
Mechanical alloying and characteristics of spark plasma sintering of Ti-Al composite powders
CHEN Hua(陈 华), XUE Tao(薛 涛), MEN Hong-zhi(门洪志)
School of Materials Science and Engineering, Changchun University of Technology, Changchun 130012, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The mechanical alloying process of Ti-Al composite powders were carried out by use of high energy ball-milling machine. Structure variations of powder mixtures during mechanical alloying and characteristic of spark plasma sintering were investigated. The results show that during milling, TiAl, Ti3Al and Ti2Al phase intermetallic compounds are formed, simultaneously with powder refinement for the (TiH2-45Al-0.2Si-5Nb) and (TiH2-45Al-0.2Si-7Nb) mixtures. The particle sizes of two powder mixtures are less than 300 nm after milling for 30 h. Sintering process of the milled powder can be completed in a short time by spark plasma sintering, and the sintering microstructure is composed of fine and homogeneous TiAl and Ti3Al phase.
Key words:
mechanical alloying; TiAl intermetallics; spark plasma sintering; microstructure;
1 Introduction
TiAl-based alloys are desirable for high temperature structural parts due to their low density, high strength to mass ratio and excellent oxidation resistance, and that has great advantages in the lightmass design of aerospace and automotive parts. However, single phase TiAl(gammar)-based alloys have high brittleness, and are difficult to deform at room temperature. Previously a great number of investigations show that low-temperature deformability of duplex phase TiAl alloys with TiAl (gammar) and Ti3Al (alpha2) can be considerably improved. The objective of this study focuses on duplex phase TiAl alloys in industrial applications[1-2].
Duplex phase TiAl alloys can be obtained by powder metallurgy (PM) technology. However, during traditional PM, the diffusion coefficients of Ti and Al atoms are different and the reactive diffusion distance of atoms is long, leading to Kirkendall effect. Thus, a great number of diffusion pores exist in sintering microstructure, and it is difficult[3-4] to obtain TiAl alloys with high performance. Although, before reaction sintering, Ti, Al and other elements can be refined by larger deformation, e.g., extrusion or rolling process, and the ultimate porosity of sintered parts can be decreased, this way is difficult to realize in actual industry application[5-6].
Before reactive sintering, cool welding-fracturing -cold welding process of mixture powder can be repeatedly carried out by use of high energy milling. Under high energy striking condition, finer compound particles can be formed. This is an effective method which can obtain fine powders and microstructure. Compared with traditional extrusion or rolling process, the milling process is more simple and possible[7-8].
In this work the authors focused on the mechanical alloying and characteristics of spark plasma sintering of Ti-Al composite powders.
2 ExperimentalIn this experiment, two kinds of component Ti-Al mixture powders were investigated. Particle sizes and purity of mixture powders are shown in Table 1. The powder particles were mixed according to the compositions of (TiH2-45Al-0.2Si-5Nb) and (TiH2- 45Al-0.2Si-7Nb) (mole fraction, %). The final products after sintering were expected to be TiAl and Ti3Al phase.
Ball mill operating in an inert atmosphere was used. The powders were weighed in a glove box filled with high purity argon, ball-to-powder mass ratio of about 10,to avoid excessive increase of the local temperature inside the vial and powder, a rest of 60 min/10 h was applied during milling. A milling speed of 360 r/min was chosen in this study.
Table 1 Particle size and purity of testing powders

Powder milled for 40 h was sintered at 1 200 ℃ for 10 min by use of spark plasma sintering (SPS), rate of temperature rising was 50-80 ℃/min, and hot pressure was 30-35 MPa.
The as-milled powder and sintered microstructure were examined and analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
3 Results and discussion3.1 Morphology and particle size of milled powder
Under high energy mechanical milling condition, (TiH2, Al, Si and Nb) mixture powders undergo great plastic deformation, thus it is clearly seen that the particle size gradually decreases and more homogenizes with increasing milling time. Fig.1 shows SEM images of the (TiH2-45Al-0.2Si-5Nb) mixture milled for 5, 15, 25, 30, 35 and 40 h, respectively. After milling for 5 h (see Fig.1(a)), most of powder particles still keep initial morphology; the average particle size is 40-50 μm. After milling for 15 h, the mixture powder is obviously refined (see Fig.1(b)), an average particle size of 8-10 μm can be obtained.

Fig.1 SEM images of (TiH2-45Al-0.2Si-5Nb) mixture milled for different times: (a) 5 h; (b) 15 h; (c) 25 h; (d) 30 h; (e) 35 h; (f) 40 h
When milling time increases to 25 and 30 h (see Figs.(1) and (d)), the mostly particles size are less than 0.5 μm, and are even in the nanometer-scale range. After milling for 35 h, particle size increases a little due to the agglomerating and welding of fine powder (see Fig.1(e)). By further milling, the powder agglomerated and welded is broken up, particle size is reduced again (see Fig.1(f)).
The test results of the (TiH2-45Al-0.2Si-7Nb) mixture powder are similar to one of the (TiH2-45Al-0.2Si-5Nb) mixture powder. However, after milling for 40 h, particle size of the (TiH2-45Al- 0.2Si-7Nb) mixture powder is less than one of the (TiH2-45Al-0.2Si-5Nb) mixture powder. The particle sizes observed by SEM are mostly powder groups due to agglomerating and welding, especially, in the late period of milling,
Fig.2 shows the variation curves of particles size with increasing milling time. It can be seen that the size variation trend of the (TiH2-45Al-0.2Si-7Nb) mixture is identical with one of the (TiH2-45Al-0.2Si-5Nb) mixture. At early stage of milling (milling for 5-10 h), particles size rapidly decreases with increasing milling time, after milling for 30 h, the minimum particles size can be obtained. After further milling, change of particles size is little.
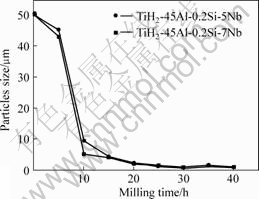
Fig.2 Variation curves of particles size with milling time
From the above analysis, it is obvious that the size of Ti-Al mixture powder possesses a critical value during milling, because mechanical alloying occurs while the particle sizes are refined. And that mechanical alloying provides both chemical composition and microstructure modifications.
At early stage of milling, the process of particle size refinement is dominant status, powder mixtures are broken into granule or flake under continuous impacting, shearing, rubbing and compressing condition. Because the materials with bcc crystal structure have a relatively large number of slip systems, the deformation is easy. However the slip systems are few for materials with hcp crystal structure, thus they are more easily broken into granules by applied force[9]. Moreover, during milling, workhardening has good influence on powder refinement.
However, after milling long time, the surface energy of powder particles is increased, powder particles can be easily gathered and combined. In this case, mechanical alloying process is the dominant status.
Micro-strain is reduced, and the variation of particle size is little, thus the cool welding and fracturing process of mixture powder attain a relative move-balance.
3.2 Structure variation of milled powder
Fig.3 shows the TEM image and selected area electron diffraction pattern of the (TiH2-45Al-0.2Si-5Nb) mixture milled for 35 h and 40 h, respectively. It can be seen that the lamellar structure is formed after milling for 35 h (see Fig.3(a) arrow). This occurs because strong extrusion and impact lead to welding of mixture powder and forming of the lamellar structure of “sandwich”. The result of diffraction shows that arrow region is Ti2Al phase. Ti2Al phase is metastable. This result proved that metastable phase is formed during milling. Between Ti and Al element can form various metastable phases, however, in this experiment, other metastable phases have not appeared.
Fig.3(b) shows the TEM morphology of powder milled for 40 h. It can be seen that particles size are very fine, and are about 100 nm. The result of TEM diffraction analysis shows forming TiAl and Ti3Al phases, obviously, milling process can form TiAl intermetallic compounds.
Fig.4 shows the TEM image and selected area electron diffraction patterns of the (TiH2-45Al- 0.2Si-7Nb) mixture milled for 35 h and 40 h, respectively. After milling for 35 h, it can be seen that a lot of fine particles exist on the powder slice, fine particle sizes are about 10 nm (see Fig.4(a)), the diffraction pattern of fine particle shows the discontinuous polycrystalline ring is diffraction spot of TiAl and Ti3Al phase. After milling for 40 h, powder particle sizes are 200-300 nm (see Fig.3(b)), and amorphous phase does not appear.
Fig.5 shows the XRD pattern of the unmilled powder and powder milled for 40 h. It is noted that the X-ray patterns of both (TiH2-45Al-0.2Si-5Nb) and (TiH2-45Al-0.2Si-7Nb) unmilled powders are almost identical (see Fig.5(a)) and reveal TiH2, Al, Si and Nb phase. However, after milling for 40 h, the diffraction peaks of Al, Nb and Si disappear, new phases of TiAl, Ti3Al, Ti2Al and NbH are formed (see Fig.5(b)), and the diffraction peaks are broadened and their intensities slightly decreased. This results from particle size refinement and the accumulation of mechanical strains[10-12].

Fig.3 TEM images and selected area electron diffraction patterns of (TiH2-45Al-0.2Si-5Nb) powder milled for 35 h(a) and 40 h(b)
After milling for long time, it is noted that Ti-Al, Al-Nb or Nb-Si intermetallic compounds have not formed. Si and Nb elements are dissolved into TiAl, Ti3Al or TiH2 phases. HELLWIG et al[13] have thought that contents of Nb in TiAl and Ti3Al phase are equal, and the maximum solid solution content of Nb in TiAl phase can reach 9%(mole fraction), the contents of Si in TiAl and Ti3Al phase are less than 2%. Nb content is 7%, and Si content is 0.2%, hence, they are fully dissolved into TiAl, Ti3Al or TiH2 phases.

Fig.4 TEM images and selected area electron diffraction patterns of (TiH2-45Al-0.2Si-7Nb) mixture milled for 35 h(a); 40 h(b)
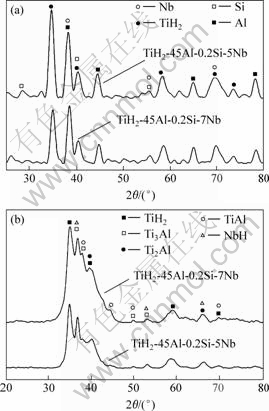
Fig.5 XRD patterns for mixture powders: (a) Unmilled; (b) Milled for 40 h
3.3 Microstructure characteristics of spark plasma sintering
The spark plasma sintering process has three characteristics. First, the mixture powders can be rapidly heated by spark discharge between the particles. Because
of reducing diffused free energy of atoms, diffusion processes of atoms are accelerated, and rapid sintering process can be obtained.
Second, during sintering, high energy particles strike interface of powder particles, leading to surface evaporation of powder particles, thereby SPS process can affect the depuration and activation.
Third, by use of SPS, powders can be compressed at a certain pressure and thus high density, even full density TiAl alloy can be obtained[14-16].
Because powders are struck during milling, lattice defects and in-stress of powders increase, and the activity of milled powder is improved. The much finer the particle are, the more higher the activity is. In addition, fine powders increase diffusion surface, and diffusion distance of powders are short, thus the full sintering time is significantly shortened. Ordinary vacuum sintering process often needs 2 h or more time for Ti alloy, however, sintering process can be completed in 10 min at 1 200 ℃ by SPS.
Fig.6 shows the micrographs of the (TiH2-45Al- 0.2Si-5Nb) and (TiH2-45Al-0.2Si-7Nb) mixture sintered at 1 200 ℃ for 10 min. It can be seen that fine, dense and homogeneous microstructure are formed by SPS.

Fig.6 Microstructures of sintered samples by SPS: (a) TiH2-45Al-0.2Si-5Nb; (b) TiH2-45Al-0.2Si-7Nb
Sintering microstructure of the (TiH2-45Al-0.2Si-7Nb) mixture has higher densification, and grains are finer. Most of the grains of the (TiH2-45Al-0.2Si-5Nb) are smaller than 2 ?m, the other particles are 2-4 μm (see Fig.6(a)). The grains of the (TiH2-45Al-0.2Si-7Nb) mixture are smaller than 2 ?m and very homogeneous (see Fig.6(b)).
Grains sizes of sintering materials depend on both powder characteristic and sintering manners. Before sintering, powders milled for 40 h are very fine, and fine intermetallic compounds are formed. It is known that fine powder has larger growing trend. However, interface bond occurs among powder particles with SPS at short time, i.e., diffusion and migration of atoms just occur in boundaries between particles. In this state, recrystallization process has not yet been carried out, thus fine globular grains are obtained.
XRD diffraction analyses of the (TiH2-45Al- 0.2Si-5Nb) and the (TiH2-45Al-0.2Si-7Nb) milled powders show that TiAl and Ti3Al phases are formed after SPS at 1 200 ℃ for 10 min (see Fig.7).
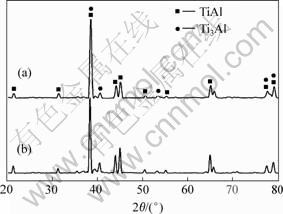
Fig.7 XRD patterns of sintered samples: (a) TiH2-45Al- 0.2Si-5Nb; (b) TiH2-45Al-0.2Si-7Nb
4 Conclusions1) During milling, TiAl, Ti3Al and Ti2Al phase intermetallic compounds are formed and the (TiH2-45Al-0.2Si-5Nb) and the (TiH2-45Al-0.2Si-7Nb) mixtures are refined. In the initial stages of milling, powder sizes are rapidly decreased, powder refinement process plays an important role. After milling for 30 h, powder sizes change a little. Mechanical alloying is predominant process. Two powder sizes reach the minimum after milling for 30 h, and are smaller than 300 nm.
2) Sintering process can be completed in short time by spark plasma sintering. Sintering microstructure is composed of fine and homogeneous TiAl and Ti3Al phase.
References
[1] WANG G W, DAHMSM M. TiAl-based alloys prepared by elemental powder metallurgy[J]. Powder Metallurgy, 1992, 24(4): 219-255.
[2] KIM Y W. Effects of microstructure on the deformation and fracture of γ-TiAl alloy[J]. Materials Science and Engineering A, 1995, 192/193: 591-538.
[3] YAN Yun-qu, ZHANG Zhen-qi. Prepared of TiAl-based alloy[J]. Titanium Industruy Progress, 2000, 1(2): 33-38. (in Chinese)
[4] LIU J, RAKOWSKI M, PETTIT F S, MEIE G H R, DETTENWANGER F, SCHUMANN E. The effect of nitrogen on the oxidation of γ-TiAl[J]. Scripta Materialial, 1996, 33(6): 997-1003.
[5] LIU Y, HUANG B Y, HE Y H. Synthesis of Ti247Al22Cr22Nb through elemental powder metallurgy[J]. Trans Nonferrous Met Soc China, 1998, 8(4): 570-575.
[6] JIANG Yao, HE Yue-hui, TANG Yi-wu, LI Zhi, HUANG Bai-yun. Fabrication of Ti-Al alloy sheets by element powder cold roll forming and reactive synthesis[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(9): 1501-1507. (in Chinese)
[7] LIU Wen-sheng, HUANG Bai-yun, TANG Jian-cheng, ZHOU Ke-chao. Lamellae growth in fully lamellar TiAl alloys and its factors[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(1): 154-157. (in Chinese)
[8] LI Xiao-qiang, HU Lian-xi, WANG Er-de. Prepation of TiAl-based alloys by mechanical milling and reactive sintering[J]. Powder Metallurgy Technology, 2001, 19(3): 131-135.
[9] HE Wen-xiang, LI Xiao-qiang, HU Lian-xi, WANG Er-de. Effect of mechanical milling on diffusive reactive of TiAl composite powders[J]. Materials Science and Engineering, 2000, 8(4): 26-29.
[10] LIU Zhi-jian, QU Hua. Yield strength calculation of fully lamellar TiAl–Nb alloys with valence-bond theory[J]. Rare Metal Materials and Engineering, 2006, 35(6): 855-859.
[11] STOLYAROY V V, ZHU Y T, ALEXANDROY L V, LOVE T C, VALIEY R Z. Grain refinement and properties of pure Ti processed by warm ECAP and cold rolling[J]. Materials Science and Engineering A, 2003, 343: 43-50.
[12] GERASIMOY K B, PAVLOV S V. Metastable Ti-Al phase obtained by mechanical alloying[J].Journal of Alloys and Compounds,1996, 242: 136-142.
[13] HELLWIG A, PALM M, INDEN G. Characterisation of structural stability of (Ti(H2)+22Al+23Nb)powder mixtures during mechanical alloying[J]. Intermetallics, 1998, 6: 79-83.
[14] FENG Hai-bo, ZHOU Yu, JIA De-chang, MENG Qing-chang. Rapid synthesis of Ti alloy with B addition by spark plasma sintering[J]. Materials Science and Engineering A, 2005, 390: 344-349.
[15] LIU Y, CHEN L F, TANG H P. Design of powder metallurgy titanium alloys and composites[J].Materials Science and Engineering A, 2006, 418: 25-35.
[16] MICHAEL F B, MARK A C, JOHN A W. The enhanced work hardening rates of the constituent TiAl and Ti3Al phases in a lamellar microstructure[J]. Materials Science and Engineering A, 1995, 201: 24-31.
Foundation item: Project (20050513) supported by the Science and Technology Development Program of Jilin Province, China
Corresponding author: CHEN Hua; Tel: +86-431-85716421; E-mail: chenhua63017@126.com


