
J. Cent. South Univ. (2021) 28: 1901-1918
DOI: https://doi.org/10.1007/s11771-021-4738-2
Arsenic availability and transportation in soil-rice system affected by iron-modified biochar
QIAN Zi-yan(钱子妍)1, XUE Sheng-guo(薛生国)1, CUI Meng-qian(崔梦倩)1,WU Chuan(吴川)1, LI Wai-chin(李伟展)2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Department of Science and Environmental Studies, the Education University of Hong Kong,Tai Po, Hong Kong, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
Iron-modified biochar (FeOS) is known to be effective at immobilization of arsenic (As) in soils. A pot experiment was conducted to investigate the effects of FeOS on As availability and ttransportation in the soil-rice system at different growth stages of rice with different pollution levels. The results showed that Fe concentration decreased and As concentration increased in paddy soils with the FeOS addition, especially in 120 mg/kg As treatment, the As concentration decreased by 16.46% and 30.56% at the maturity stage with 0.5% and 1% FeOS additions, respectively. Compared with the control, the application of FeOS reduced the arsenic content in rice tissues and increased the biomass, with the root biomass increased by 12.68% and the shoot biomass was increased by 8.94% with the addition of 1% FeOS. This may be related to the promotion of iron plaque formation and the transformation of microbial community structure in FeOS treatments, in accordance with the result of gene abundance and Fe/As contents of iron plaque in the study. This study is expected to provide further support and theoretical basis for the application of FeOS in the remediation of As contaminated paddy soil.
Key words:
arsenic; rice; iron-modified biochar (FeOS); iron plaque; speciation; uptake;
Cite this article as:
QIAN Zi-yan, XUE Sheng-guo, CUI Meng-qian, WU Chuan, LI Wai-chin. Arsenic availability and transportation in soil-rice system affected by iron-modified biochar [J]. Journal of Central South University, 2021, 28(6): 1901-1918.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4738-21 Introduction
Arsenic (As), a carcinogenic metalloid, exerts strong physiological toxicity on most living organisms [1]. Due to natural activities such as volcanic eruptions, as well as the effects of mining, smelting, and fertilizer application, soil and groundwater have been contaminated [2], which led to the As contamination of rice [3]. In some areas such as Bangladesh, As concentrations in groundwater has reached 2 mg/L [4], which is far beyond the limit set by WHO (0.01 mg/L). The groundwater is often used for irrigation of paddy fields [5], so polluted grounderwater poses a certain threat to soil quality and food safety [6, 7]. Arsenic exposure has threatened human health in many regions of the world [8], and the frequent occurrence of arsenic-contaminated rice events has attracted much attention of researchers in recent years [9]. China is one of the countries with the most serious arsenic pollution in the world, especially in Hunan,Zhejiang, Yunnan, Guangxi and Guangdong provinces with many mines, resulting in the irrigation water in its rice fields being polluted by arsenic-containing wastewater released during mining and smelting [10]. Rice is the most important grain crop in China [11], which produces the greatest rice production in the world, and the total outputs are over two hundred million tons of rice per year [12]. Unfortunately, rice has greater capability to uptake and transport As than other crops, which is mainly due to the flooded growth condition. Therefore, there is an urgent requirement to control the level of As pollution in paddy soils [13].
Biochar, known as “black gold”, is produced by pyrolysis of biological organic materials (such as wood chips and crop straws) in an oxygen-free or low-oxygen environment [14]. It has many benefits, including a wide range of raw materials, low cost, ecological safety, and can be spread over large areas [15, 16]. In recent years, amendment of soils with biochar has been seen in many aspects: 1) to improve carbon sequestration, mainly due to its inert physical properties (large surface area and porous structure); 2) to improve soil health (by improving soil fertility and increasing crop yields) [17]; 3) to significantly increase the content of organic carbon and nitrogen in soil particulates, which means that biochar can reduce nutrient leaching and increase nutrient recycling [18]. Furthermore, the surface of biochar has a large number of organic functional groups such as COOH- and OH-, which can strongly adsorb heavy metal cations onto its surface [19, 20], such as cadmium [21].
Biochar materials can be modified by changing the pore structure, increasing the surface area and specific adsorption sites, as well as improving the surface activity. Thus some new structure biochars can be created to improve the adsorption abilities for pollutants [22]. Iron oxide is an important component of soil colloids with relatively stable chemical properties, large specific surface area and numerous adsorption sites [23]. The combination of biochar and iron oxide has been shown to reduce the mobility of As in soils by making full use of the ion exchange effect of iron oxides. The presence of surface hydroxyl groups can effectively adsorb As, whilst the mechanism of adsorption and co-precipitation allows for arsenite and arsenate to maintain their original redox states on the surface of the iron oxide and form inner sphere coordination complexes on its surface which slowly form precipitates [24].
Our previous studies showed that red mud modified biochar and iron-modified biochar could reduce arsenic availability in soils and change the microbial community structure [17, 25]. The present study further approved that the iron-modified biochar could significantly reduce total As and inorganic As in rice plants, and it is potential for the remediation of As contaminated paddy soils. Moreover, our previous study has shown that biochar modified with Fe-oxyhydroxy sulfate (biochar-FeOS) could decrease As availability in soils [25]. However, whether the iron-modified biochar could affect As behavior in rice rhizosphere as well as As accumulation and speciation in rice is still uncertain. In addition, how the iron-modified biochar affects Fe and As related genes in paddy soils is also needed further research. The present study focused on effects of iron-modified biochar on: 1) As and Fe concentration in non-rhizosphere and rhizosphere soil solution in different growth stages of rice; 2) As and Fe related gene abundances in soils; 3) As accumulation and speciation in rice plants.
2 Materials and methods
2.1 Materials
Uncontaminated soil was collected from a paddy field in the Wangcheng district of Changsha, Hunan Province, China. Surface soil samples were collected from 0-20 cm depth. Soils were subsequently air-dried and then crushed and sieved (<0.15 mm). Selected soil properties are presented in Table 1. In the environmental quality standard for agricultural soil in China (GB 15618—2008), pH≤6.5, total As≤30 mg/kg. Rice genotype Shenyou 9586, was used in this investigation, which was obtained from Hunan Agricultural University, China.
Table 1 Basic physical and chemical properties of soil

Biochar was produced using rice straw, which was pyrolysed at 300 °C for 1 h (17 °C/min) in a high-performance muffle furnace (SX 2-5-12, Yuandong Therm Corporation in Changsha, China). Biochar (10 g) was added to a solution of FeSO4·7H2O (100 mL, 0.75 mol/L) at a volume ratio of 1:0.5 with H2O2 added slowly (30%,0.4 mL/min) [25]. The solution was stirred for 24 h with a magnetic stirrer (85-2, Zhongda Instrument Corporation in Jintan), filtered, and dried at 30 °C. Analytical grade H2O2 and ferrous sulphate heptahydrate (FeSO4·7H2O) were used to prepare the modified biochars. The properties and characteristics of tested raw rice straw biochar and iron-modified biochar have been provide in our previous study [25], with basic chemical compositions, SEM morphology, XRD spectra analysis and element contents.
All seeds were sterilized with 30% H2O2 for 15 min and then thoroughly washed with deionized water. They were then germinated in Petri dishes, each containing a piece of moist filter paper. After 2 d, the germinated seeds were transplanted to a nutrient solution for two weeks. Seedlings of uniform growth were then selected and transplanted to the pot experiment [26].
2.2 Pot experiment
Exogenous As was added to the soil as an arsenate solution (Na2HAsO4·12H2O) at 40, 80 and 120 mg/kg As. Subsequently, modified biochar was hand-mixed with the paddy soils as follows:
Control: CK; CK+1 vol% FeOS; CK+0.5 vol% FeOS.
Treatment A: 40 mg/kg As; 40 mg/kg As+1 vol% FeOS; 40 mg/kg As+0.5 vol% FeOS.
Treatment B: 80 mg/kg As; 80 mg/kg As+1 vol% FeOS; 80 mg/kg As+0.5 vol% FeOS.
Treatment C: 120 mg/kg As; 120 mg/kg As+1 vol% FeOS; 120 mg/kg As+0.5 vol% FeOS.
The N, P and K (N as CO(NH2)2 at 0.2 g/kg N, P as CaH2PO4·H2O at 0.15 g/kg P2O5 and K as KCl at 0.2 g/kg K2O) were applied as basal fertilizers and thoroughly mixed with soils to ensure adequate nutrition for the growth of rice plants [2]. Soils were equilibrated for 2 weeks, and then placed in polyethylene pots (5 kg of soil, 20 cm diameter,26 cm height). At the same time, 400 mesh (30 mm) nylon meshes as rhizosphere bags were used to make a root pocket, and the root pocket was placed in the polyethylene pots. The treated soil was evenly packed into a Polyvinyl chloride (PVC) pot to keep the soil inside and outside the rhizosphere bag at the same level. The soil in the rhizosphere bag was regarded as the rhizosphere soil, and the periphery is considered as the non-rhizosphere soil. Three seedlings were planted per pot with three replicates per treatment.
2.3 Pore water and chlorophyll sampling and analysis
During the various periods of rice growth (tillering, jointing, heading, filling and maturity stages), the water level was maintained at 2-3 cm above the soil surface until harvest [27]. Leaf chlorophyll content was determined using a SPAD meter (SPAD-502, Japan). Rhizon soil moisture samplers were inserted at a depth of 10 cm into the pots; pore water was sampling and analyzed for Fe by inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin Elmer Optima 5300 DV, American) [28]. Hydride generation atomic fluorescence spectrometer (HG-AFS,AFS-8230, Beijing Jitian Instruments Co., China) was used to determine As contents.
2.4 Soil DNA extraction and quantitative real-time PCR analysis
Rhizosphere and non-rhizosphere soils were analyzed by quantitative PCR (qPCR) for arsenic functional genes and iron reduction genes for the four rice genotypes at the 15th day (tillering stage), 30th day (jointing stage), 75th day (filling stage) and 105 d (maturity stage). Total microbial DNA extraction and gene abundance amplification were according to the method of Refs. [29-31]. Arsenite oxidase genes (aioA), arsenate reductase gene (arsC), arsenite methyltransferase gene (arsM) and iron reduction gene (Geo) abundances were studied.
2.5 Extraction of iron plaque from rice roots
Extraction of Fe and As from iron plaque on rice root surfaces was conducted using the dithionite-citrate-bicarbonate (DCB) extraction method [32, 33]. The rice roots were immersed in 30 mL of DCB solution (0.03 mol/L sodium citrate and 0.125 mol/L sodium bicarbonate) at room temperature (25 °C) for extraction for 30 min, then 0.6 g sodium dithionite was added, followed by rinsing the extracted rice roots three times with deionized water, adding the wash solution to the extract, and equilibrating to 50 mL. The Fe concentration was measured by ultraviolet spectrophotometry, and the concentration of As was determined using hydride generation atomic fluorescence spectrometry (HG-AFS).
2.6 Analysis of total As and As speciation
After the plants were harvested, they were washed with deionized water. The plants were then divided into two parts: roots and shoots. After separation, part of the samples were oven-dried at 75 °C until a constant weight was obtained; the samples were then analyzed for total As. The remaining samples were freeze-dried for the determination of As species. Samples were ground after drying.
Oven-dried samples (0.2 g) were weighed into digestion tubes and digested with 15 mL acid (v(HNO3):v(HCCO4)=4:1). The solution was digested on an electric hotplate at 120 °C and left to stand overnight at room temperature (approximately 25 °C) until the solution became clear. After cooling, the digestion was filtered (0.45 μm) and diluted to 20 mL with deionized water. Total As concentration was measured using hydride generation atomic fluorescence spectrometry (HG-AFS, AFS-8230, Beijing Jitian Instruments Co., China)
Samples (0.2 g) for As speciation were weighed into 50 mL tubes and extracted with 20 mL of 1% HNO3 on heating blocks at 95 °C for 1.5 h and centrifuged at 5000 r/min (10 min). The supernatant solution was then passed through a 0.22 μm filter membrane. Arsenic speciation in the filtrate was determined by HLCP-HG-AFS (Shimadzu LC-15C Suzhou Instruments Co., China and HG-AFS, AFS-8230, Beijing Jitian Instruments Co., China).
2.7 Statistical analysis
Analysis of data was conducted using Microsoft Excel 2010 and SPSS 13.0. Significant difference between different treatments was analyzed using one-way analysis of variance (ANOVA, Tukey) by SPSS 13.0 at p<0.05 level. And the correlation analysis between Fe/As concentration in soil solutions and the DCB extractable Fe/As concentrations in Fe plaque has also been conducted on SPSS 13.0 by Pearson correlation at p<0.05 level (two-tailed). All figures were created with Origin 9.0.
3 Results and discussion
3.1 Effect of iron-modified biochar on leaf chlorophyll content
The adverse effects of arsenic on physiological and agronomical parameters are related to the basic photochemical reaction in plants, the photosynthesis. The leaf is the main organ that performs photosynthesis [34], and chlorophyll content is regarded as an important index to show the As phytotoxicity on rice plant [35]. In this study, the relative chlorophyll content (η) in leaves increased at first but then decreased throughout the growth period of rice. The content reached the maximum at heading time, approximately 38-45 (vs SPAD), and then reached the minimum, approximately 34–40 during the maturity stage, because the leaves started to age and their physiological functions weakened [36], which is consistent with the results in Ref. [37]. Heavy metal stress causes larger poisonous effect so that it destroys the chlorophyll synthesis with the increasing concentrations of heavy metals. However, in treatments with FeOS, chlorophyll content was higher than that of control groups, and in some specific growth stages, it showed significant differences as shown in Figures 1(a)-(c). In different treatments, the order of chlorophyll content was as follows: 0.5 vol% FeOS≈1 vol% FeOS>CK. While from Figure 1(d), the application of iron-modified biochar significant increased the relative chlorophyll contents at each growth stage in soils with 120 mg/kg As. The relative content of chlorophyll is as follows: 1 vol% FeOS≥0.5 vol% FeOS>CK.
The results demonstrated that iron-modified biochar could significantly increase the relative chlorophyll content of rice leaves under relatively serious arsenic pollution, and the treatment effect of 1 vol% FeOS was slightly better than that of 0.5 vol% FeOS. The effect of FeOS was associated with Fe in it [25]. On one hand, Fe was an essential element for chlorophyll synthesis, which can promote photosynthesis, alleviate arsenic poisoning symptoms and increase chlorophyll content in plants. On the other hand, Fe was oxidized in soil to form iron hydroxide to adsorb arsenic, or dissolved ferrous iron co-precipitated with arsenic, reducing the availability of arsenic in soil and effectively alleviateing As toxicity in rice [38].

Figure 1 Dynamic trend of chlorophyll relative contents (vs SPAD) in rice leaves with different treatments:
3.2 Arsenic, iron concentrations and pH in soil solutions
The pH of rhizosphere and non-rhizosphere pore waters firstly increased and then decreased, and it reached a maximum at the jointing stage, approximately 6.84 (rhizosphere) and 6.89 (non-rhizosphere) in control treatment, and then decreased during the heading, filling and maturing stages as shown in Tables 2 and 3. With the increase of incubation time, the microbial activity and respiration of root system were enhanced, which promoted the generation of CO2 and lowered the soil pH gradually [39]. Meanwhile, the oxidation of ferrous iron (Fe2+) by O2 secreted by root system also led to the decrease of rhizosphere pore water pH [40]. The reaction formula is as follows: 4Fe2++O2+ 10H2O=4Fe(OH)3+8H+. However, the addition of modified materials slightly increased the pH of rice soil solution at maturing stages, which was supposed to be the slow release of hydroxyl and other functional groups in modified materials. With paddy soil as a buffer system, the change of pH is in a dynamic equilibrium. Compared with controls, the addition of modified biochars did not significantly change pore water pH, which is consistent with results from previous soil cultivation experiments [25]. It showed minimal changes in the soil pH when adding the modified biochars, suggesting the less damage to the equilibrium of soil [41].
The dynamic effects of iron-modified biochar on As concentration in rhizosphere and non-rhizosphere soil solution during various rice growth stages are presented in Figure 2.As concentration in both rhizosphere and non-rhizosphere soil solutions firstly increased, but then decreased and reached a maximum during the heading stage. In 40 mg/kg As treatment, the addition of 1% and 0.5% iron-modified biochar reduced As concentration in rhizosphere soil solutions by 8.4% and 17.6 %, respectively. In 80 mg/kg As treatment, it declined by 17.3% and 24.7% respectively with the addition of 1% and 0.5% iron-modified biochar, and in 120 mg/kg As trreatment, by 36% and 31%, respectively. While in non-rhizosphere soil solutions, As concentration declined by 9.1% and 5.7% respectively with the addition of 1% and 0.5% iron-modified biochar in 40 mg/kg As treatment; 31.4% and 17.1% in 80 mg/kg As treatment; 22.1% and 12.5% in 120 mg/kg As treatment. Generally speaking, arsenic concentration in soil solution was higher in rhizosphere than in non-rhizosphere. In the contaminated soils with 40, 80 and 120 mg/kg exogenous As treatment, the arsenic concentration in soil solution was significantly reduced at certain specific rice growth stages for 1% and 0.5% FeOS treatments. The effect of FeOS was stronger in treatments with higher arsenic pollution levels, and seemed to be slightly greater in rhizosphere than in non-rhizosphere. However, biochar could promote the reduction of arsenic and iron in soil, causing the desorption and mobilization of As [42]. While in this study, ferrous materials changed the chemical properties of biochar such as adsorption sites and functional groups. It was found that the XRD pattern of FeOS has a mineral peak of jarosite mineral (KFe3(SO4)2(OH)6), which may lead to adsorption and immobilization of arsenic [33, 43]. Furthermore, when the dissolved Fe(II) was oxidized and newly formed, scantly soluble Fe compounds precipitated, scavenging As from the solution by coprecipitation. Studies reported that Fe compound amendments could promote the transformation of As fractions from mobile As to poorly crystalline/crystalline Fe oxide-bound As [44]. In our previous study, FeOS application can reduce the concentration of non-specific adsorbed and specific adsorbed As fractions, and increase poorly crystalline/crystalline Fe oxide-bound As and residual As fractions in soil [25]. In addition, iron redox cycling micro-organisms in paddy soils, including iron reducing bacteria and iron oxidizing bacteria, play an important role in the coupled arsenic mobilization process. Coprecipitation of Fe and As could occur in the generation of Fe(III) oxide by their mediated oxidation of Fe(II) or in the formation of secondary Fe minerals during the reduction of Fe(III). Similarly, SO42- in FeOS could act as an electron acceptor to couple the oxidation of Fe(II) and accelerate the synthesis rate of iron oxide, thus realizing immobilization. In addition, the stress of exogenous pollutants stimulated the secretion of organic acids in rhizosphere soil solution [45], and the decrease of pH enhanced the adsorption of arsenic. It might be the reason why the reduction of arsenic concentration in rhizosphere soil solution was slightly larger than that in non-rhizosphere.
Table 2 Effects of FeOS on rhizosphere soil pH

Table 3 Effects of FeOS on non-rhizosphere soil pH

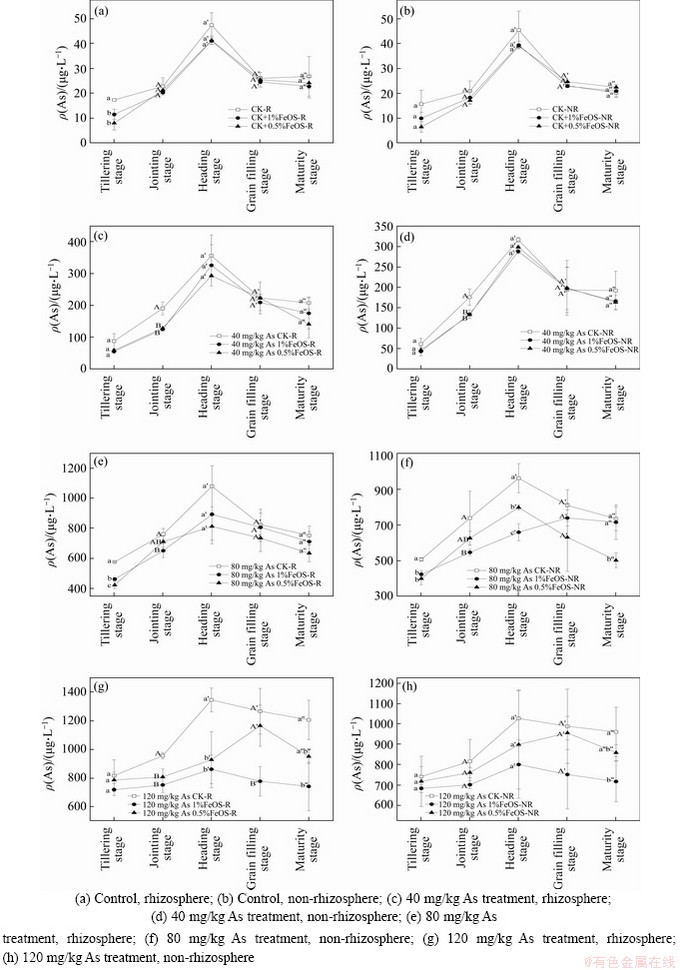
Figure 2 Changes of As concentrations in soil solutions with different treatments:
Differences in Fe concentration are presented in Figure 3, revealing that application of FeOS contributed to a higher Fe concentration in soil. The change trend of iron concentration was consistent with arsenic, increasing first and then decreasing, which was consistent with the research in Ref. [46]. During the jointing and filling stages, the iron concentrations under all treatment showed an increasing trend, and they decreased slightly at the maturity stage. As the demand for Fe increased during the period of vigorous growth, the Fe(III) in the system was reduced to Fe(II) subsequently releasing As. In addition, there appeared to be a lagging trend in the change of As in soil solutions compared to Fe with the application of FeOS treatments. It could be assumed that the application of FeOS firstly affects the concentration distribution of Fe in soil. For example, Fe (II) in iron oxide formed iron plaques on the root surface through rhizosphere radial oxygen loss and microbial oxidation, and then the arsenic concentration in rhizosphere soil solution decreased through the adsorption and coprecipitation. The decrease in pH was then accompanied by a gradual decrease in As and Fe concentrations in pore waters, indicating that acidic conditions are more conducive to the formation of Fe-As coprecipitates, which is consistent with Ref. [47]. Futhermore, the concentration of As and Fe showed a positive correlation in the soil solutions (Figure 4), consistent with previous studies [44, 48], reflecting the high affinity between iron and arsenic. These results revealed that the addition of iron source remediation materials might first affect the distribution of iron in soil, and then indirectly affect the distribution of arsenic in soil and the bioavailability of arsenic in rice plants.
3.3 Quantitative PCR analysis of arsenic metabolism and iron redox genes
Quantitative PCR analysis of arsenite oxidase genes (aioA), arsenate reductase gene (arsC), arsenite methyltransferase gene (arsM) and iron reduction gene (Geo) abundances in four growth stage of two treatments (80 mg/kg As and 80 mg/kg As+1% FeOS) is shown in Figure 5. The copy number of aioA gene decreased firstly and then increased slowly during the whole growth period, and reached the lowest at jointing stage. AioA gene refers to the oxidation process from As(III) to As(V) under the action of oxidase. Studies have proved that some rhizosphere microorganisms can promote the oxidation process of arsenic under aerobic or anaerobic conditions, thus reducing the content of As(III) [44, 49]. The addition of FeOS made aioA gene abundance higher than that of the control, and the aioA gene was significantly increased in the soil amended with 1%FeOS at maturity stage, suggesting that the abundance and activity of arsenic-oxidizing microorganisms in soil increased. The copy number of arsC gene increased first and then decreased and reached the maximum at the filling stage. The total As/Fe and As(III) concentration in soil solution was the main factor affecting the abundance of AsrC [50]. ArsC was an arsenic-resistant microbial gene, which reduces As(V) to As(III), reducing the intracellular toxicity of arsenizc but not producing energy. It is distinguish able from the mechanism of dissimilated arsenic reduction [29]. The copy number of arsM gene decreased slowly and then increased significantly during the growth period. Methylation of arsenic is a detoxification process because the toxicity of organic arsenic is much less than that of inorganic arsenic [2, 6]. Geo and arsC, arsM gene showed a similar trend in rice growth period with the treatmemt of 1% FeOS. It was reported that iron reduction in soil can promote the reduction and methylation of As [18, 50]. CHEN et al [42] indicated that enhanced activity and abundance of Fe(III)-reducing bacteria led to the reductive dissolution of As-bearing Fe(III) minerals, which related to iron reduction and the As(III) releasing. As a result of iron reduction, increased As in soil solution may trigger microbial detoxification and promote methylation of As through the challenger pathway: As(V)→As(III)→monomethyl arsenic pentavalent (MMA(V))→monomethyl arsenic trivalent (MMA(III))→dimethyl arsenic pentavalent (DMA(V))→dimethyl arsenic trivalent (DMA(III))→trimethylarsine oxide pentavalent (TMAO(V))→trimethylarsine oxide trivalent (TMA(III)), resulting in an increase of methylation of As [31].
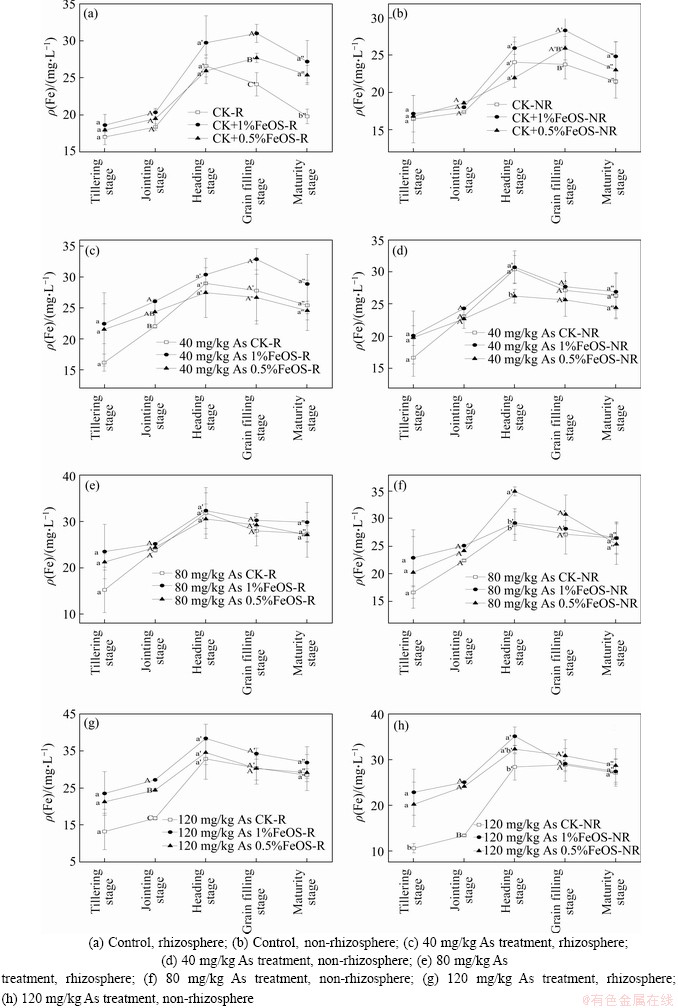
Figure 3 Changes of Fe concentration in soil solutions with different treatments:

Figure 4 Correlations between Fe and As concentrations in soil solutions:
Overall, the increase of four genes abundance in rhizosphere and non-rhizosphere soil revealed that the addition of FeOS improved the activity of micro-organisms which was related to iron and arsenic transformation in soils. QIAO et al [51] reported that biochar and lactate provided nutrition for micro-organisms and changed the microbial community structure by producing dissolved carbon and changing the pH value of soil, thereby stimulating the activity of bacteria containing arsenic reduction genes ArrA or arsC, such as Geobater sp. and Enterobacteriaceae sp. [52]. The FeOS combined the characteristics of Fe material and biochar, and the addition of FeOS introduces dissolved Fe(II) into soil, resulting in the increase of the abundance of iron redox cycling micro-organisms in the soils. The dissimilatory iron reduction process mediated by iron reducing bacteria might result in the release of As(V) originally adsorbed on iron (hydroxide) oxides, thus indirectly increasing the concentration of As in the soils and promoting the speciation transformation processes of As such as redox and methylation. It coincides with the results in Ref. [50] that the abundance of AioA, asrC and asrM gene was significantly positively correlated with Geo. Besides, the reduction of Fe(III) to Fe(II) by iron reducing bacteria also might form new secondary minerals and re-sequestrated As. Moreover, the quinolinone and phenazine groups on biochar can also serve as electron shuttles to accelerate the electron transfer between bacteria and iron minerals, thus promoting the migration and transformation of iron and arsenic in soils [53].
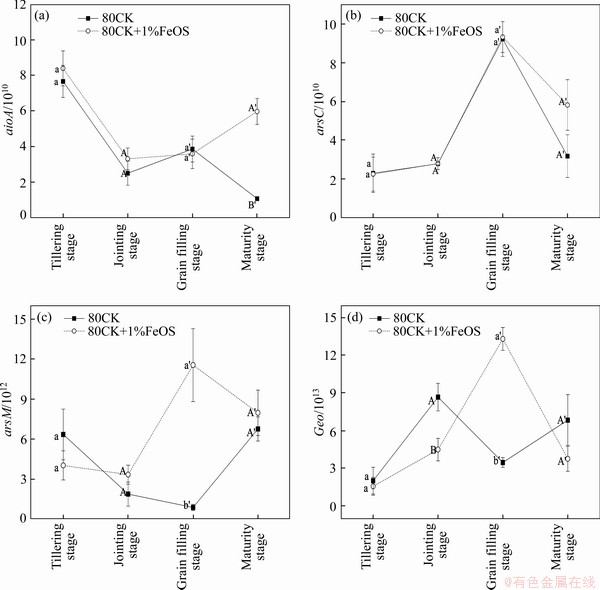
Figure 5 Copy numbers of aioA (a), arsC (b), arsM (c), Geo genes (d) present in soil samples in four growth stages in 80 mg/kg As treatment
3.4 Arsenic accumulation and speciation
Arsenic content and speciation in rice plant tissues are important factors in human health risk assessment. Since inorganic arsenic is generally considered to be more harmful than arsenic organic, the toxicity and bioavailability of arsenic to rice plant depends not only on its total content, but also on its speciation. Arsenic species, including As(III), As(V), DMA and MMA, were analyzed in the rice tissues for all treatments. Inorganic As species (As(III) and As(V)) were the main species in rice roots and shoots, whereas the two organic As species, DMA and MMA, accounted for only a small proportion of the total amount of As, in the order of different species As as follows: As(III)≥As(V)> DMA>MMA (Figure 6), which was consistent with results in Ref. [54]. It reported that the majority of the As presented as inorganic As species (ranged from 46% to 87%) and subsequently DMA (ranged from 14% to 32%) in stems and leaves. The preponderance of As(III) in plants was attributed to its higher bioavailability and its predominance in flooded paddy soils. On the other hand, studies have reported that plants have the ability to mediate arsenate reduction [55], which may be due to the self-defense mechanism of plants, that is, As(V) can occupy phosphate binding sites in the ATP cycle, thus affecting the production of adenosine triphosphate. In addition, arsenate is easier to combine with iron plaque, while arsenite has lower affinity with iron oxide, which makes it easier for arsenite to enter rice tissue.

Figure 6 Proportions of As species in roots and shoots with different treatments:
Arsenic content in the roots was greater than that in shoots (Figure 6). Many other researches have investigated the As concentration in rice tissue, in the order as grain≤leaf≤stem≤root [52]. The much higher accumulation in roots than other tissues was attributed to the inability of the plant to translocate As beyond the roots. The transformation efficiency of arsenic in rice tissues is also related to arsenic speciation. DMA is much more efficient than inorganic arsenic in transferring from rice roots to shoots [56] and subsequently from shoots to grains [57], explaining why the ratio of DMA to inorganic arsenic was higher in shoots than in roots (Figure 6). In the soil with higher arsenic pollution, the addition of FeOS significantly decreased inorganic arsenic contents in roots and shoots of rice plant. It was speculated that the decrease of As content was related to iron plaque in root surface, which acts as a barrier to prevent plants from arsenic, inhibiting the uptake of arsenic in rice plants.
3.5 Effect of iron-modified biochar on As sequestration in iron plaque
Table 4 presents the Fe and As contents in iron plaques. In different arsenic pollution soils, iron content in iron plaque was increased by adding FeOS, indicating that it promoved the formation of iron plaque, but there was no significant difference in the groups (p<0.05). It may be attributed to the increase of iron content in soil by adding iron oxide, which provided an iron source for the formation of iron plaque on the root surface. On the other hand, FeOS affected the abundance of some iron oxidizing bacteria or iron reducing bacteria such as Leptothrix sp., Gallionella sp. or Geobacter sp., Anaeromyxobacter sp., etc, in soil [42, 58], thus affecting the formation/dissolution of iron plaque.
Iron plaque is mainly composed of ferrihydrite, containing less goethite and a small amount of siderite [31]. The development of aerenchyma tissue allows the roots to secrete oxygen, which contributes to the conversion of water-soluble ferrous iron to form ferric oxide on root surfaces [2, 6]. The formation of iron plaque on root surface was reported to prevent As from transferring into the root system and play an important role in reducing As uptake according to some greenhouse and field experiments [48, 59, 60]. However, other studies revealed that the accumulation of As by iron plaques on the root surface promoted the absorption and accumulation of As by the roots and hence aerial tissues [32]. LIU et al [61] investigated that iron plaque increased arsenic uptake, but inhibited arsenic transfer from root to shoot. In the present study, the concentration of As and Fe showed a significantly positive correlation (p<0.001) in the iron plaque (Figure 7), combined with the results of arsenic content in roots and shoots of rice, indicating that iron plaque can immobile arsenic in soil and separate arsenic from plants, which have considerable implications for the environmental fate of As. Moreover, as that concentration of exogenous arsenic in soil increased, the iron content in the iron plaque also increased significantly, showing that the formation of iron plaque was related to the concentration of arsenic in soil. Soil polluted with higher concentration arsenic tended to form more iron plaques.
Table 4 Fe and As concentration on Fe plaque of rice roots with different treatments
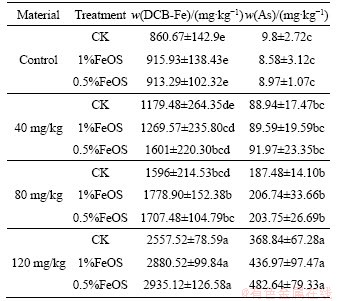

Figure 7 Correlations between Fe and As concentrations on Fe plaque of rice roots
3.6 Effect of iron-modified biochar on rice plant biomass
Rice biomass in different treatments is shown in Table 5. Arsenic pollution in soil had a major influence on the normal growth of rice, and the biomass of roots and shoots decreased significantly with the increase of arsenic concentration in soil (p<0.05). The biomass of roots and shoots of rice plants reached the maximum in the soil without exogenous arsenic, which was 48.90 g/pot and 268.12 g/pot, respectively. The biomass of roots with FeOS treantments was higher than that of control, although it showed no significant difference. For shoots, the addition of FeOS significantly increased the biomass in the soil with higher concentration of arsenic (80 and 120 mg/kg). In 80 mg/kg As treatment, adding 1% or 0.5% FeOS, the shoot biomass increased by 13.66% and 12.17%, respectively. In 120 mg/kg As treatment, adding 1% or 0.5% FeOS, the shoot biomass increased by 15.14% and 10.63%, respectively.
Table 5 Effect of iron-modified biochars on rice biomass of root and shoot (g/pot)
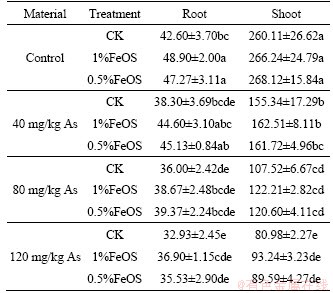
XIAO et al [62] reported that some iron-oxiding bacteria have plant growth-promoting traits. For example, some of them could mediate phosphate solubilization and indoleacetic acid molecule production. Therefore, compared to the control, the explanations for the increase of biomass might be related to the changes of microbial community structure and microbial activity with FeOS treatments. Moreover, the addition of FeOS deceased the As content in soil solution and stimulated the formation of iron plaque, which alleviates the stress of arsenic pollution on rice growth. WU et al [7] also investigated that the dry biomasses of rice roots, shoots and grains were slightly increased with amendments of iron-modified biochar in the heavy metal contaminated soil. Similarly, it also reported that addition of ferromanganese oxide biochar composites increased the rice roots, stems, leaves, and grains biomass in As-contaminated paddy soils [63].
4 Conclusions
The application of iron modified biochar could reduce the bioavailability of arsenic in rice plants. In this study, the application of FeOS increased the concentration of Fe and decreased the concentration of As in soil solution, and it promoted the formation of iron plaque on root surface which chould sequestrated As. The chlorophyll content and shoot biomass in rice plant with FeOS treatments were higher and the As concentrations in different parts of rice were lower than those of the control, and it showed significant differences in the soil with higher As content. Moreover, the addition of FeOS changed the abundance of iron reduction, arsenic reduction and arsenic methylation genes, affecting the abundance and activity of micro-organisms related to iron and arsenic metabolism in soil.
Contributors
The overarching research goals were developed WU Chuan, QIAN Zi-yan, and CUI Meng-qian. QIAN Zi-yan conducted the literature review, analyzed the data and wrote the first draft of the manuscript. XUE Sheng-guo guided the study, and reviewed the manuscript. CUI Meng-qian provided the experimental approaches and carried out experiments. WU Chuan reviewed the manuscript, and proposed some important advices. LI Wai-chin reviewed the manuscript.
Conflict of interest
QIAN Zi-yan, XUE Sheng-guo, CUI Meng-qian, WU Chuan, and LI Wai-chin declare that they have no conflict of interest.
References
[1] PAN Wei-song, WU Chuan, XUE Sheng-guo, HARTLEY W. Arsenic dynamics in the rhizosphere and its sequestration on rice roots as affected by root oxidation [J]. Journal of Environmental Sciences, 2014, 26(4): 892-899. DOI: 10.1016/S1001-0742(13)60483-0.
[2] WU Chuan, ZOU Qi, XUE Sheng-guo, PAN Wei-song, YUE Xu, HARTLEY W, HUANG Liu, MO Jing-yu. Effect of silicate on arsenic fractionation in soils and its accumulation in rice plants [J]. Chemosphere, 2016, 165: 478-486. DOI: 10.1016/j.chemosphere.2016.09.061.
[3] SINGH R, SINGH S, PARIHAR P, SINGH V P, PRASAD S M. Arsenic contamination, consequences and remediation techniques: A review [J]. Ecotoxicology and Environmental Safety, 2015, 112: 247-270. DOI: 10.1016/j.ecoenv.2014. 10.009.
[4] MEHARG A A, RAHMAN M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption [J]. Environmental Science & Technology, 2003, 37(2): 229-234.
[5] LI R Y, STROUD J L, MA J F, MCGRATH S P, ZHAO F J. Mitigation of arsenic accumulation in rice with water management and silicon fertilization [J]. Environmental Science & Technology, 2009, 43(10): 3778-3783. DOI: 10.1021/es803643v.
[6] WU Chuan, ZOU Qi, XUE Sheng-guo, PAN Wei-song, HUANG Liu, HARTLEY W, MO Jing-yu, WONG Ming-hung. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL) [J]. Environmental Pollution, 2016, 212: 27-33. DOI: 10.1016/j.envpol.2016.01.004.
[7] WU Chuan, SHI Li-zheng, XUE Sheng-guo, LI Wai-chin, JIANG Xing-xing, RAJENDRAN M, QIAN Zi-yan. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils [J]. Science of the Total Environment, 2019, 647: 1158-1168. DOI: 10.1016/j.scitotenv.2018.08.087.
[8] WANG Jun, CHENG Qing-yu, XUE Sheng-guo, RAJENDRAN M, WU Chuan, LIAO Jia-xin. Pollution characteristics of surface runoff under different restoration types in manganese tailing wasteland [J]. Environmental Science and Pollution Research, 2018, 25(10): 9998-10005. DOI: 10.1007/s11356-018-1338-2.
[9] CUI Meng-qian, WU Chuan, JIANG Xing-xing, LIU Zi-yu, XUE Sheng-guo. Bibliometric analysis of research on soil arsenic during 2005-2016 [J]. Journal of Central South University, 2019, 26(2): 479-488. DOI: 10.1007/s11771-019-4020-z.
[10] XUE Sheng-guo, SHI Li-zheng, WU Chuan, WU Hui, QIN Yan-yan, PAN Wei-song, HARTLEY W, CUI Meng-qian. Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines [J]. Environmental Research, 2017, 156: 23-30. DOI: 10.1016/j.envres.2017.03.014.
[11] QIAO Jiang-tao, LI Xiao-min, LI Fang-bai. Roles of different active metal-reducing bacteria in arsenic release from arsenic-contaminated paddy soil amended with biochar [J]. Journal of Hazardous Materials, 2018, 344: 958-967. DOI: 10.1016/ j.jhazmat.2017.11.025.
[12] RAJENDRAN M,ANWen-hui, LI Wai-chin, PERUMAL V, WU Chuan, SAHI S V, SARKAR S K. Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides(L.) Roberty) [J]. Journal of Central South University, 2019, 26(2): 489-500. DOI: 10.1007/s11771-019-4021-y.
[13] WU Chuan, HUANG Liu, XUE Sheng-guo, PAN Wei-song, ZOU Qi, HARTLEY W, WONG Ming-hung. Oxic and anoxic conditions affect arsenic (As) accumulation and arsenite transporter expression in rice [J]. Chemosphere, 2017, 168: 969-975. DOI: 10.1016/j.chemosphere.2016.10.114.
[14] SU Hui-jie, FANG Zhan-qian, TSANG P E, FANG Jian-zhang, ZHAO Dong-ye. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil [J]. Environmental Pollution, 2016, 214: 94-100. DOI: 10.1016/j.envpol.2016.03.072.
[15] AHMAD M, RAJAPAKSHA A U, LIM J E, ZHANG M, BOLAN N, MOHAN D, VITHANAGE M, LEE S S, OK Y S. Biochar as a sorbent for contaminant management in soil and water: A review [J]. Chemosphere, 2014, 99(3): 19-33. DOI: 10.1016/j.chemosphere.2013.10.071.
[16] VITHANAGE M, HERATH I, JOSEPH S, BUNDSCHUH J, BOLAN N, OK Y S, KIRKHAM M B, RINKLEBE J. Interaction of arsenic with biochar in soil and water: A critical review [J]. Carbon, 2017, 113: 219-230. DOI: 10.1016/ j.chemosphere.2013.10.071.
[17] SMEBYE A, ALLING V, VOGT R D, GADMAR T C, MULDER J. Biochar amendment to soil changes dissolved organic matter content and composition [J]. Chemosphere, 2015, 142: 100-105. DOI: 10.1016/j.chemosphere.2015.04. 087.
[18] WANG Ning, XUE Xi-xie, JUHASZ A L, CHANG Zhi-zhou, LI Hong-bo. Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic [J]. Environmental Pollution, 2017, 220: 514-522. DOI: 10.1016/j.envpol.2016.09.095.
[19] BEESLEY L, MARMIROLI M, PAGANO L, PIGONI V, FELLET G, FRESNO T, VAMERALI T, BANDIERA M, MARMIROLI N. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanumlycopersicum L.) [J]. Science of the Total Environment, 2013, 454-455(5): 598-603. DOI: 10.1016/j.scitotenv2013.02.047.
[20] RAJENDRAN M,SHI Li-zheng,WU Chuan,LI Wai-chin,AN Wen-hui,LIU Zi-yu,XUE Sheng-guo. Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil-rice system [J]. Chemosphere, 2019, 222: 314-322. DOI: 10.1016/ j.chemosphere.2019.01.149.
[21] XU Yan-zhe, FANG Zhan-qing, TSANG E P. In situ immobilization of cadmium in soil by stabilized biochar-supported iron phosphate nanoparticles [J]. Environmental Science and Pollution Research, 2016, 23: 19164-19172. DOI: 10.1007/s11356-016-7117-z.
[22] WANG Shen-sen, GAO Bin, ZIMMERMAN A R, LI Yun-cong, MA Le-na, HARRIS W G, MIGLIACCIO K W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite [J]. Bioresource Technology, 2015, 175: 391-395. DOI: 10.1016/j.biortech.2014.10.104.
[23] YAN X L, LIN L Y, LIAO X Y, ZHANG W B, WEN Y. Arsenic stabilization by zero-valent iron, bauxite residue, and zeolite at a contaminated site planting Panax notoginseng [J]. Chemosphere, 2013, 93(4): 661-667. DOI: 10.1016/ j.chemosphere.2013.05.083.
[24] YANG Zhi-hui, LIU Lin, CHAI Li-yuan, LIAO Ying-ping, YAO Wen-bin, XIAO Rui-yang. Arsenic immobilization in the contaminated soil using poorly crystalline Fe-oxyhydroxy sulfate [J]. Environmental Science and Pollution Research, 2015, 22: 12624-12632. DOI: 10.1007/s11356-015-4455-1.
[25] WU Chuan, CUI Meng-qian, XUE Sheng-guo, LI Wai-chin, HUANG Liu, JIANG Xing-xing, QIAN Zi-yan. Remediation of arsenic-contaminated paddy soil by iron-modified biochar [J]. Environmental Science and Pollution Research, 2018, 25: 20792-20801. DOI: 10.1007/s11356-018-2268-8.
[26] WU Chuan, WANG Qiong-li, XUE Sheng-guo, PAN Wei-song, LOU Lai-qing, LI Dao-jun, HARTLEY W. Do aeration conditions affect arsenic andphosphate accumulation and phosphate transporter expression in rice (Oryza sativa L.)? [J]. Environmental Science and Pollution Research, 2018, 25(1): 43-51. DOI: 10.1007/s11356-016-7976-3.
[27] FITZ W J, WENZEL W W. Arsenic transformation in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation [J]. Journal of Biotechnology, 2002, 99(3): 259-278. DOI: 10.1016/S0168-1656(02)00218-3.
[28] ZHU Feng, CHENG Qing-yu, XUE Sheng-guo, LI Chu-xuan, HARTLEY W, WU Chuan, TIAN Tao. Influence of natural regeneration on fractal features of residue microaggregates in bauxite residue disposal areas [J]. Land Degradation & Development, 2018, 29: 138-149. DOI: 10.1002/ldr.2848.
[29] SUN Y M, POLISHCHUK E A, RADOJA U, CULLEN W R. Identification and quantification of arsC genes in environmental samples by using real-time PCR [J]. Journal of Microbiological Methods, 2004, 58: 335-349. DOI: 10.1016/j.mimet.2004.04.015.
[30] JIA Yan, HUANG Hai, ZHONG Min, WANG Feng-hua, ZHANG Li-mei, ZHU Yong-guan. Microbial arsenic methylation in soil and rice rhizosphere [J]. Environmental Science & Technology, 2013, 47: 3141-3148. DOI: 10.1021/es303649v.
[31] SOMENAHALLY A C, HOLLISTER E B, LOEPPERT R H, YAN W G, GENTRY T J. Microbial communities in rice rhizosphere altered by intermittent and continuous flooding in fields with long-term arsenic application [J]. Science of the Total Environment, 2011, 43: 1220-1228. DOI: 10.1016/ j.soilbio.2011.02.011.
[32] LEE C, HSIEH Y, LIN T, LEE D. Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice [J]. Plant and Soil, 2013, 363(1, 2): 231-241. DOI: 10.1007/s11104-012-1308-2.
[33] SYU C H, JIANG P Y, HUANG H H, CHEN W T, LIN T H, LEE D Y. Arsenic sequestration in iron plaque and its effect on As uptake by rice plants grown in paddy soils with high contents of As, iron oxides, and organic matter [J]. Soil Science and Plant Nutrition, 2013, 59(3): 463-471. DOI: 10.1080/00380768.2013.784950.
[34] VARAPRASAD B, DAUGHTRY C S, CODLING E E, HANSEN D Y, SUSAN W H, GREEN C E. Evaluating leaf and canopy reflectance of stressed rice plants to monitor arsenic contamination [J]. International Journal of Environmental Research and Public Health, 2016, 13(6): 606-621. DOI: 10.3390/ijerph13060606.
[35] RAHMAN M A, HASEGAWA H, RAHMAN M M, ISLAM M N, MIAH M A M, TASMEN A. Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh [J]. Chemosphere, 2007, 67: 1072-1079. DOI: 10.1016/j.chemosphere.2006. 11.061.
[36] KIM H B, KIM S H, JEON E K, KIM D H, TSANG D C W, ALESSI D S, KWON E E, BAEK K. Effect of dissolved organic carbon from sludge, Rice straw and spent coffee ground biochar on the mobility of arsenic in soil [J]. Science of the Total Environment, 2018, 636: 1241-1248. DOI: 10.1016/j.scitotenv.2018.04.406.
[37] CUI Da-lian, MA Yu-xin, PANG Cai-jiu, DING Xue-jiao. The effects of Zn2+, Cd2+ Pollution on physiological and biochemical characters of Sesbania Cannabina Pers [C]. Advanced Materials Research, 2012, 6: 518-523. DOI: 10.4028/www.scientific.net/AMR.518-523.2039.
[38] JR V U U, NAKAYAMA A, TANAKA S, KANG Y, SAKURAI K, IWASAKI K. Potential for the alleviation of arsenic toxicity in paddy rice using amorphous iron-(hydr)oxide amendments [J]. Soil Science and Plant Nutrition , 2010, 55(1): 160-169. DOI: 10.1111/j.1747-0765.2008. 00341.x.
[39] TAO S, CHEN Y J, XU F L, CAO J, LI B G. Changes of copper speciation in maize rhizosphere soil [J]. Environmental Pollution, 2003, 122(3): 447-454. DOI: 10.1016/S0269-7491(02)00313-5.
[40] DAKORA F D, PHILLIPS D A. Root exudates as mediators of mineral acquisition in low-nutrient environments [J]. Plant and Soil, 2002, 245(1): 201-213. DOI: 10.1023/A: 1020809400075.
[41] GOLDBERG S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals [J]. Soil Science Society of America Journal, 2002, 66: 413-421. DOI: 10.2136/ sssaj2002.4130.
[42] CHEN Zheng, WANG Yuan-peng, XIA Dong, JIANG Xiu-li, FU Dun, SHEN Liang, WANG Hai-tao, LI Qing-biao. Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition [J]. Journal of Hazardous Materials, 2016, 311: 20-29. DOI: 10.1016/j.jhazmat.2016.02.069.
[43] OUYANG Bing-jie, LU Xian-cai, LIU Huan, LI Juan, ZHU Ting-ting, ZHU Xiang-yu, LU Jian-jun, WANG Ru-cheng. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization [J]. Geochimica et Cosmochimica Acta, 2014, 124(1): 54-71. DOI: 10.1016/j.gca.2013.09.020.
[44] YU Huan-yun, WANG Xiang-qin, LI Fang-bai, LI Bin, LIU Chuan-ping, WANG Qi, LEI Jing. Arsenic mobility and bioavailability in paddy soil under iron compound amendments at different growth stages of rice [J]. Environmental Pollution, 2017, 224: 136-147. DOI: 10.1016/j.envpol.2017.01.072.
[45] ZHANG Jun, ZHOU Wu-xian, LIU Bing-bing, HE Jian, SHEN Qi-rong, ZHAO Fang-jie. Anaerobic arsenite oxidation by an autotrophic arsenite-oxidizing bacterium from an arsenic-contaminated paddy soil [J]. Environmental Science & Technology, 2015, 49: 5956-5964. DOI: 10.1021/ es506097c.
[46] GARNIER J M, TRAVASSAC F, LENOBLE V, ROSE J, ZHENG Y, HOSSAIN M S, CHOWDHURY S H, BISWAS A K, AHMED K M, CHENG Z, GEEN A V. Temporal variations in arsenic uptake by rice plants in Bangladesh: the role of iron plaque in paddy fields irrigated with groundwater [J]. Science of the Total Environment, 2010, 408(19): 4185-4193. DOI: 10.1016/j.scitotenv.2010.05.019.
[47] EHLERT K, MIKUTTA C, KRETZSCHMAR R. Mineralogical controls on the bioaccessibility of Arsenic in Fe(III)-As(V) coprecipitates [J]. Environmental Science & Technology, 2018, 52(2): 616-627. DOI: 10.1021/acs.est. 7b05176.
[48] ZOU Qi, AN Wen-hui, WU Chuan, LI Wai-chin, FU An-qin, XIAO Rui-yang, CHEN Hui-kang, XUE Sheng-guo. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition [J]. Environmental Chemistry Letters, 2018, 16: 615-622. DOI: 10.1007/s10311-017-0688-1.
[49] ZECCHIN S, CORSINI A, MARTIN M, ROMANI M, BEONE G M, ZANCHI R, ZANZO E, TENNI D, FONTANELLA M C, CAVALCA L. Rhizospheric iron and arsenic bacteria affected by water regime: Implications for metalloid uptake by rice [J]. Soil Biology & Biochemistry, 2017, 106: 129-137. DOI: 10.1016/j.soilbio.2016.12.021.
[50] XUE Sheng-guo, JIANG Xing-xing, WU Chuan, HARTLEY W, QIAN Zi-yan, LUO Xing-hua, LI Wai-chin. Microbial driven iron reduction affects arsenic transformation and transportation in soil-rice system [J]. Environmental Pollution, 2020, 260: 114010. DOI: 10.1016/j.envpol.2020.114010.
[51] QIAO Jiang-tao, LI Xiao-min, Hu Min, LI Fang-bai, YONG L Y, SUN Wei-min, HUANG Weinlin, CUI Jiang-hu. Transcriptional activity of arsenic-reducing bacteria and genes regulated by lactate and biochar during arsenic transformation in flooded paddy soil [J]. Environmental Science & Technology, 2018, 52(1): 61-70. DOI: 10.1021/acs.est. 7b03771.
[52] ZHAO Can-can, FU Sheng-lei, MATHEW R P, LAWRENCE K S, FENG Yu-cheng. Soil microbial community structure and activity in a 100-year-old fertilization and crop rotation experiment [J]. Journal of Plant Ecology, 2015, 8(6): 623-632. DOI: 10.1093/jpe/rtv007.
[53] WU Chuan, AN Wen-hui, LIU Zi-yu, LIN Jun, QIAN Zi-yan, XUE Shen-gguo. The effects of biochar as the electron shuttle on the ferrihydrite reduction and related arsenic (As) fate [J]. Journal of Hazardous Materials, 2020, 390: 121391. DOI: 10.1016/j.jhazmat.2019.121391.
[54] SMITH E, JUHASZ A L, WEBER J, NAIDU R. Arsenic uptake and speciation in rice plants grown under greenhouse conditions with arsenic contaminated irrigation water [J]. Science of the Total Environment, 2008, 392(2, 3): 277-283. DOI: 10.1016/j.scitotenv.2007.11.023.
[55] WEBB S M, GAILLARD J F, MA L Q, TU C. XAS speciation of arsenic in a hyper-accumulating fern [J]. Environmental Science & Technology, 2003, 37(4): 754-760. DOI: 10.1021/es0258475.
[56] RAAB A, WILLIAMS P N, MEHARG A, FELDMANN J. Uptake and translocation of inorganic and methylated arsenic species by plants [J]. Environmental Chemistry, 2007, 4: 197-203. DOI: 10.1071/EN06079.
[57] CAREY A M, SCHECKEL K G, LOMBI E, NEWVILLE M, CHOI Y, NORTON G J, CHARNOCK J M, FELDMANN J, PRICE A H, MEHARG A A. Grain unloading of arsenic species in rice [J]. Plant Physiology, 2010, 152: 309-319. DOI:10.1104/pp.109.146126.
[58] ZHANG Chun-hua, GE Ying, YAO Huan, CHEN Xiao, HU Min-kun. Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review [J]. Frontiers of Environmental Science & Engineering, 2012, 6(4): 509-517. DOI: 10.1007/s11783-012-0394-y.
[59] WU Chuan, ZOU Qi, XUE Sheng-guo, PAN Wei-song, HUANG Liu, HARTLEY W, MO Jing-yu, WONG Ming-huang. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL) [J]. Environmental Pollution, 2016, 212: 27-33. DOI: 10.1016/j.envpol.2016.01.004.
[60] HU Min, LI Fang-bai, LIU Chuan-ping, WU Wei-jian. The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice [J]. Scientific Reports, 2015, 5: 13611-13620. DOI: 10.1038/ srep13611.
[61] LIU W J, ZHU Y G, HU Y, WILLIAMS P N, GAULT A G, MEHARG A A, CHARNOCK J M, SMITH F A. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza Sativa L.) [J]. Environmental Science & Technology, 2006, 40(18): 5730-5736. DOI: 10.1021/es060800v.
[62] XIAO An-wen, LI Wai-chin, YE Zhi-zhong. Effects of Fe-oxidizing bacteria (FeOB) on iron plaque formation, As concentrations and speciation in rice (Oryza sativa L.) [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110136. DOI: 10.1016/j.ecoenv.2019.110136.
[63] LIN Li-na, GAO Min-ling, QIU Wei-wen, WANG Di, HUANG Qing, SONG Zheng-guo. Reduced arsenic accumulation in indica rice (Oryza sativa L.) cultivar with ferromanganese oxide impregnated biochar composites amendments [J]. Environmental Pollution, 2017, 231: 479-486. DOI: 10.1016/j.envpol.2017.08.001.
(Edited by ZHENG Yu-tong)
中文导读
铁改性生物炭对土壤-水稻系统中砷有效性和迁移的影响
摘要:铁改性生物炭(FeOS)能有效地促进土壤中砷的固定。本文通过盆栽实验研究了铁改性生物炭对砷在土壤-水稻系统中有效性和迁移的影响。本实验研究了不同砷污染水平下,施用铁改性生物炭对水稻土壤溶液、铁膜中砷和铁的含量的影响,以及对水稻根、茎生物量和砷积累量的影响。结果表明,施用铁氧化物提高了土壤溶液中铁的浓度,降低了砷的浓度;特别是在120 mg/kg As处理中,添加0.5%和1% FeOS后,成熟期砷浓度分别降低16.46%和30.56%。与对照相比,施用FeOS降低了水稻组织中的砷含量,增加了水稻根和茎的生物量。添加1% FeOS后,水稻根系生物量增加12.68%,地上部生物量增加8.94%。这可能与FeOS的添加促进了铁膜形成和微生物群落结构的转变有关,与本实验中土壤的铁砷相关基因丰度和铁膜的铁含量所呈现的结果一致。该研究可为FeOS在砷污染水稻土修复中的应用提供进一步的支持和理论依据。
关键词:砷;水稻;铁改性生物炭;铁膜;形态;吸收
Foundation item: Project(2019YFC1803601) supported by the National Key Research and Development Program of China; Project(41771512) supported by the National Natural Science Foundation of China; Project(2018RS3004) supported by Hunan Science & Technology Innovation Program, China
Received date: 2020-09-07; Accepted date: 2021-01-15
Corresponding author: WU Chuan, PhD, Professor; Tel: +86-15273172436; E-mail: wuchuan@csu.edu.cn; ORCID: https://orcid.or 0000-0002-5259-3130
Abstract: Iron-modified biochar (FeOS) is known to be effective at immobilization of arsenic (As) in soils. A pot experiment was conducted to investigate the effects of FeOS on As availability and ttransportation in the soil-rice system at different growth stages of rice with different pollution levels. The results showed that Fe concentration decreased and As concentration increased in paddy soils with the FeOS addition, especially in 120 mg/kg As treatment, the As concentration decreased by 16.46% and 30.56% at the maturity stage with 0.5% and 1% FeOS additions, respectively. Compared with the control, the application of FeOS reduced the arsenic content in rice tissues and increased the biomass, with the root biomass increased by 12.68% and the shoot biomass was increased by 8.94% with the addition of 1% FeOS. This may be related to the promotion of iron plaque formation and the transformation of microbial community structure in FeOS treatments, in accordance with the result of gene abundance and Fe/As contents of iron plaque in the study. This study is expected to provide further support and theoretical basis for the application of FeOS in the remediation of As contaminated paddy soil.


