Trans. Nonferrous Met. Soc. China 27(2017) 369-376
Effect of nickel phosphide nanoparticles crystallization on hydrogen evolution reaction catalytic performance
Ya-qiong CHEN1,2, Jin-feng ZHANG1,2, Lei WAN1,2, Wen-bin HU1,2,3,4, Lei LIU1,2, Cheng ZHONG3,4, Yi-da DENG3,4
1. School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China;
3. School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China;
4. Key Laboratory of Advanced Ceramics and Machining Technology (Ministry of Education), and Tianjin Key Laboratory of Composite and Functional Materials, Tianjin University, Tianjin 300072, China
Received 15 December 2015; accepted 3 May 2016
Abstract:
In order to investigate the effect of nickel phosphide nanoparticles’ (Ni-P NPs) crystallization on hydrogen evolution reaction (HER) catalytic performance, amorphous Ni-P NPs and crystalline Ni12P5 were synthesized by a simple and low-cost autocatalytic reduction method and heat treatment process. The result of electrochemical tests shows that crystalline Ni12P5 has much higher HER catalytic activity than the amorphous one. X-ray diffraction, transmission electron microscopy and X-ray photoelectron spectroscopy revealed that Ni-P bond formed during crystallization, making Ni positively charged and P negatively charged. This charged nature of Ni12P5 is similar to [NiFe] hydrogenase and its analogous, which make the removal of H2 less energy-cost.
Key words:
hydrogen evolution reaction; nickel phosphide nanoparticles; Ni12P5; catalyst; crystallization;
1 Introduction
With the decreasing reserves-to-production ratio of traditional fossil fuels and increasingly serious environment issues of pollution [1-3], a numerous researches concerning the development and utilization of renewable clean energy have been conducted [4-8]. It is widely considered that hydrogen is a promising energy carrier because of its larger storage density and longer storage time [9,10]. Among all kinds of hydrogen production methods, water electrolysis is a clean, simple and fast way to produce hydrogen on large scale [11]. According to the volcano plot described by TRASATTI [12] and  et al [13,14], the hydrogen evolution reaction (HER) catalytic performance is related to the binding strength between the metal surface and the absorbed hydrogen (M-Hads). Unfortunately, the best electrocatalyst for HER is some noble metals, such as Pt, which have optimal binding strength but cannot be used on large scale due to their high price and low store in earth. Therefore, finding an inexpensive and abundant catalyst could facilitate the industrialization of hydrogen production. Many related researches have been conducted [15-18].
et al [13,14], the hydrogen evolution reaction (HER) catalytic performance is related to the binding strength between the metal surface and the absorbed hydrogen (M-Hads). Unfortunately, the best electrocatalyst for HER is some noble metals, such as Pt, which have optimal binding strength but cannot be used on large scale due to their high price and low store in earth. Therefore, finding an inexpensive and abundant catalyst could facilitate the industrialization of hydrogen production. Many related researches have been conducted [15-18].
In recent years, metal phosphide nanoparticles as HER electrocatalyst have received a lot of concern because of its excellent catalytic activity [19-23]. Among all these metal phosphides, it has been demonstrated that crystalline nickel phosphide nanoparticles (Ni-P NPs) have superior electrocatalytic performance for HER. POPCZUN et al [24] proved that the hexagonal structure Ni2P exhibits extraordinary HER catalytic activity because of its large surface area and exposed (0 0 1) active facets. Additionally, HUANG et al [25] reported that Ni12P5 is a promising HER catalyst both in electrolysis and photoelectrolysis. PAN et al [22] compared the electrocatalytic properties of nickel phosphides with different phases and demonstrated that the crystalline phase is very important to electrocatalytic performance.
These reported researches suggested that the morphology and phases of Ni-P NPs play important roles in their HER catalytic performance. However, some other important factors, such as crystallization effect and particles size effect, have not been studied. MAO et al [26] demonstrated that the crystallinity plays an important role in the capacitor performance. JOO et al [27] proved that optimal crystallinity of TiO2 would enhance its catalytic activity. Thus, we think that it is significant to investigate the effect of Ni-P NPs’ crystallinity on HER performance. In this work, Ni-P NPs with different crystallinities were prepared by a simple and low-cost method. And the effect of crystallization of Ni-P NPs was systematically investigated for HER catalytic performance.
2 Experimental
2.1 Chemicals and materials
Nickel sulfate (NiSO4·6H2O), sodium hypophosphite (NaH2PO2·H2O), sodium hydroxide (NaOH) were purchased from the National Chemical Reagent Ltd., Shanghai, China. All chemicals were analytical grade and used without further purification. Solutions of NiSO4 (2 mol/L), NaOH (4 mol/L), NaH2PO2 (4 mol/L) were prepared by dissolving certain quantities of these chemicals in deionized water, respectively.
2.2 Synthesis of Ni-P NPs
An autocatalytic reduction method was used to prepare Ni-P nanoparticles [28]. Firstly, all the needed reagents were preheated to 90 °C. Secondly, together 15 mL NiSO4 solution, 7.5 mL NaH2PO2 solution and 22.5 mL deionized water were mixed together, then the mixture was constantly stirred violently and kept it at 90 °C. After stirring for 5 min, 30 mL NaOH solution and 50 mL NaH2PO2 solution were added to the mixture. Then, the dark-gray powder dispersion liquid would be obtained in 5 min. Finally, the dispersion was washed repeatedly with deionized water 6 times and the fully-washed powers were dried in vacuum furnace at 100 °C for 6 h.
2.3 Preparation of crystalline Ni-P NPs
Heat treatment was applied to crystallizing synthesized Ni-P NPs. The heat treatment temperature was set at 350 °C, which is above crystallization temperature of synthesized Ni-P NPs according to the differential scanning calorimetry (DSC) and thermogravimetry (TG), as shown in Fig. 1. The samples were heat treated from 20 to 350 °C with a heating rate of 10 °C/min. In order to obtain samples with different crystallinities, the samples were kept at 350 °C for different periods of time. The heat treating condition is in an oven full of 5%H2/Ar [24].
2.4 Characterization
XRD was performed by D/max-IIIA X-ray diffractometer with Cu Kα (λ=1.54  ) as irradiation source. The samples were scanned from 10° to 90° in 2θ with scan rate of 10 (°)/min. The morphology of samples was investigated by a field-emission transmission electron microscopy (JEM-2100F) with set working voltage of 200 kV. And the TEM samples were prepared by dispersing various Ni-P NPs in ethanol and depositing the dispersion liquid into a carbon copper grid. The crystallization temperature was characterized by DSC/TGA curves which were obtained by a Pyris 1 TGA Thermo Gravimetric. Besides, XPS data were obtained by testing the samples in a Kratos AXIS Ultra X-ray photoelectron spectroscope with an incident radiation of Monochromatic Al KR X-ray at 150 W.
) as irradiation source. The samples were scanned from 10° to 90° in 2θ with scan rate of 10 (°)/min. The morphology of samples was investigated by a field-emission transmission electron microscopy (JEM-2100F) with set working voltage of 200 kV. And the TEM samples were prepared by dispersing various Ni-P NPs in ethanol and depositing the dispersion liquid into a carbon copper grid. The crystallization temperature was characterized by DSC/TGA curves which were obtained by a Pyris 1 TGA Thermo Gravimetric. Besides, XPS data were obtained by testing the samples in a Kratos AXIS Ultra X-ray photoelectron spectroscope with an incident radiation of Monochromatic Al KR X-ray at 150 W.
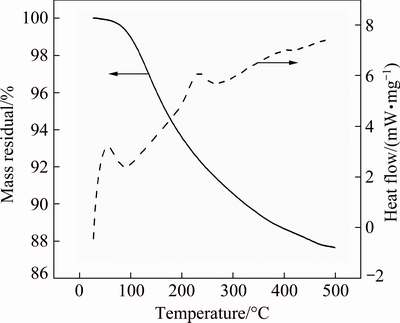
Fig. 1 DSC and TG curves of synthesized Ni-P NPs
2.5 Preparation of working electrodes
Different Ni-P catalysts and commercial Pt/C catalyst (Johnson Matthey, Hispec 3000, 20%) were prepared as electrode material. Catalyst (7 mg) and Nafion solution (2%, 100 μL) were dispersed in ethanol (1 mL) by ultrasonication, and a homogeneous ink would be formed. Then, 7 μL of catalyst ink was loaded onto a glass carbon electrode (GCE, 2.5 mm in diameter).
2.6 Electrochemical measurements
Electrochemical measurements were performed using an electrochemical workstation (CHI 660D) in a typical three-electrode glass cell. The catalysts deposited glassy carbon electrode served as the working electrode, a platinum foil and Ag/AgCl with saturated KCl were used as the counter and reference electrode, respectively. The electrolyte was oxygen-free 0.5 mol/L H2SO4 solutions, which is achieved by bubbling N2 for 20 min before the experiments.
Catalytic activity to HER was analyzed by means of cyclic voltammetry (CV), linear sweep voltammetry (LSV) and potentiostatic electrolysis. CV curves were scanned at a scan rate of 50 mV/s without accounting for uncompensated resistance. 1000 potential cycles of CV were carried out for the stability test. The LSV was conducted at a scan rate of 10 mV/s after a certain number of CV scan cycles. Potentiostatic electrolysis was carried out at 0.12 V vs RHE.
3 Results and discussion
3.1 Morphology and structure
X-ray diffraction (XRD) patterns of the before and after heat treated Ni-P NPs are shown in Fig. 2. Before heat treatment, only a single broad profile was found in the XRD pattern, indicating that the synthesized nanoparticles are amorphous. After heat treatment at 350 °C for 30 min, some peaks appeared, which means that crystallization occurred at this temperature. With increasing heat treatment time, more keen-edged peaks corresponding to Ni12P5 appeared and the peaks were largely enhanced until heat treatment for 100 min. This means that the crystallinity of Ni-P NPs would increase with increasing heat treatment time. The samples heat treated at 350 °C for 180 min show perfect peaks characteristics of tetragonal Ni12P5 phase (PDF No. 22- 1190). The peaks at 2θ values of 20.3°, 29.2°, 32.7°,35.8°, 38.4°, 41.3°, 41.7°, 44.4°, 47.0°, 49.0°, 54.0°, 56.2°, 60.1°, 68.6°, 74.0° and 74.8° correspond to the (1 0 1), (2 2 0), (3 1 0), (3 0 1), (1 1 2), (2 0 2), (4 0 0), (3 3 0), (4 2 0), (3 1 2), (5 1 0), (5 0 1), (4 2 2), (6 2 0), (5 3 2) and (0 0 4) crystalline phases, respectively. With further increase of heat treatment time, the crystal phase will not be changed.
Figures 3(a) and (b) present transmission electron microscope (TEM) images of the amorphous Ni-P NPs.
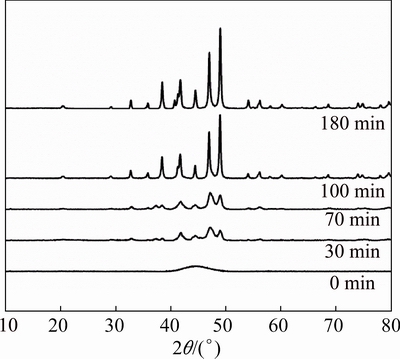
Fig. 2 XRD patterns of Ni-P NPs with different heat treatment time
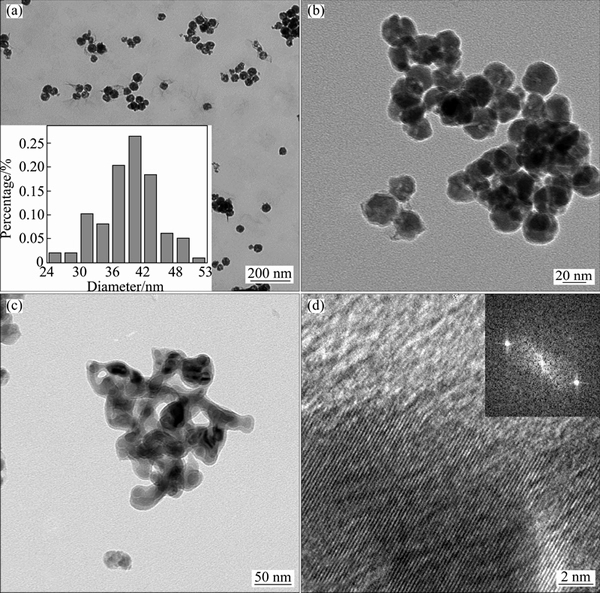
Fig. 3 TEM images (a, b) of amorphous nickel phosphide nanoparticles with inset size distribution pattern, TEM image (c) and HRTEM image (d) of well crystallized Ni12P5 nanoparticles with FFT pattern
The amorphous Ni-P NPs exhibited the polydisperse channeled nanosphere morphology with average diameter of about 40.44 nm. After being heat treated at 350 °C for 100 min, the regular nanospheres transformed to some bigger irregular particles within nanoscale dimension (Fig. 3(c)), which may be due to the high temperature during the heat treatment. From the high resolution transmission electron microscope (HRTEM) image (Fig. 3(d)) of well crystallized Ni12P5, clear lattice fringes were observed, and the interplane distance was calculated according to the single-dimensional fast Fourier transform (FFT) pattern, which is 0.186 nm. This interplane distance corresponds to the (3 1 2) crystallinity phase of Ni12P5, which demonstrates that the 100 min heat treated sample, Ni12P5, is single crystalline.
3.2 Electrocatalytic properties
In order to test electrocatalytic performance, amorphous Ni-P NPs, various crystalline Ni-P NPs with different heat treatment time and commercial Pt/C catalyst (20%) were deposited on glass carbon electrode (GCE). Because of the high temperature during synthesis, amorphous and crystalline Ni-P NPs might have oxides on their surface. Thus, all the electrochemical tests would start after the 50th cyclic voltammetric (CV) sweeps, which will help to remove oxide from the electrode surface [29].
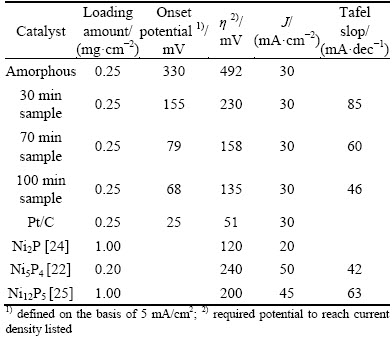
The HER catalytic performance of electrodes was judged by the overpotential required to reach an operating current density, which is determined by both its Tafel slope and onset potential [30]. Figure 4 displays the typical polarization curves of various electrodes and bare GCE in 0.5 mol/L H2SO4. For comparison, the amount of samples loaded on GCE was fixed at 0.25 mg/cm2. As expected, bare GCE showed negligible current in the given potential range. Thus, it is apparent that the cathodic current in that potential range was largely attributed to the catalytic activity of various Ni-P catalysts and commercial Pt/C catalyst. The onset potentials is the potential at which catalytic current is first observed, and we defined it on the basis of 5 mA/cm2. According to Fig. 4, the onset potentials for amorphous sample, 30 min treated sample, 70 min treated sample and 100 min treated sample were 330, 155, 79 and 68 mV, respectively. Clearly, the amorphous sample had the largest onset potential. And after crystallization, the onset potential largely decreased, indicating that the catalytic performance was enhanced. When the current density reached 30 mA/cm2, the potentials required for amorphous sample, 30 min treated sample, 70 min treated sample and 100 min treated sample were 492, 235, 158 and 140 mV, respectively.
The kinetic behavior of the various Ni-P NPs in 0.5 mol/L H2SO4 at 30 °C was studied by Tafel plots, as shown in Fig. 5. By fitting the linear part of the Tafel plots to Tafel equation, Tafel slop could be obtained. The Tafel slops of samples heat-treated for 30, 70 and 100 min were 85, 60 and 46 mA/dec, respectively. This means that the HER rates were determined by both Heyrovsky and Tafel reactions. And the samples heat-treated for 100 min had the smallest Tafel slop, demonstrating that its catalytic activity for HER is excellent.
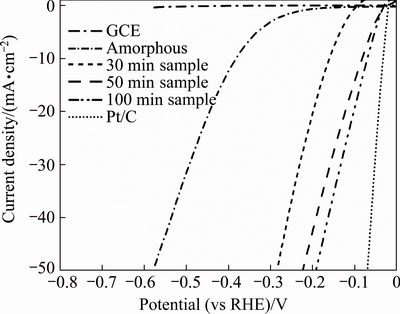
Fig. 4 Polarization curves of various Ni-P NPs, commercial Pt/C and bare GCE
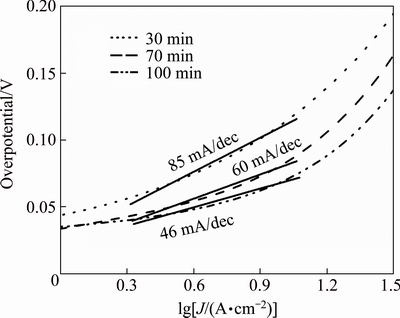
Fig. 5 Tafel plots of samples heat treated for 30, 70 and 100 min
Apparently, the HER catalytic performance of crystalline Ni-P NPs is much better than that of the amorphous one. After heat treatment, the electrocatalytic performance of Ni-P NPs was largely enhanced, which will increase with the increased crystallinity. The hydrogen evolution activity data for the tested catalysts and several recently reported Ni-P catalysts were compared in Table 1.
To further demonstrate the high electrocatalytic performance of well crystallized Ni12P5, the accelerated degradation studies of 100 min treated sample (Ni12P5) have been conducted. The linear sweep voltammetry (LSV) curves were tested after different cyclic voltammetric (CV) sweeps (Fig. 6(a)), and the long-term stability of Ni12P5 was also evaluated by electrolysis at a fixed potential (vs RHE) of 120 mV (Fig. 6(b)). It is obvious that the LSV curves after the 200th cycle, 600th cycle, and 1000th cycle were very close and Ni12P5 can remain catalytic current density of 18 mA/cm2 over 10000 s. Therefore, it indicates that the well crystallized Ni12P5 has a good stability in acid condition.
Table 1 Summary and comparison of HER catalysis data
3.3 X-ray photoelectron spectroscopy
In order to investigate the effect of crystallization, X-ray photoelectron spectroscopy (XPS) was conducted to amorphous Ni-P NPs and well crystallized Ni12P5. The XPS spectra of the Ni 2p and P 2p regions in amorphous Ni-P NPs and well crystallized Ni12P5 are shown in Fig. 7. In Ni 2p region (Figs. 7(a) and (c)), three peaks at Ni 2p3/2 energy level were found. The peaks at 853.1/853.0 eV are close to the peaks of Ni with zero valence state [31], thus it can be speculated that it corresponds to the weakly positive charged Ni (Niδ+) in Ni12P5 [25]. And comparing contribution at around 853 eV, the intensity of Niδ+ in Ni12P5 is much larger than that in amorphous Ni-P NPs, indicating only a few of Ni12P5 species formed in Ni-P NPs, which may be due to the high temperature during synthesis procedure. The peaks at 856.0/856.8 eV correspond to Ni2+ species due to a superficial passivation, and the 860.2/861.5 eV is the broad shake-up satellite due to the presence of Ni2+ species [32]. The remaining peaks at Ni 2p1/2 energy level (870.5/870.3, 875.4/875.1 and 879.0/880.0 eV) were the Ni 2p1/2 doublets of the prior bands. As to P 2p spectrum (Figs. 7(b) and (d)), the peaks at 129.6/129.5 eV are assigned to weakly negative charged P (Pδ-) in phosphide, and 133.5/133.4 eV corresponds to surface phosphate due to the superficial oxidation [33]. Similarly, the intensity of Pδ- in crystalline Ni12P5 is much larger than that in amorphous Ni-P NPs. In Fig. 7(d), two peaks at 130.3 and 134.3 eV are the doublets P 2p3/2-2p1/2 with a separation of 0.84 eV. By comparing XPS spectra of amorphous and crystalline Ni-P NPs, we can see that much more Ni12P5 formed during crystallization, which means that partial electron transfer occurred from P to Ni, making Ni positively charged.
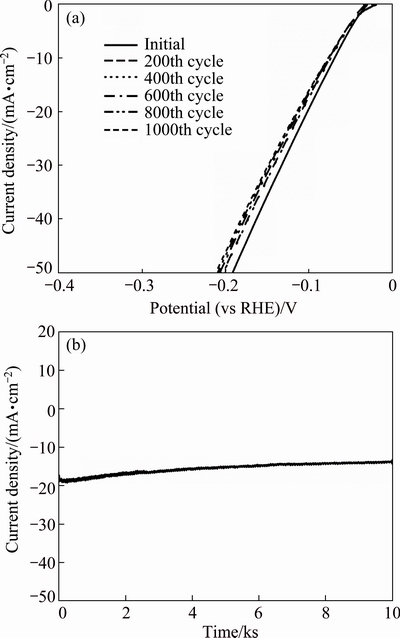
Fig. 6 LSV curves of well crystallized Ni12P5 after 200, 400, 600, 800 and 1000 cycles (a) and time-dependent current density curve of Ni12P5 heat treated for 10000 s (b)
3.4 Discussion
The HER in acid solution is thought to involve three steps [34,35]:
Volmer step: H++e+*→H* (1)
Heyrovsky step: H++H*→H2 (2)
Tafel step: 2H*→H2+2* (3)
where “*” represents a free active site, and “H*” represents the hydrogen bonded to this site. A good HER catalyst should have the ability to trap protons, bond the atomic hydrogen strongly, and desorb H2 as well [19].
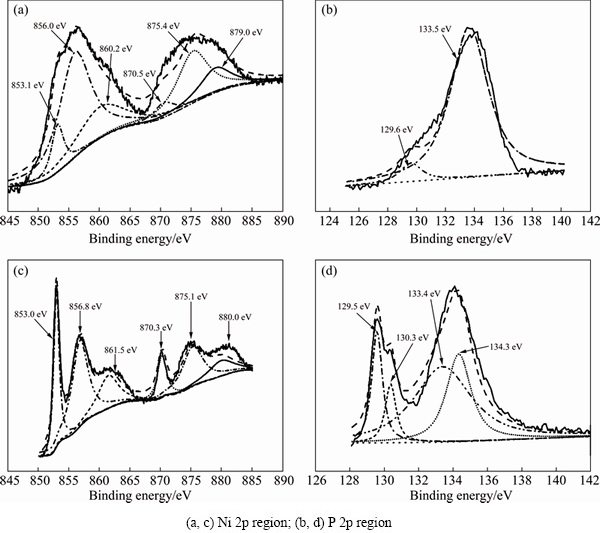
Fig. 7 XPS spectra of synthesized NPs (a, b) and Ni12P5 (c, d)
As reported, amorphous Ni-P electrodes for HER are active only with low phosphorus content (to 3%) [36]. When phosphorus content is high, it would strongly bound hydrogen, resulting in the high energy cost for the removal of H2 and the high overvoltage for H2 evolution [13,19,36]. In our study, the phosphorus content of amorphous Ni-P NPs is about 18%, therefore we speculate that its poor HER catalytic activity can be related to its strong bond with hydrogen.
From the XPS spectra, it is clear that electron transfer occurred from P to Ni during crystallization. Ni in Ni12P5 has a very small positive charge, and P has a very small negative charge. This property is very familiar to [NiFe] hydrogenase and its molecular analogues, which have high HER catalytic activity [37,38]. Hydrogenase and its analogues have hydride acceptor site (positively charged Ni in [Ni(PS3*)(CO)]- and [Ni(PNP)2]2+) to moderately bond the hydrogen, and proton acceptor site (negatively charged S in [Ni(PS3*)(CO)]- and N in [Ni(PNP)2]2+) to strongly trap the protons [19,39,40]. These two sites work co-ordinately, resulting to its HER catalytic activity. Besides, Ni2P has also been proved to be a highly active catalyst for HER because of its similar properties to [NiFe] hydrogenase [19,22,24]. Thus, it is reasonable to speculate that the high HER catalytic performance of crystalline Ni12P5 is related to the similar charged nature. After crystallization, Ni-P bond formed in Ni12P5 and it has a weak “ligand effect”, which make Ni hollow sites strong hydride acceptors [41], and negatively charged P can trap protons just like [NiFe] hydrogenase and its analogues. And according to Ref. [19] P occupied Ni hollow sites, making the removal of H2 less energy-cost.
4 Conclusions
1) Amorphous Ni-P NPs and well crystallized Ni12P5 were successfully synthesized by autocatalytic reduction method and heat treatment process.
2) The electrochemical tests results show that the amorphous Ni-P NPs have poor HER catalytic activity, while crystalline Ni-P NPs are highly active HER catalysts.
3) During crystallization, electron transfer occurred from P to Ni, P occupied Ni hollow sites and Ni-P bond formed, which make the charged nature of Ni12P5 similar to [NiFe] hydrogenase and its analogous. This property makes the removal of H2 less energy-cost and is very beneficial to HER catalytic activity.
References
[1] WIGLEY T M, RICHELS R, EDMONDS J A. Economic and environmental choices in the stabilization of atmospheric CO2 concentrations [J]. Nature, 1996, 379: 240-243.
[2] FEYGIN M, SATKIN R. The oil reserves-to-production ratio and its proper interpretation [J]. Natural Resources Research, 2004, 13: 57-60.
[3] ARMAROLI N, BALZANI V. The future of energy supply: Challenges and opportunities [J]. Angewandte Chemie International Edition, 2007, 46: 52-66.
[4] KAMAT P V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion [J]. The Journal of Physical Chemistry C, 2007, 111: 2834-2860.
[5] FURUKAWA H, YAGHI O M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications [J]. Journal of the American Chemical Society, 2009, 131: 8875-8883.
[6] YAO Mao-hai, TANG You-gen, ZHANG Li, YANG Hai-hua, YAN Jian-hui. Photocatalytic activity of CuO towards HER in catalyst from oxalic acid solution under simulated sunlight irradiation[J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1944-1949.
[7] LI Bao-song, LIN An, GAN Fu-xing. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 1193-1199.
[8] WU J, ZHOU C, ZHAO Y, SHANG L, BIAN T, SHAO L, SHI F, WU L Z, TUNG C H, ZHANG T. One-pot hydrothermal synthesis and photocatalytic hydrogen evolution of pyrochlore type K2Nb2O6 [J]. Chinese Journal of Chemistry, 2014, 32: 485-490.
[9] DUNN S. Hydrogen futures: Toward a sustainable energy system [J]. International Journal of Hydrogen Energy, 2002, 27: 235-264.
[10] YILDIZ B, KAZIMI M S. Efficiency of hydrogen production systems using alternative nuclear energy technologies [J]. International Journal of Hydrogen Energy, 2006, 31: 77-92.
[11]  Hydrogen generation from water electrolysis-possibilities of energy saving [J]. Journal of Power Sources, 2003, 118: 315-319.
Hydrogen generation from water electrolysis-possibilities of energy saving [J]. Journal of Power Sources, 2003, 118: 315-319.
[12] TRASATTI S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1972, 39: 163-184.
[13]  J K, BLIGAARD T, LOGADOTTIR A, KITCHIN J, CHEN J, PANDELOV S, STIMMING U. Trends in the exchange current for hydrogen evolution [J]. Journal of the Electrochemical Society, 2005, 152: J23-J26.
J K, BLIGAARD T, LOGADOTTIR A, KITCHIN J, CHEN J, PANDELOV S, STIMMING U. Trends in the exchange current for hydrogen evolution [J]. Journal of the Electrochemical Society, 2005, 152: J23-J26.
[14] GREELEY J,  J K, KIBLER L A, EL-AZIZ A M, KOLB D M. Hydrogen evolution over bimetallic systems: Understanding the trends [J]. Chemical Physics and Physical Chemistry, 2006, 7: 1032-1035.
J K, KIBLER L A, EL-AZIZ A M, KOLB D M. Hydrogen evolution over bimetallic systems: Understanding the trends [J]. Chemical Physics and Physical Chemistry, 2006, 7: 1032-1035.
[15] CHEN W F, SASAKI K, MA C, FRENKEL A I, MARINKOVIC N, MUCKERMAN J T, ZHU Y, ADZIC R R. Hydrogen-evolution catalysts based on non-noble metal nickel-molybdenum nitride nanosheets [J]. Angewandte Chemie International Edition, 2012, 51: 6131-6135.
[16] LEDENDECKER M, CLAVEL G, ANTONIETTI M, SHALOM M. Highly porous materials as tunable electrocatalysts for the hydrogen and oxygen evolution reaction [J]. Advanced Functional Materials, 2015, 25: 393-399.
[17] YAN Y, XIA B, XU Z, WANG X. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction [J]. ACS Catalysis, 2014, 4: 1693-1705.
[18] ZHOU S M. Studies on structure and electrocatalytic hydrogen evolution of nanocrystalline Ni-Mo-Fe alloy electrodeposit electrodes [J]. Chinese Journal of Chemistry, 2003, 21: 382-386.
[19] LIU P, RODRIGUEZ J A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: The importance of ensemble effect [J]. Journal of the American Chemical Society, 2005, 127: 14871-14878.
[20] LI Q, XING Z, ASIRI A M, JIANG P, SUN X. Cobalt phosphide nanoparticles film growth on carbon cloth: A high-performance cathode for electrochemical hydrogen evolution [J]. International Journal of Hydrogen Energy, 2014, 39: 16806-16811.
[21] HUANG Z, CHEN Z, CHEN Z, LV C, HUMPHREY M G, ZHANG C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction [J]. Nano Energy, 2014, 9: 373-382.
[22] PAN Y, LIU Y R, ZHAO J C, YANG K, LIANG J L, LIU D D, HU W H, LIU D P, LIU Y Q, LIU C G. Monodispersed nickel phosphide nanocrystals with different phases: Synthesis, characterization and electrocatalytic properties for hydrogen evolution [J]. Journal of Materials Chemistry A, 2015, 3: 1656-1665.
[23] KIBSGAARD J, JARAMILLO T F. Molybdenum phosphosulfide: An active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction [J]. Angewandte Chemie International Edition, 2014, 53: 14433-14437.
[24] POPCZUN E J, MCKONE J R, READ C G, BIACCHI A J, WILTROUT A M, LEWIS N S, SCHAAK R E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction [J]. Journal of the American Chemical Society, 2013, 135: 9267-9270.
[25] HUANG Z, CHEN Z, CHEN Z, LV C, MENG H, ZHANG C. Ni12P5 nanoparticles as an efficient catalyst for hydrogen generation via electrolysis and photoelectrolysis [J]. ACS Nano, 2014, 8: 8121-8129.
[26] MAO L, ZHANG K, CHAN HSO, WU J. Nanostructured MnO2/graphene composites for supercapacitor electrodes: The effect of morphology, crystallinity and composition [J]. Journal of Materials Chemistry, 2012, 22: 1845-1851.
[27] JOO J B, ZHANG Q, DAHL M, LEE I, GOEBL J, ZAERA F, YIN Y. Control of the nanoscale crystallinity in mesoporous TiO2 shells for enhanced photocatalytic activity [J]. Energy & Environmental Science, 2012, 5: 6321-6327.
[28] DENG Y D, WANG H R, XU L Y, WU Y T, ZHONG C, HU W B. Autocatalytic-assembly based on self-decomposing templates: A facile approach toward hollow metal nanostructures [J]. RSC Advances, 2013, 3: 4666-4672.
[29] CAI J, XU J, WANG J, ZHANG L, ZHOU H, ZHONG Y, CHEN D, FAN H, SHAO H, ZHANG J, CAO C N. Fabrication of three-dimensional nanoporous nickel films with tunable nanoporosity and their excellent electrocatalytic activities for hydrogen evolution reaction [J]. International Journal of Hydrogen Energy, 2013, 38: 934-941.
[30] BENCK J D, HELLSTERN T R, KIBSGAARD J, CHAKTHRANONT P, JARAMILLO T F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials [J]. ACS Catalysis, 2014, 4: 3957-3971.
[31] MANDALE A, BADRINARAYANAN S, DATE S, SINHA A. Photoelectron-spectroscopic study of nickel, manganese and cobalt selenides [J]. Journal of Electron Spectroscopy and Related Phenomena, 1984, 33: 61-72.
[32] OYAMA S T, WANG X, LEE Y K. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques [J]. Journal of Catalysis, 2002, 210: 207-217.
[33] SONG H, DAI M, GUO Y T, ZHANG Y J. Preparation of composite TiO2-Al2O3 supported nickel phosphide hydrotreating catalysts and catalytic activity for hydrodesulfurization of dibenzothiophene [J]. Fuel Processing Technology, 2012, 96: 228-236.
[34] KUNIMATSU K, SENZAKI T, TSUSHIMA M, OSAWA M. A combined surface-enhanced infrared and electrochemical kinetics study of hydrogen adsorption and evolution on a Pt electrode [J]. Chemical Physics Letters, 2005, 401: 451-454.
[35] CURTIS C J, MIEDANER A, ELLIS W W, DUBOIS D L. Measurement of the hydride donor abilities of [HM (diphosphine)2]+ complexes (M=Ni, Pt) by heterolytic activation of hydrogen [J]. Journal of the American Chemical Society, 2002, 124: 1918-1925.
[36] PASEKA I. Evolution of hydrogen and its sorption on remarkable active amorphous smooth NiP(x) electrodes [J]. Electrochimica Acta, 1995, 40: 1633-1640.
[37] TARD C, LIU X, IBRAHIM S K. Synthesis of the H-cluster framework of iron-only hydrogenase [J]. Nature, 2005, 433: 610-613.
[38]  C, DEMENTIN S, BERTRAND P, ROUSSET M, GUIGLIARELLI B. Inhibition and aerobic inactivation kinetics of Desulfovibrio fructosovorans NiFe hydrogenase studied by protein film voltammetry [J]. Journal of the American Chemical Society, 2004, 126: 12162-12172.
C, DEMENTIN S, BERTRAND P, ROUSSET M, GUIGLIARELLI B. Inhibition and aerobic inactivation kinetics of Desulfovibrio fructosovorans NiFe hydrogenase studied by protein film voltammetry [J]. Journal of the American Chemical Society, 2004, 126: 12162-12172.
[39] CURTIS C J, MIEDANER A, CIANCANELLI R, ELLIS W W, NOLL B C, RAKOWSKI DUBOIS M, DUBOIS D L. [Ni(Et2PCH2NMeCH2PEt2)2]2+ as a functional model for hydrogenases [J]. Inorganic Chemistry, 2003, 42: 216-227.
[40] NGUYEN D H, HSU H F, MILLAR M, KOCH S A, ACHIM C, BOMINAAR E L,  E. Nickel (II) thiolate complex with carbon monoxide and its Fe (II) analog: Synthetic models for CO adducts of nickel-iron-containing enzymes [J]. Journal of the American Chemical Society, 1996, 118: 8963-8964.
E. Nickel (II) thiolate complex with carbon monoxide and its Fe (II) analog: Synthetic models for CO adducts of nickel-iron-containing enzymes [J]. Journal of the American Chemical Society, 1996, 118: 8963-8964.
[41] RODRIGUEZ J A, KIM J Y, HANSON J C, SAWHILL S J, BUSSELL M E. Physical and chemical properties of MoP, Ni2P, and MoNiP hydrodesulfurization catalysts: Time-resolved X-ray diffraction, density functional, and hydrodesulfurization activity studies [J]. The Journal of Physical Chemistry B, 2003, 107: 6276-6285.
镍磷纳米颗粒的晶化对其催化析氢性能的影响
陈亚琼1,2,张金凤1,2,万 磊1,2,胡文彬1,2,3,4,刘 磊1,2,钟 澄3,4,邓意达3,4
1. 上海交通大学 材料科学与工程学院,上海 200240;
2. 上海交通大学 金属基复合材料重点实验室,上海 200240;
3. 天津大学 材料科学与工程学院,天津 300072;
4. 天津大学 先进陶瓷与加工教育部重点实验室及天津市复合与功能材料重点实验室,天津 300072
摘 要:为了研究镍磷纳米颗粒(Ni-P)的晶化对其催化析氢性能的影响,利用自催化还原反应及热处理工艺制备非晶Ni-P纳米颗粒及晶态Ni12P5。通过对样品进行电催化析氢性能分析,发现晶化的Ni12P5与晶化前的Ni-P相比,其催化析氢性能有了极大的提升。X射线衍射、透射电子显微镜及X射线光电子能谱分析结果表明,Ni12P5中形成了Ni-P键,使其中的Ni、P分别带有微弱的正、负电荷,[NiFe]氢化酶及其同系物的这种性质类似,会减少析氢过程中氢气脱离的能量消耗,Ni12P5的高效催化析氢性能可能与此有关。
关键词:析氢反应;镍磷纳米颗粒;Ni12P5;催化;结晶
(Edited by Xiang-qun LI)
Foundation item: Project (51125016) supported by the National Science Fund for Distinguished Young Scholars, China; Projects (51371119, 51571151) supported by the National Natural Science Foundation of China
Corresponding author: Yi-da DENG; Tel: +86-13501735862; E-mail: yida.deng@tju.edu.cn
DOI: 10.1016/S1003-6326(17)60041-4
Abstract: In order to investigate the effect of nickel phosphide nanoparticles’ (Ni-P NPs) crystallization on hydrogen evolution reaction (HER) catalytic performance, amorphous Ni-P NPs and crystalline Ni12P5 were synthesized by a simple and low-cost autocatalytic reduction method and heat treatment process. The result of electrochemical tests shows that crystalline Ni12P5 has much higher HER catalytic activity than the amorphous one. X-ray diffraction, transmission electron microscopy and X-ray photoelectron spectroscopy revealed that Ni-P bond formed during crystallization, making Ni positively charged and P negatively charged. This charged nature of Ni12P5 is similar to [NiFe] hydrogenase and its analogous, which make the removal of H2 less energy-cost.


