 4.45×10-7A even after 2.5 V.
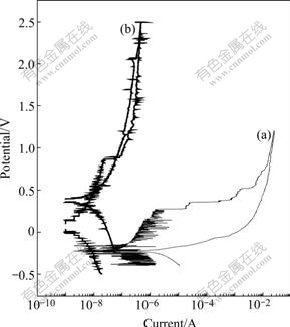
Fig.4 Electrochemical testing results of chemically-polished NiTi SMA (a) and NiTi SMA with nanostructured titania film produced by Fenton’s oxidation method (b)
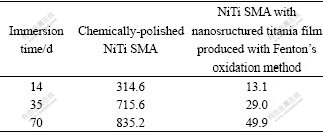
Table 1 summarizes the ICPMS results of the SBFs after immersion tests. For all immersion time, the amount of Ni leached from the chemically-polished NiTi SMA is about 20 times higher than that of the oxidized NiTi SMA. Thus, the nanostructure titania film as a barrier layer is evidently effective in mitigating Ni from the substrate. As a well-established implant material, the major concern is the release of harmful Ni from NiTi SMA. The data provide strong evidence that Fenton’s oxidation process can effectively impede Ni release from NiTi SMA to the SBF.
Table 1 Concentrations of Ni ions detected in SBF by ICPMS for chemically-polished NiTi SMA (a) and NiTi SMA with nanostructured titania film produced by Fenton’s oxidation method after different immersion time (b) (10-6)
Fenton’s reagent consists of a mixture of H2O2 and trace amounts of ferrous ions. It can lead to different results depending on parameters such as the pH value, H2O2 to FeSO4 ratio, and reaction temperature[14-15]. In order to achieve effective Fenton’s oxidation and the highest treatment efficiency of the Fenton’s reagent, the optimal parameters reported previously are adopted [14-15]. Fenton’s oxidation is proceeded by the catalytic decomposition of H2O2 into hydroxyl radicals (OH?) according to the following reaction:
Fe2++H2O2→Fe3++OH?+OH- (1)
H2O2+Fe3+→HOO?+H++Fe2+ (2)
The ferrous ion (Fe2+) initiates and catalyzes the decomposition of H2O2, resulting in the generation of hydroxyl radicals. Hydroxyl radicals have one unpaired electron and are strong, highly reactive, only inferior to elemental fluorine in reactivity.
Ti has a stronger affinity than Ni to O chemisorption because formation enthalpy of TiO2 (-956 kJ/mol) is four times of that for NiO (-241 kJ/mol). Therefore, Ti on NiTi surface can be oxidized by ·OH to form TiO2, while Ni may remain unchanged and could be removed from Ni—Ti bond due to H2O2-etching into aqueous solution. SEM, XRD and XPS results also reveal that a nano-structured titania film composed of mainly rutile and anatase TiO2 phases is formed by Fenton’s oxidation method. However, the surface morphology and structure of the nano-structured titania film formed on NiTi by Fenton’s oxidation method are quite different from those of titania films produced on Ti or NiTi by other oxidation methods including H2O2 oxidation[3,9,12-13]. This result may indicate a different formation mechanism of titania film during Fenton’s oxidation. The oxidation mechanism of NiTi SMA at high temperature in air has been discussed by CHU et al[9]. According to their model, titanium oxide grows due to the outward diffusion of Ti from the metal at high temperature, leading to the formation of the TiNi3 or Ni phase in a Ni-rich layer between the oxide and NiTi substrate. However, no intermediate Ni-rich layer cannot be detected between the nano-structured titania film prepared by Fenton’s oxidation method and NiTi substrate in this study. Moreover, there is a clear Ni-free region in the nano-structured titania film. The detailed mechanism is not completely known and further work is needed in the future.
The NiTi SMA after Fenton’s oxidation for 24 h has a much higher breakdown potential of about 866 mV than the chemically-polished one, and the corrosion currents remain at low levels below 5.0×10-7A even after 2.5 V. Thus Fenton’s oxidation can improve corrosion resistance of NiTi SMA. And the improvement effectiveness to corrosion resistance of NiTi SMA by Fenton’s oxidation method is similar to that by oxygen plasma immersion ion implantation reported earlier[18]. This may be attributed to the lower electrical conductivity of the nano-structured titania film in comparison with the chemically-polished NiTi. The exchange of electrical charges on the oxidized sample surface is reduced and consequently electrochemical corrosion is less severe. Furthermore, titania film is inherently chemically inert. This may play an important role in its good corrosion resistance as well. However, in spite of the encouraging results, the electrochemical test in this work is accelerated and the corrosion conditions of NiTi SMA implants are different in vitro and in vivo. Therefore, more studies are needed to investigate the long-term effects.
In order to find wide acceptance in cardiovascular products, the release of harmful Ni from the NiTi SMA must be significantly mitigated. The results reported here indicate that the nano-structured titania film produced by Fenton’s oxidation method is more effective in impeding the outward-diffusion of Ni from NiTi SMA during the entire ten week immersion period. The reduction in Ni release by Fenton’s oxidation method is comparable to that by oxygen plasma immersion ion implantation reported earlier[18]. This may be attributed to the two advantages provided by Fenton’s oxidation method to NiTi SMA: the formation of a Ni-free region zone on the surface and absence of the intermediate Ni rich layer.
SME and SE of NiTi alloy are associated with reversible martensitic transformation, which is very sensitive to the treatment temperature[1]. However, many reported surface modifications involve high- temperature process. The thermal effect produced by these technologies at the same time of enhancing surface properties can affect properties of NiTi SMA including SME and SE. Fenton’s oxidation is usually conducted at a relatively low temperature less than 200 ℃. It is reasonable that such treatment technique can improve surface properties and does not degrade properties of NiTi SMA.
4 Conclusions
1) Fenton’s oxidation could result in in situ synthesis of a nanostructured titania film with a Ni-free zone near its top surface on NiTi SMA, which notably improves corrosion resistance and decreases the leaching of harmful Ni ions from NiTi SMA in simulated body fluids.
2) The improvement of effectiveness to corrosion resistance and the reduction in Ni release of NiTi SMA by Fenton’s oxidation method are comparable to those by oxygen plasma immersion ion implantation reported earlier.
3) Fenton’s oxidation is a promising low- temperature surface modification way to NiTi SMA for medical applications.
References
[1] Otsuka K, Wayman C M. Shape memory materials [M]. Cambridge: Cambridge University Press, 1998.
[2] WEVER D J, VELDHUIZEN A G, DE VRIES J, BUSSCHER H J, UGES D R A, VAN HORN JR. Electrochemical and surface characterization of a nickel-titanium alloy [J]. Biomaterials, 1998, 19: 761-769.
[3] FIRSTOV G S, VITCHEV R G, KUMAR H, BLANPAIN B, HUMBEECK J V. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23: 4863-4871.
[4] Poon R W Y, Yeung K W K, Liu X Y, CHU P K, CHUNG C Y, LU W W, CHEUNG K M C, CHAN D. Carbon plasma immersion ion implantation of nickel-titanium shape memory alloys [J]. Biomaterials, 2005, 26: 2265-2272.
[5] Choi J, Bogdanski D, K?ller M, Esenwein SA, Müller D, Muhr G, Epple M. Calcium phosphate coating of nickel-titanium shape-memory alloys: Coating procedure and adherence of leukocytes and platelets [J]. Biomaterials, 2003, 24: 3689-3696.
[6] Kokubo T, Miyaji F, Kim H M, Nakamura T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals [J]. J Am Ceram Soc, 1996, 79: 1127-1129.
[7] Habibovic P, Barr?re F, van Blitterswijk C A, de Groot K, Layrolle P. Biomimetic hydroxyapatite coating on metal implants [J]. J Am Ceram Soc, 2002, 85: 517-22.
[8] Shabalovskaya S A, Anderegg J W. Surface spectroscopic characterization of TiNi nearly equiatomic shape memory alloys for implants [J]. J Vac Sci Technol, 1995, A13(5): 2624-2632.
[9] Chu C L, Wu S K, Yen Y C. Oxidation behavior of equiatomic TiNi alloy in high temperature air environment [J]. Mater Sci Eng A, 1996, 216: 193-200.
[10] Wang X X, Hayakawa S, Tsuru K, Osaka A. Improvement of bioactivity of H2O2/TaCl5-treated titanium after subsequent heat treatments [J]. J Biomed Mater Res, 2000, 52: 171-176.
[11] Kaneko S, Tsuru K, Hayakawa S, Takemoto S, Ohtsuki C, Ozaki T, Inoue H, Osaka A. In vivo evaluation of bone-bonding of titanium metal chemically treated with a hydrogen peroxide solution containing tantalum chloride [J]. Biomaterials, 2001, 22: 875-881.
[12] CHU C l, ZHOU J, CHUNG C Y, PU Y p, LIN P h. In situ formation of titania film on NiTi alloy treated with hydrogen peroxide solution at low temperature [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 834-838.
[13] Hu T, Chu c l, Yin l h, Pu y p, Dong y s, guo c, Sheng x b, Chung c y, Chu p k. In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation [J]. Trans Nonferrous Met Soc China, 2007, 17(3): 553-557.
[14] Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique [J]. Journal of Hazardous Materials, 2003, B98: 33-50.
[15] Bergendahl J A, Thies T P. Fenton’s oxidation of MTBE with zero-valent iron [J]. Water Research, 2004, 38: 327-334.
[16] Starosvetsky D, Gotman I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy [J]. Biomaterials, 2001, 22(13): 1853-1859.
[17] Cho S B, Nakanishi K, Kokubo T, Soga N. Dependence of apatite formation on silica gel on its structure: Effect of heat treatment [J]. J Am Ceram Soc, 1995, 78: 1769-1774.
[18] Poon R W Y, Ho J P Y, Liu X Y, Chung C Y, Chu P K, Yeung K W K, Lu W W, Cheung K M C. Improvements of anti-corrosion and mechanical properties of NiTi orthopedic materials by acetylene, nitrogen and oxygen plasma immersion ion implantation [J]. Nuclear Instruments and Methods in Physics Research, 2005, B237: 411-416.
Foundation item: Project supported by Program for New Century Excellent Talents(NCET) in University of Ministry of Education of China; Project(50501007) supported by the National Natural Science Foundation of China; Project(BK2007515) supported by the Natural Science Foundation of Jiangsu Province, China; Project(7001999) supported by SRG Grant from the Research Committee of the CityU of HK
Corresponding author: CHU Cheng-lin; Tel: +86-25-52090683; E-mail: clchu@seu.edu.cn


