文章编号:1004-0609(2015)04-1093-10
采用阴离子膜电解法从红土镍矿常压浸出液中制备金属镍
孟 龙1, 2, 3,曲景奎2, 3,谢克强1,张培育2, 3,韩林芯2, 3,郭 强2, 3,齐 涛2, 3,张国之2, 3
(1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190;
3. 中国科学院 过程工程研究所 绿色过程与工程重点实验室,北京 100190)
摘 要:
摘 要:以红土镍矿常压酸浸净化液为原料,采用阴离子交换膜与电解结合的方法制备金属镍。分别研究电解液成分、温度、时间、电流密度和pH等因素对阴极电流效率、回收率、能耗以及槽电压的影响。结果表明:含Ni2+溶液浓度为64 g/L、H3BO4浓度为40 g/L、H2SO4浓度为0.1 mol/L、温度为40 ℃、电流密度为 300 A/m2、pH为5.2时,阴极电流效率达到98.47%,能耗为3470 kW·h/t,得到的金属镍纯度达到99.9%以上,能够满足工业上的要求。
关键词:
中图分类号:TF815 文献标志码:A
Preparation of Ni from nickel laterite leaching solution by anion membrane electrolysis method
MENG Long1, 2, 3, QU Jing-kui2, 3, XIE Ke-qiang1, ZHANG Pei-yu2, 3, HAN Lin-xin2, 3,
GUO Qiang2, 3, QI Tao2, 3, ZHANG Guo-zhi2, 3
(1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,
Kunming 650093, China;
2. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
3. Key Laboratory of Green Process and Engineering, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China)
Abstract: Ni was prepared by the method combining anionexchange membrane with electrolysis using nickel laterite atmospheric acid leaching purifying solution as main raw material. The influences of parameters including the electrolyte composition, temperature, time, current density, pH and other factors on the cathodic current efficiency, recovery rate, energy consumption and cell voltage were explored, respectively. The results indicate that the cathode current efficiency and the energy consumption are 98.47%, 3470 kW·h/t at Ni concentration of 64 g/L, H3BO4 concentration of 40 g/L, H2SO4 concentration of 0.1 mol/L, temperature of 40 ℃, pH of 5.2 and cathode current density of 300 A/m2, respectively,and the purity of this nickel can achieve more than 99.9%, which can meet the industrial requirements.
Key words: purifying solution; anion exchange membrane; electrolysis; Ni; current efficiency; energy consumption
镍是一种银白色重金属,以其优良的特性,被用于制造不锈钢、耐热合金、抗腐蚀合金、磁性合金和特种合金等方面,是一种重要的战略资源[1]。镍资源主要有硫化镍矿和红土镍矿两种,其中红土镍矿占镍资源总量的60%以上。随着高品位含镍硫化矿的日益枯竭和社会发展对镍需求量的不断增加,高效低成本地开发利用红土镍矿具有重要的意义。
目前,红土镍矿的处理工艺分为火法和湿法两种。火法工艺主要包括镍铁工艺和镍锍工艺[2-3]。湿法工艺主要包括还原焙烧-氨浸[4-6]、高压酸浸[7-9]和常压酸 浸[10-14]等。其中,常压酸浸工艺具有投资少、技术风险小、易于操作、适用矿种广、收益率较高等优点,成为非常具有吸引力的一种工艺选择[1]。但到目前为止,常压酸浸工艺还未实现大规模工业应用,其主要问题在于酸耗大、浸出液难以处理。红土镍矿中的镍主要以晶格取代的形式赋存在铁矿物中,为使镍完全浸出,需消耗大量酸,且伴随大量的铁浸出。若采用普通中和剂进行沉淀除铁,则有沉淀量较大,过滤困难、镍/钴夹带损失大等问题;若采用萃取方式除铁,则萃取剂用量较大。因此,如何解决常压酸浸过程酸耗大,浸出液中铁、镍分离的难题是常压酸浸工艺走向工业化的主要瓶颈。针对上述不足,中国科学院过程工程研究所湿法冶金清洁生产技术国家工程实验室研发了红土镍矿盐酸常压酸浸新工艺,工艺原则流程如图1所示[15]。新工艺既实现镍、钴与铁的高效分离,又实现盐酸的再生循环。工艺得到的镍、钴溶液经进一步除杂、萃取分离及硫酸转型后,最终得到用于本研究电解的硫酸镍溶液。
传统制备金属Ni的方法为电解工艺,电解液主要包括氯化体系[16-18]和硫酸体系[19-21]。氯化体系的优点是溶液导电性较好、能耗较低,缺点是反应有氯气释放,污染和腐蚀较严重,劳动条件差[22];硫酸体系是常用的电解体系,它的优点是反应稳定、污染低、缺点是溶液导电性稍差、电流效率较低[23]。离子膜技术如今已成功用于氯碱和电解行业,该技术显示的高效、优质、污染少的特性引起了人们的高度重视[24]。离子交换膜中的交换基团较稳定,膜能耐化学腐蚀且对物质有高度的选择透过性,在电化学中可促进电解的复分解反应,制取固体高聚物电解质[25]。离子膜电解技术具有很广阔的应用前景,在于离子膜可以将电解池的阴、阳极室隔开,维持极室间良好的导电性,解决传统电解过程中导电性差的问题,同时具有提高电流效率和增加产物纯度的优点[26-27]。本文作者采用膜电解方法,重点研究电解液成分、温度、时间、电流密度、pH等因素对电流效率及能耗的影响,优化电解工艺条件,制备出合格的电解镍产品,为常压酸浸工艺的进一步完善和工业应用提供理论支持。

图1 常压酸浸工艺流程图
Fig. 1 Flow chart of atmospheric acid leaching
1 实验
1.1 实验原料及装置
红土镍矿常压浸出液经中和除杂、镍/钴分离等工艺后,得到纯净的氯化镍溶液,进一步经中和、硫酸转型后,制得所需浓度的硫酸镍溶液。以此硫酸镍溶液为原料,进行阴离子交换膜电解制备金属镍。
实验采用聚四氟乙烯电解槽(自制),装置示意图如图2所示,其中隔膜是阴离子交换膜(IONSEPTM,杭州埃尔环保科技有限公司生产)。阳极是耐腐蚀不锈钢(316L,有效面积是40 mm×60 mm),阴极是多孔不溶性镀钌铱钛阳极(有效面积是40 mm×75 mm),阳极液是稀硫酸溶液,阴极液是上述硫酸镍溶液。
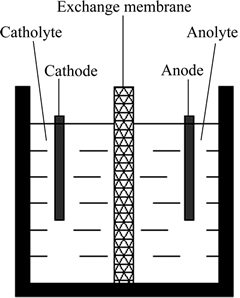
图2 电解槽装置示意图
Fig. 2 Schematic diagram of electrolysis cell
1.2 实验原理及步骤
电解过程阴、阳极的反应如式(1)和(2)所示。
阴极反应:
Ni2++2e→Ni2H++2e→H2 ↑ (1)
阳极反应:
2H2O-4e→O2↑+4H+ (2)
离子交换膜的微观结构类似于离子交换树脂,可分为基膜和活性基团两大部分。基膜是具有立体网状结构的高分子化合物,活性基团是具有交换作用的阳(或阴)离子与基膜相连的固定阴(或阳)离子组成[28]。离子交换膜的最基本性能是对不同性质的离子具有选择透过性。以阳膜为例,膜中的活性基团在电离之后带有电荷,在固定基团附近与电解质溶液中带相反电荷(可交换)的离子形成双电层,此时固定基团构成足够强的负电场,使膜外溶液中带正电荷的离子容易被吸入空隙中透过阳膜,而排斥带负电荷的离子使之不能进入、透过阳膜,电解质溶液中的离子与膜内离子发生交换作用,最后达到平衡,构成平衡体系;阴膜的情况正好相反;从而使离子交换膜具有选择透过性[29-30]。
实验前,将阴极板一面用AB胶涂抹均匀,放入干燥箱中烘干;用砂纸将另一板面打磨、抛光,稀盐酸清洗其表面,放入超声波中进行超声,去离子水清洗干净,再在干燥箱中烘干。将两个电极分别放入两个极室,保持一定的极距,随后将电解槽放入恒温水浴中,阴、阳极室中分别加入300 mL硫酸镍溶液和稀硫酸溶液进行电解。
1.3 分析方法
实验在恒定的电流下进行,每隔30 min记录电压的变化,取其平均值;实验得到的电解金属镍用SEM(FEI MLA 250,美国FEI电子光学公司生产)观察其表面形貌;阴极电流效率η、能耗W和回收率ε分别由式(3)~式(5)计算所得。
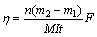 (3)
(3)
式中:m1为阴极板反应前的质量,g;m2为阴极板反应后的质量,g;M为镍的摩尔质量,g/mol;F为法拉第常数,96485 C/mol;n为电子转移个数;I为电流强度,A;t为电解时间,h。
 (4)
(4)
式中:U为平均槽电压,V;η为阴极电流效率。
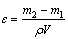 (5)
(5)
式中: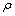 为反应前阴极电解液中Ni2+的浓度,g/L;V为反应前阴极电解液的体积,L。
为反应前阴极电解液中Ni2+的浓度,g/L;V为反应前阴极电解液的体积,L。
2 结果与分析
2.1 普通电解与膜电解的对比
在H2SO4浓度0.1 mol/L,H3BO4浓度40 g/L,电流密度300 A/m2,温度35 ℃,电极间距30 mm和电解时间3 h的条件下,改变Ni2+初始浓度,分别采用普通电解与膜电解进行实验。主要考察电流效率和能耗的影响,对比结果如图3所示。
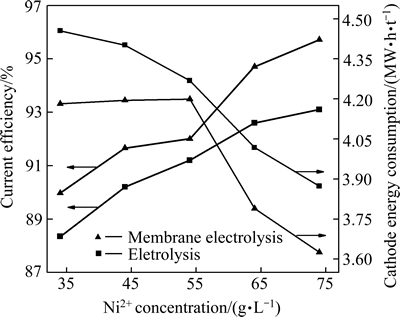
图3 电流效率和能耗在普通电解和膜电解下的对比
Fig. 3 Comparison of current efficiency and energy consumption under common electrolysis and membrane electrolysis
通过对比可知,在相同条件下,膜电解能够提高电解电流效率,降低阴极能耗。此外,阴离子交换膜电解的方法可以将阴、阳极室隔开,维持阴、阳极室之间良好的导电性,同时,反应后的镍溶液与硫酸溶液位于不同极室,很容易分离,便于循环利用,提高资源的综合利用率,因此,本实验中采用阴离子交换膜电解制备金属镍。
2.2 H3BO4浓度的影响
硼酸在电解液中有3个作用:1) 作为电解液的缓冲剂,可以保持电解液pH值的稳定;2) 促使镍的沉积电位正移,抑制析氢反应的发生,利于电沉积反应的进行;3) 可以减少阴极电解镍的脆性, 使电解镍表面平整光滑[31-33]。在Ni2+浓度34 g/L、H2SO4浓度0.1 mol/L、电流密度200 A/m2、温度35 ℃、电极间距30 mm和电解时间4 h的条件下,考察H3BO4浓度对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图4所示。从图4(a)中可以看出,随着H3BO4浓度的增加,电流效率和回收率先下降后上升,当H3BO4的浓度为40 g/L时,达到最大值。从图4(b)中可以看出,能耗和槽电压随着H3BO4浓度的增加,先升高后下降,在40 g/L时,能耗和槽电压最低,因此,选择H3BO4浓度为40 g/L对电解更有利。

图4 H3BO4浓度对电流效率、回收率、能耗和槽电压的影响
Fig. 4 Effect of H3BO4 concentration on current efficiency, recovery rate, energy consumption and cell voltage
2.3 电解时间的影响
在Ni2+浓度34 g/L、H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流密度200 A/m2、温度35 ℃、pH值3.4和电极间距25 mm的条件下,考察电解时间对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图5所示。从图5(a)中可以看出,电解2 h时,电流效率接近100%,随着时间的延长,电流效率大幅下降,但镍回收率呈现上升趋势。由图5(b)可知,电解3 h时,电解能耗及槽电压均最低,能耗仅为2837.9 kW·h/t,综合考虑,选择电解3 h较好。

图5 时间对电流效率、回收率、能耗和槽电压的影响
Fig. 5 Effect of time on current efficiency, recovery rate, energy consumption and cell voltage
2.4 电流密度的影响
在Ni2+浓度34 g/L、H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流时间3 h、温度35 ℃、pH值3.4和电极间距30 mm的条件下,考察电流密度对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图6所示。从图6(a)中可以看出,随着电流密度的增加,电流效率逐渐降低,电流效率从200 A/m2时的95.38%降低到400 A/m2时的90.06%,这可能是由于电流密度的增加,导致析氢反应加剧,使得镍沉积反应速率降低,因而电流效率逐渐降低[34],但镍的回收率逐渐增加。从图6(b)中可以看出,随着电流密度的增加,槽电压和阴极能耗显著上升,电流密度从200 A/m2增加到400 A/m2,槽电压从3.60 V升高到4.42 V,阴极能耗从3446.92 kW·h/t升高到4482.04 kW·h/t,主要原因是当电流密度升高时, 极化现象加剧, 极化电位升高,导致槽电压的数值增大。而槽电压是影响阴极能耗的首要因素,因此,电流密度增加,槽电压升高,阴极能耗增大,增加了生产成本[35-36]。综合考虑,电流密度选择300 A/m2为较好。
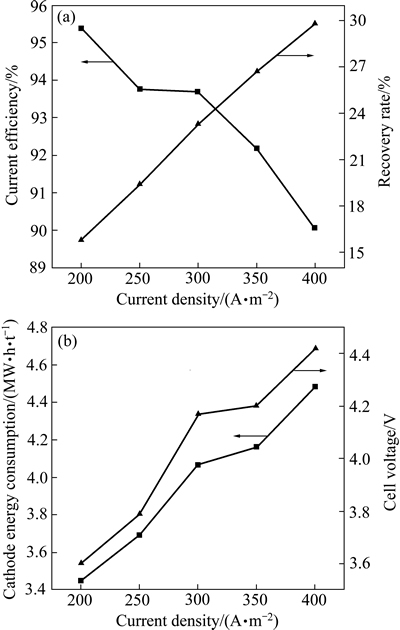
图6 电流密度对电流效率、回收率、能耗和槽电压的影响
Fig. 6 Effect of current density on current efficiency, recovery rate, energy consumption and cell voltage
2.5 Ni2+浓度的影响
在H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流密度300 A/m2、电流时间3 h、温度35 ℃和电极间距30 mm的条件下,考察Ni2+浓度对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图7所示。从图7(a)中可以看出,随着Ni2+浓度的增加,电流效率逐渐升高,回收率逐渐降低。这是由于在低浓度下,H+相对较多,而Ni2+与H+电位相近,使得H+放电比较严重,因此,电流效率较低;当Ni2+浓度逐渐增加后,溶液中大部分被Ni2+占据,促使镍沉积进行,电流效率随之增加[37]。回收率随着Ni2+浓度的增加而降低,是由于电沉积出的镍量小于初始镍溶液添加的量。从图7(b)中可知,随着Ni2+浓度的增加,能耗及槽电压先缓慢升高后逐渐降低,这是由于在Ni2+浓度较低条件下,阴极析氢严重,电解液电阻比较大,导致槽电压升高,耗能增加。此外,溶液导电性增强,槽电压逐渐降低,因而阴极能耗也随之降低。综合考虑,64 g/L为较优Ni2+浓度。
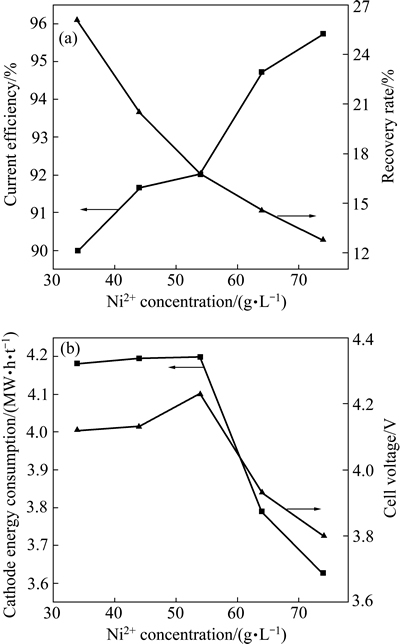
图7 Ni2+浓度对电流效率、回收率、能耗和槽电压的影响
Fig. 7 Effect of Ni2+ concentration on current efficiency, recovery rate, energy consumption and cell voltage
2.6 H2SO4浓度的影响
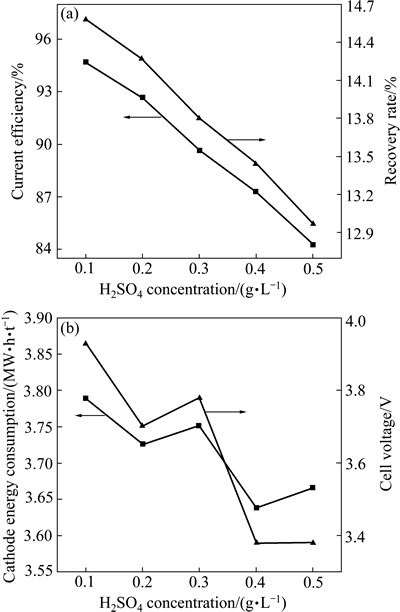
图8 H2SO4浓度对电流效率、回收率、能耗和槽电压的影响
Fig. 8 Effect of H2SO4 concentration on current efficiency, recovery rate, energy consumption and cell voltage
在Ni2+浓度64 g/L、H3BO4浓度40 g/L、电流时间3 h、电流密度300 A/m2、温度35 ℃、pH值3.4和电极间距30 mm的条件下,考察H2SO4浓度对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图8所示。由图8(a)可知,随着H2SO4浓度的增加,电流效率大幅下降,从0.1 mol/L时的94.78%一直降低到0.5 mol/L时的84.22%,同时,镍回收率也呈现下降的趋势。这可能是由于阳极液中SO42-浓度的增加阻碍了阴极液中阴离子向阳极液的迁移,导致阴极液中离子浓度相对增加,导电性变差,因而电流效率降低[38]。由图8(b)可知,阴极能耗和槽电压随着H2SO4浓度的增加呈下降趋势,但有波动变化,这可能是由阴极电流效率与阳极电流效率共同作用的结果。综合考虑,选择H2SO4浓度为0.1 mol/L,有利于电解的进行。
2.7 温度的影响
在Ni2+浓度64 g/L、H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流密度300 A/m2、电流时间3 h、pH值3.4和电极间距30 mm的条件下,考察电解温度对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图9所示。从图9中可以看出,随着温度的升高,电流效率和回收率均逐渐增加,能耗和槽电压逐渐降低。这是由于温度升高可提高溶液中反应离子在界面与扩散层的扩散系数,减小扩散层的厚度,加快离子向电极表面的迁移,分子运动加剧,使反应速率增加;而且,升温有利于降低电解液的浓差极化,有利于Ni2+扩散和沉积,以及电解液导电率的提高[39-41],因此,随着电流效率的增加,反应能耗反而降低。但是,考虑到阴离子交换膜的寿命(膜在不高于40 ℃正常工作),选择40 ℃为较优电解温度。
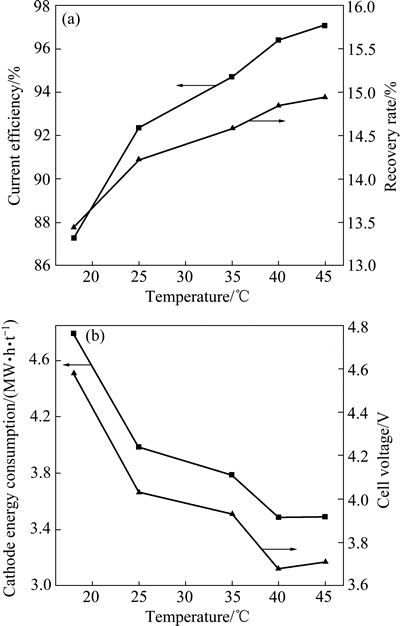
图9 温度对电流效率、回收率、能耗和槽电压的影响
Fig. 9 Effect of temperature on current efficiency, recovery rate, energy consumption and cell voltage
2.8 电极间距的影响
电极间距可以改变电极间的电阻。在Ni2+浓度64 g/L、H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流密度300 A/m2、温度40 ℃、电流时间3 h和pH值3.4条件下考察电极间距对电流效率、镍回收率以及电解能耗和槽电压的影响,其结果如图10所示。从图10(a)中看出,随着极距的增加,电流效率和回收率略微增加后保持不变。从图10(b)中可以看出,随着极距的增加,电解能耗和槽电压逐渐升高;这是由于电解液电阻增大,导致槽电压增加,因而能耗也随之增加[42],因此,选择电极间距为30 mm。
2.9 pH的影响及金属镍纯度的测定
在Ni2+浓度64 g/L、H2SO4浓度0.1 mol/L、H3BO4浓度40 g/L、电流密度300 A/m2、温度40 ℃、电流时间3 h和电极间距30 mm的条件下,考察pH值对电流效率、镍回收率以及电解能耗、槽电压和表面形貌的影响,其结果如图11和12所示。从图11(a)中可以看出,随着pH值的增大,电流效率和镍回收率均呈现增大趋势;当pH值从2.9增大到5.2时,电流效率从93.69%增加到98.47%。从图11(b)中可以看出,电解能耗和槽电压随着pH值增加,呈现先升高后降低的趋势。这是由于电解液pH值较小时,溶液中发生析氢副反应,有大量的氢气放出,此时,析出的氢气一部分进入到金属镍中,造成镍板出现气泡和孔洞,使得镍板表面形貌粗糙(见图12(a)),因此,电流效率较低,电解液电阻增大,导致槽电压升高、耗能增加;当pH值增大时,沉镍反应逐渐替代析氢反应,使得电流效率增加,槽电压和能耗也逐渐回落,有利电解进行[34, 43]。此外,金属镍的表面形貌也更加平整、光滑、致密(见图12(b))。综合考虑,pH=5.2为较优条件,此时,电流效率为98.47%,回收率为15.2%,能耗为3470 kW·h/t,槽电压为3.74 V。

图10 电极间距对电流效率、回收率、能耗和槽电压的影响
Fig. 10 Effect of electrode distance on current efficiency, recovery rate, energy consumption and cell voltage
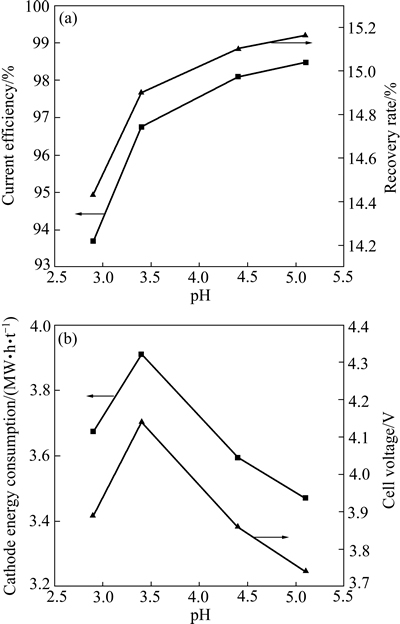
图11 pH对电流效率、回收率、能耗和槽电压的影响
Fig. 11 Effect of pH on current efficiency, recovery rate, energy consumption and cell voltage

图12 pH对金属镍表面形貌的影响
Fig. 12 Effect of pH on nickel surface morphology
在Ni2+浓度64 g/L、H3BO4浓度40 g/L、H2SO4浓度0.1 mol/L、电解时间3 h、电解温度40 ℃、电流密度300 A/m2、pH值5.2和电极间距为30 mm最优条件下制备金属镍,称取少量制备的金属镍用盐酸加热溶解,用ICP(Optima 5300DV, Perkin Elmer)测定其含量,经测定计算,其金属镍纯度达到99.9%以上,能够满足工业上的应用。
3 结论
1) 膜电解实验能够提高电解的电流效率,降低阴极能耗。离子膜可以将阴、阳极室隔开,维持阴、阳极室之间良好的导电性,便于反应后阴、阳极室电解液的分离,使其能够循环利用,提高资源的综合利用率。
2) 在Ni2+浓度64 g/L、H3BO4浓度40 g/L、H2SO4浓度0.1 mol/L、电解时间3 h、电解温度40 ℃、电流密度300 A/m2、pH值5.2和电极间距为30 mm的条件下,电解制备金属镍效果较好。此时,阴极电流效率可以达到98.47%,能耗降低到3470 kW·h/t,回收率为15.2%,槽电压为3.74 V。
3) 电解得到的金属镍纯度达到99.9%以上,能够满足工业上应用。
REFERENCES
[1] 王宝全. 褐铁型红土镍矿常压盐酸浸出工艺研究[D]. 北京: 中国科学院, 2012: 1-9.
WANG Bao-quan. Study on the atmospheric hydrochloric acid leaching of limonitic laterite[D]. Beijing: Chinese Academy of Sciences, 2012: 1-9.
[2] 李小明, 唐 琳, 刘仕良. 红土镍矿处理工艺探讨[J]. 铁合金, 2007(4): 28-30.
LI Xiao-ming, TANG Lin, LIU Shi-liang. Technology of treating nickel-bearing laterite[J]. Ferro-Alloys, 2007(4): 28-30.
[3] 陆 琳. 红土镍矿火法冶炼镍铁-金属镁联产工艺分析[J]. 现代冶金, 2012, 40(2): 33-36.
LU Lin. Analysis of pyrometallurgical nickel laterite ferronickel- magnesium generation process[J]. Modern Metallurgy, 2012, 40(2): 33-36.
[4] 尹 飞, 阮书锋, 江培海, 王成彦, 陈永强. 低品位红土镍矿还原焙砂氨浸试验研究[J]. 矿冶, 2007, 16(3): 29-33.
YIN Fei, RUAN Shu-feng, JIANG Pei-hai, WANG Cheng-yan, CHEN Yong-qiang. Experimental study on roasted ore of poor nickeliferous laterite ore with ammonia leaching technology[J]. Mining & Metallurgy, 2007, 16(3): 29-33.
[5] 胡长松, 贾彦忠, 梁德兰, 张丽肖, 王祥臻, 李仕超. 红土镍矿还原焙烧的机理研究[J]. 中国有色冶金, 2012, 41(1): 72-75.
HU Chang-song, JIA Yan-zhong, LIANG De-lan, ZHANG Li-xiao, WANG Xiang-zhen, LI Shi-chao. Studying on reductive roasting mechanism about nickel laterite[J]. China Nonferrous Metallurgy, 2012, 41(1): 72-75.
[6] 刘 慧. 还原-氨浸法富集会理含镍蛇纹石中镍的研究[D]. 沈阳: 东北大学, 2012: 23-48.
LIU Hui. The study for extract of nickel in serpentine from Huili by reduction-ammonia leaching[D]. Shenyang: Northeastern University, 2012: 23-48.
[7] LIU Kui, CHEN Qi-yuan, HUA Hui-ping, DING Zhi-ying, YIN Zhou-lan. Characteristics of scales formed from pressure leaching of Yuanjiang laterite[J]. Hydrometallurgy, 2011, 109(1/2): 131-139.
[8] 李少龙, 傅建国, 殷书岩. 红土镍矿高压酸浸工程设计综述[J]. 中国有色冶金, 2013, 42(5): 18-22.
LI Shao-long, FU Jian-guo, YIN Shu-yan. Engineering design summary on HPAL process of nickel laterite[J]. China Nonferrous Metallurgy, 2013, 42(5): 18-22.
[9] QUENEAU P B, DOANE R E, COOPERRIDER M W, BERGGREN M H, REY P. Control of autoclave scaling during acid pressure leaching of nickeliferous laterite ores[J]. Metallurgical Transactions B, 1984, 15: 433-440.
[10] 郭学益, 吴 展, 李 栋, 石文堂, 田庆华. 红土镍矿常压盐酸浸出工艺及其动力学研究[J]. 矿冶工程, 2011, 31(4): 69-76.
GUO Xue-yi, WU Zhan, LI Dong, SHI Wen-tang, TIAN Qing-hua. Atmospheric leaching of nickel laterite by hydrochloride acid and its kinetics[J]. Mining and Metallurgical Engineering, 2011, 31(4): 69-76.
[11] 周晓文. 常压酸浸法从含镍红土矿中提取镍的研究[D]. 赣州: 江西理工大学, 2009: 31-42.
ZHOU Xiao-wen. The study of the process and technology of constant-pressured acid leaching of nickel extraction from laterite-nickel ore[D]. Ganzhou: Jiangxi University of Science and Technology, 2009: 31-42.
[12] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review. Part Ⅰ. Sulphuric acid technologies[J]. Hydrometallurgy, 2008, 91(1/4): 35-55.
[13] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review. Part Ⅱ. Chloride and biotechnologies[J]. Hydrometallurgy, 2008, 91(1/4): 56-69.
[14] BUYUKAKINCI E, TOPKAYA Y A. Extraction of nickel from lateritic ores at atmospheric pressure with agitation leaching[J]. Hydrometallurgy, 2009, 97(1/2): 33-38.
[15] 郭 强, 曲景奎, 齐 涛, 张培育, 时美玲, 孟 龙. 一种红土镍矿盐酸常压浸出过程铁与镍、钴、硅分离与综合利用的清洁生产方法: 中国, CN 103757261[P]. 2014-04-30.
GUO Qiang, QU Jing-kui, QI Tao, ZHANG Pei-yu, SHI Mei-ling, MENG Long. A method of hydrochloric acid atmospheric nickel laterite leaching process of Ni/Co/Fe/Si separation and utilization of clean production: China, CN 103757261[P]. 2014-04-30.
[16] 谷晴晴, 李成威, 王飞飞, 陈雪婷, 姚 丹, 马 壮. 电解法制备微细镍粉的实验研究[J]. 宁夏工程技术, 2013, 12(3): 255-256.
GU Qing-qing, LI Cheng-wei, WANG Fei, CHEN Xue-ting, YAO Dan, MA Zhuang. Experimental study on electrolytic method of fine nickel powder preparation[J]. Ningxia Engineering Technology, 2013, 12(3): 255-256.
[17] 姜力强, 张晓忠, 毛信表, 莫一平. 超细镍粉电解制备工艺研究[J]. 材料科学与工艺, 1999, 17(1): 87-91.
JIANG Li-qiang, ZHANG Xiao-zhong, MAO Xin-biao, MO Yi-ping. Study on electrolysis process for superfine nickel powders[J]. Material Science & Technology, 1999, 17(1): 87-91.
[18] 郭学益, 黄 凯, 刘志宏, 张多默, 陈慧光. 氯化体系阴极还原电化学行为[J]. 有色金属, 2000, 52(1): 55-58.
GUO Xue-yi, HUANG Kai, LIU Zhi-hong, ZHANG Duo-mo, CHEN Hui-guang. Electrochemical behavior of nickel cathode reduction in chloride solution under different conditions[J]. Nonferrous Metals, 2000, 52(1): 55-58.
[19] LU Jing, YANG Qi-hua, ZHANG Zhao. Effects of additives on nickel electrowinning from sulfate system[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s97-s101.
[20] MOHANTY U S, TRIPATHY B C, DAS S C, SINGH P, MISRA V N. Effect of sodium lauryl sulphate (SLS) on nickel electrowinning from acidic sulphate solutions[J]. Hydrometallurgy, 2009, 100: 60-64.
[21] ALFANTAZI A M, SHAKSHOUKI A. The effects of chloride ions on the electrowinning of nickel from sulfate electrolytes[J]. Journal of the Electrochemical Society, 2002, 149: C506-C510.
[22] 赵奇金, 李日辉, 赵德厚, 董俊卿. 低氯硫酸镍电解液生产镍粉[J]. 粉末冶金技术, 1997, 15(3): 186-187.
ZHAO Qi-jin, LI Ri-hui, ZHAO De-hou, DONG Jun-qing. The production of nickel powder with nickel sulphate electrolyte containing low chloride[J]. Powder Metallurgy Technology, 1997, 15(3): 186-187.
[23] MOSKALYK R R, ALFANTAZI A M. Nickel laterite processing and electro-winning practice[J]. Minerals Engineering, 2002, 15(8): 593-605.
[24] 谭翎燕, 王训遒. 离子交换膜电解技术在湿法冶金中的应用[J]. 化工进展, 2002, 21(12): 912-914.
TAN Yu-yan, WANG Xun-qiu. Study on ion exchange membrane electrolysis technology in hydrometallurgy[J]. Chemical Industry and Engineering Process, 2002, 21(12): 912-914.
[25] 杜 杰. 离子膜电解同时制备金属锰与二氧化锰[D]. 哈尔滨: 哈尔滨工业大学, 2007: 11-12.
DU Jie. Electrodeposition of manganese metal and simultaneous production of end by ion-exchange membrane electrolysis[D]. Harbin: Harbin Institute of Technology, 2007: 11-12.
[26] REN Xiu-lian, WEI Qi-feng, HU Su-rong, WEI Si-jie. The recovery of zinc from hot galvanizing slag in an anion-exchange membrane electrolysis reactor[J]. Journal of Hazardous Materials, 2010, 181(1/3): 908-915.
[27] WEI Qi-feng, REN Xiu-lian, DU Jie, WEI Si-jie, HU Su-rong. Study of the electrodeposition conditions of metallic manganese in an electrolytic membrane reactor[J]. Minerals Engineering, 2010, 23(7): 578-586.
[28] 陆九芳. 分离过程化学[M]. 北京: 清华大学出版社, 1993: 220-222.
LU Jiu-fang. Chemical separation process[M]. Beijing: Tsinghua University Press, 1993: 220-222.
[29] WISNIEWSKI N J, WISNIEWSKA G. Application of electrodialysis and cation exchange technique to water and acid recovery[J]. Environmental Protection Engineering, 1997, 23(3): 35-36.
[30] ZABOLOTSKY V I, NIKONENKO V V. Coupled transport phenomena in overlimiting current electrodialysis[J]. Separation and Purification Technology, 1998, 14(1/3): 255-267.
[31] 高天星, 李仕雄, 刘爱心. 镍电解阴极过程中添加剂的作用及其在线监控[J]. 中国有色金属学报, 2006, 16(10): 1806-1811.
GAO Tian-xing, LI Shi-xiong, LIU Ai-xin. Role of additive in nickel electrolysis cathodic process and its on-line control[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(10): 1806-1811.
[32] SUPICOVA M, ROZIK R, TRNKOVA L. Influence of boric acid on the electrochemical deposition of Ni[J]. Journal of Solid State Electrochemistry, 2005, 10(2): 61-68.
[33] TSURU Y, NOMURA M, FOULKES F R. Effects of boric acid on hydrogen evolution and internal stress in films deposited from a nickel sulfamate bath[J]. Journal of Applied Electrochemistry, 2002, 32: 629-634.
[34] REN Xiu-lian, WEI Qi-feng, LIU Zhe, LIU Jun. Electrodeposition conditions of metallic nickel in electrolytic membrane reactor[J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 470-474.
[35] 徐海清, 钟洪胜, 袁国伟, 赵国鹏, 胡耀红. 电化学法再生酸性氯化铜蚀刻液评述[J]. 电镀与涂饰, 2011, 30(9): 35-37.
XU Hai-qing, ZHONG Hong-sheng, YUAN Guo-wei, ZHAO Guo-peng, HU Yao-hong. Review on electrolytic regeneration of acidic cupric chloride etchant[J]. Electroplation & Finishing, 2011, 30(9): 35-37.
[36] 程进辰. 提高铜电解电流密度的新方法-喷射电解液法[J]. 有色金属(冶炼部分), 1997(4): 25-28.
CHEN Jin-chen. A new method to improve the electrolytic current density of copper--injection method of electrolyte[J]. Nonferrous Metals (Extractive Metallurgy), 1997(4): 25-28.
[37] 刘可鑫. 电解法制备细颗粒镍粉的研究[D]. 长沙: 中南大学, 2004: 22-24.
LIU Ke-xin. Research of electrolytic method of fine nickel powder preparation[D]. Changsha: Central South University, 2004: 22-24.
[38] 孟 洪, 彭昌盛, 卢寿慈. 离子交换膜的选择透过性机理[J]. 北京科技大学学报, 2002, 24(6): 656-659.
MENG Hong, PENG Chang-sheng, LU Shou-ci. Permselectivity mechanism of ion exchange membranes[J]. Journal of University of Science and Technology Beijing, 2002, 24(6): 656-659.
[39] 许姣姣. 电沉积制备纳米晶镍及其电化学性能研究[D]. 昆明: 昆明理工大学, 2007: 24-32.
XU Jiao-jiao. Preparation of electro-deposition nanocrystalline nickel and its electrochemical properties[D]. Kunming: Kunming University of Science and Technology, 2007: 24-32.
[40] SAITOU M, OSHIRO S, ASADUAL HOSSAIN S M. Effect of temperature on nickel electrodeposition from a nickel sulfamate electrolyte[J]. Journal of Applied Electrochemistry, 2008, 38: 309-31.
[41] 陈培丽, 魏国侠. 电解废镍基耐高温合金回收镍的研究[J]. 南开大学学报(自然科学版), 2011, 44(6): 78-79.
CHEN Pei-li, WEI Guo-xia. Study on recycling nickel with high temperature resistant nickel base superalloy scrap by electrolysis[J]. Acta Scientiarum Naturalium Universitatis Nankaiensis, 2011, 44(6): 78-79.
[42] 刘 军. 离子膜阴极电沉积镍板同时阳极电溶高冰镍的研究[D]. 哈尔滨: 哈尔滨工业大学, 2008: 26.
LIU Jun. Study on electrodepositing nickel on cathode and simultaneous electrodissoluting nickel matte on anode by ion-exchange membrane[D]. Harbin: Harbin Institute of Technology, 2008: 26.
[43] 侯慧芬. 镍电解精炼阴极沉积物的结构和表面缺陷[J]. 上海金属, 1988, 9(5): 3-4.
HOU Hui-fen. Structure of and defect on the surface of cathode deposits obtained in electrolytic refining of nickel[J]. Shanghai Nonferrous Metals, 1988, 9(5): 3-4.
(编辑 李艳红)
基金项目:国家杰出青年科学基金资助项目(51125018);“十二五” 国家科技支撑计划课题(2011BAC06B07);国家自然科学基金资助项目(51204153)
收稿日期:2014-08-11;修订日期:2014-12-30
通信作者:郭 强,副研究员,博士;电话:010-82544847;E-mail: qguo@ipe.ac.cn
[1] 王宝全. 褐铁型红土镍矿常压盐酸浸出工艺研究[D]. 北京: 中国科学院, 2012: 1-9.
[2] 李小明, 唐 琳, 刘仕良. 红土镍矿处理工艺探讨[J]. 铁合金, 2007(4): 28-30.
[3] 陆 琳. 红土镍矿火法冶炼镍铁-金属镁联产工艺分析[J]. 现代冶金, 2012, 40(2): 33-36.
[4] 尹 飞, 阮书锋, 江培海, 王成彦, 陈永强. 低品位红土镍矿还原焙砂氨浸试验研究[J]. 矿冶, 2007, 16(3): 29-33.
[5] 胡长松, 贾彦忠, 梁德兰, 张丽肖, 王祥臻, 李仕超. 红土镍矿还原焙烧的机理研究[J]. 中国有色冶金, 2012, 41(1): 72-75.
[6] 刘 慧. 还原-氨浸法富集会理含镍蛇纹石中镍的研究[D]. 沈阳: 东北大学, 2012: 23-48.
[8] 李少龙, 傅建国, 殷书岩. 红土镍矿高压酸浸工程设计综述[J]. 中国有色冶金, 2013, 42(5): 18-22.
[10] 郭学益, 吴 展, 李 栋, 石文堂, 田庆华. 红土镍矿常压盐酸浸出工艺及其动力学研究[J]. 矿冶工程, 2011, 31(4): 69-76.
[11] 周晓文. 常压酸浸法从含镍红土矿中提取镍的研究[D]. 赣州: 江西理工大学, 2009: 31-42.
[16] 谷晴晴, 李成威, 王飞飞, 陈雪婷, 姚 丹, 马 壮. 电解法制备微细镍粉的实验研究[J]. 宁夏工程技术, 2013, 12(3): 255-256.
[17] 姜力强, 张晓忠, 毛信表, 莫一平. 超细镍粉电解制备工艺研究[J]. 材料科学与工艺, 1999, 17(1): 87-91.
[18] 郭学益, 黄 凯, 刘志宏, 张多默, 陈慧光. 氯化体系阴极还原电化学行为[J]. 有色金属, 2000, 52(1): 55-58.
[22] 赵奇金, 李日辉, 赵德厚, 董俊卿. 低氯硫酸镍电解液生产镍粉[J]. 粉末冶金技术, 1997, 15(3): 186-187.
[24] 谭翎燕, 王训遒. 离子交换膜电解技术在湿法冶金中的应用[J]. 化工进展, 2002, 21(12): 912-914.
[25] 杜 杰. 离子膜电解同时制备金属锰与二氧化锰[D]. 哈尔滨: 哈尔滨工业大学, 2007: 11-12.
[28] 陆九芳. 分离过程化学[M]. 北京: 清华大学出版社, 1993: 220-222.
LU Jiu-fang. Chemical separation process[M]. Beijing: Tsinghua University Press, 1993: 220-222.
[31] 高天星, 李仕雄, 刘爱心. 镍电解阴极过程中添加剂的作用及其在线监控[J]. 中国有色金属学报, 2006, 16(10): 1806-1811.
[35] 徐海清, 钟洪胜, 袁国伟, 赵国鹏, 胡耀红. 电化学法再生酸性氯化铜蚀刻液评述[J]. 电镀与涂饰, 2011, 30(9): 35-37.
[36] 程进辰. 提高铜电解电流密度的新方法-喷射电解液法[J]. 有色金属(冶炼部分), 1997(4): 25-28.
[37] 刘可鑫. 电解法制备细颗粒镍粉的研究[D]. 长沙: 中南大学, 2004: 22-24.
[38] 孟 洪, 彭昌盛, 卢寿慈. 离子交换膜的选择透过性机理[J]. 北京科技大学学报, 2002, 24(6): 656-659.
[39] 许姣姣. 电沉积制备纳米晶镍及其电化学性能研究[D]. 昆明: 昆明理工大学, 2007: 24-32.
[41] 陈培丽, 魏国侠. 电解废镍基耐高温合金回收镍的研究[J]. 南开大学学报(自然科学版), 2011, 44(6): 78-79.
[42] 刘 军. 离子膜阴极电沉积镍板同时阳极电溶高冰镍的研究[D]. 哈尔滨: 哈尔滨工业大学, 2008: 26.


