文章编号:1004-0609(2016)-12-2596-09
MnO2@graphene复合材料的制备及其对微生物燃料电池阴极氧还原反应的催化活性
王亚光1, 2,何则强1, 2,龙秋萍1, 2,熊利芝1, 2
(1. 吉首大学 生物资源与环境科学学院,吉首 416000;
2. 湖南省2011计划“锰锌钒产业技术”协同创新中心,吉首 416000)
摘 要:
以高锰酸钾、草酸锰、石墨烯为原料,采用化学共沉淀法制备MnO2@graphene 复合材料,用X射线衍射、红外光谱、拉曼光谱、扫描电镜、比表面测定等对其进行表征。以MnO2@graphene为MFC阴极氧还原反应催化剂,采用循环伏安法和电化学阻抗法研究MnO2@graphene催化电极对氧还原反应的催化活性。结果表明:粒度为400 nm左右的MnO2颗粒通过静电相互作用均匀而牢固地分散在纸片状graphene表面,形成MnO2@graphene复合材料。循环伏安测试结果表明:当扫描速率为5 mV/s时,虽然MnO2@graphene催化电极在pH为7.0的磷酸盐缓冲体系(PBS)的氧还原反应起峰电位比Pt/C催化电极负0.048V,但其峰电位(-0.440 V)与Pt/C催化电极的起峰电位(-0.434 V)接近。随着循环次数的增加,MnO2@graphene催化电极的起峰电位稍有下降,但峰电流密度下降很小,表明MnO2@graphene催化剂具有更好的氧还原催化活性和更优秀的循环稳定性。电化学阻抗实验发现:MnO2@graphene催化电极的电荷转移阻抗为12.6 Ω,比同条件下Pt/C催化电极和MnO2催化电极的低,表明由于graphene增加MnO2的导电性,降低催化电极电荷转移阻抗,加快电子的转移速率,促进阴极氧还原反应。
关键词:
中图分类号:O646 文献标志码:A
微生物燃料电池(Microbial fuel cell,MFC)作为一种新型的能源转换装置,利用产电微生物在阳极室氧化有机物而产生电子和质子,质子通过质子交换膜扩散到阴极,而电子则通过外电路转移到阴极表面的活性中心后与电子受体(如O2、KMnO4、K3Fe(CN)6、K2CrO7等)[1-4]及质子结合产生水。MFC由于具有氧化降解有机物和产生电能的双重功能而得到研究者的广泛关注[5]。但MFC输出功率低、有机物氧化降解效率不高等因素成为其实际应用的瓶颈[6]。为此,研究者们通过选用不同的电极材料[7]、筛选高效产电微生物[8-9]、改变MFC构型[10]、优化MFC运行条件[11]等方法以改善MFC的产电性能、提高其废水处理效率。
实践证明,通过改善阴极的反应条件促进电子快速转移是提高MFC性能的有效手段[12]。但除O2外,MFC阴极通常采用的电子受体不具可再生性,且具有一定的环境危害,而O2虽然价格低廉、来源广泛,但在水中的溶解度小、阴极超电势大,导致阴极氧还原速率低,MFC产电功率不高。为了降低O2的阴极超电势,常采用金属Pt[3]或过渡金属大环络合物等催化剂(如热解酞菁铁[13]、四甲基苯卟啉钴[14]),但金属Pt价格昂贵,过渡金属大环络合物化学稳定性不高、制备条件苛刻。寻找廉价实用的新型氧还原反应催化剂以降低MFC阴极制造成本、提高氧还原速率,具有十分重要的意义。研究表明,MnO2廉价易得,环境友好、化学稳定性好、氧还原催化活性高,是一种极具开发前景的阴极催化剂[15-17]。然而,由于MnO2本身导电性差[18]以及分散性不好[19]等原因,纯MnO2催化剂的电催化化学活性不佳[20]。通过将MnO2与导电材料进行复合,可有效提高其氧还原催化活性。如利用超声波的“空化”作用将MnOx包覆于乙炔黑表面制备出MnOx/C复合材料[21-23],曾双双等[24]采用直接还原KMnO4法制备出MnO2/碳纳米管,并研究了其在超级电容器和锂离子电池中的优良特性。而ZHANG等[25]采用水热法合成的MnO2/碳纳米管作为MFC的阴极催化剂,发现该MFC的产电功率达到210 mW/m2,接近Pt/C为阴极催化剂的MFC的产电功率(229 mW/m2)。
考虑到石墨烯具有优秀的导电性、高比表面积和弹性结构等特点,可以作为一种合适的二维催化载体材料[26](如石墨烯负载Pt催化剂、石墨烯负载非Pt催化剂、氮掺杂石墨烯等),显示出良好的氧还原反应催化活性[27-28]。如将石墨烯与MnO2复合,可望制备出高催化活性的氧还原反应催化剂。WEN等[19]采用微波辐射法合成的MnO2/石墨烯作为MFC的阴极催化剂,发现MnO2/石墨烯的氧还原催化活性比纯MnO2的显著提高,甚至优于Pt/C催化剂的。本文作者采用简单的化学共沉淀法将MnO2与石墨烯进行复合得到MnO2@graphene复合材料,研究了MnO2@graphene对MFC阴极氧还原反应的催化性能。
1 实验
1.1 MnO2@graphene的制备与表征
以商用石墨为原料,采用Hummer法制备氧化石墨。然后采用高温裂解还原氧化石墨,经超声剥离、水合肼处理得到二维纸片状石墨烯(经测定,其比表面积达到67 m2/g)。将0.1 g石墨烯分散在去离子水中,加入3.5 g高锰酸钾,搅拌均匀后缓慢滴加0.01 mol/L的草酸锰溶液,控制高锰酸钾与草酸锰的物质的量比为2:3,强烈搅拌下持续滴完。待反应完成后,产品分别用100 mL蒸馏水、100 mL无水乙醇洗涤3次,并于105 ℃真空干燥4 h,得到MnO2@graphene复合材料。
采用XRD(德国Bruker公司生产的D8 Advance X射线衍射仪)分析样品的物相组成;采用SEM(日本JEOL公司生产的JSM-5600LV型扫描电子显微镜)观察样品的表面形貌并估算其颗粒尺寸;采用红外光谱(美国Thermo公司生产的Nicolet6700型傅里叶变换红外光谱仪)和拉曼光谱(法国Dilor公司生产的LABRAM-1B型拉曼光谱仪)分析样品的价键特征;采用XPS(英国Kratos公司生产的Axis Ultra(DLD) 型X射线光电子能谱仪)分析样品中Mn的价态。采用BET法分析(美国Micromeritics公司生产的GeminiVII-2390型比表面分析仪)样品的比表面积。
1.2 工作电极的制作及催化剂氧还原催化性能的测定
将MnO2@graphene或纯MnO2粉末、聚偏二氟乙烯(PVDF)、高纯石墨粉按照质量比70:15:15均匀混合后,加入适量N-甲基吡咯烷酮(NMP)作为溶剂,搅拌成均匀的糊状物,超声分散30 min后涂布到碳纤维布上,在真空干燥箱中60 ℃干燥10 h得到MFC空气阴极。MnO2@graphene或MnO2的负载量为5 mg/cm2。为了考察不同电极对MFC阴极氧还原催化性能的影响,参照文献[29]以氯铂酸为铂源、采用传统水热法制备了Pt/C电极,Pt的担载量约为50 μg/cm2。
采用三电极体系(以负载MnO2@graphene或MnO2的空气阴极为工作电极、铂电极为对电极,Ag/AgCl电极为参比电极)在电化学工作站(中国上海辰华公司生产的CHI660A型电化学工作站)上研究不同催化材料的循环伏安曲线和电化学阻抗谱。电解质溶液为由KH2PO4和NaOH组成的浓度为50 mmol/L、pH为7.0的磷酸盐缓冲溶液(PBS)。循环伏安的电压扫描范围-1.0~0.5 V (vs Ag/AgCl)。测试前,电解质溶液通过空气曝气30 min用氧气饱和。测试温度为室温,电化学阻抗测定时频率范围为0.1Hz~106 Hz,扰动幅度为5 mV。
2 结果与讨论
2.1 MnO2@graphene的表征
图1所示为合成样品的XRD谱。从图1可见,在石墨烯的XRD谱上2θ约为23.98°和43.35°处分别出现了标识石墨烯(002)和(100)晶面的特征衍射峰(JCPDS卡片号No.050661)。在MnO2的XRD谱上2θ约为12.15°、37.02°和66.44°处分别出现了标识α-MnO2(002)、(006)和(119)的特征衍射峰(JCPDS卡片号No.18-802)[30]。而在MnO2@graphene的XRD谱上2θ分别为24.27°处出现了石墨烯的特征衍射峰,而在11.92°、36.77°和66.44°出现了α-MnO2的特征衍射峰,表明MnO2@graphene材料确实是MnO2和graphene复合而成。但复合材料中在43.18°处没有出现标识石墨烯(100)晶面的特征衍射峰,而24°附近的衍射峰强度明显减弱,可能是由于MnO2纳米粒子在graphene表面沉积所致[31]。CAO等[32]研究认为,尽管由于本身导电性差以及分散不均匀等原因,MnO2的氧还原催化性能有待改善,但在α、β、γ、δ和λ 5种MnO2晶体形态中,α-MnO2具有最优的氧还原催化活性,因此,以本研究制备的α-MnO2与导电性能优良的二维石墨烯复合,可望取长补短而获得氧还原催化性能优良的催化材料。
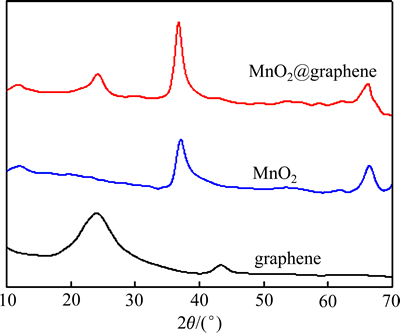
图1 样品的XRD谱
Fig. 1 XRD patterns of samples
图2所示为合成样品的红外光谱。
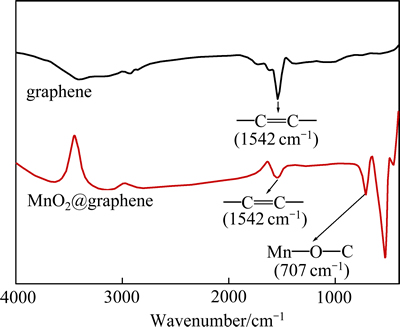
图2 样品的红外光谱
Fig. 2 FTIR spectra of samples
从图2可知,在石墨烯的FTIR谱上1542 cm-1处出现了标识石墨烯的—C=C—吸收峰[33]。而在MnO2@graphene的谱图中,该吸收峰强度较石墨烯中该吸收峰的吸收强度大大弱化,可能是石墨烯表面被MnO2颗粒覆盖所致。另外,在MnO2@graphene的FTIR图中,707cm-1左右还出现了一个较强的吸收峰,这可能是C和MnO2反应产生的结构单元Mn—O—C键的吸收振动[34],更进一步说明石墨烯和MnO2已经紧密复合。
图3所示为合成样品的拉曼光谱。很明显,MnO2@graphene的拉曼光谱中577 cm-1和648 cm-1处出现了标识MnO2中Mn—O伸缩振动的尖峰[35],而在1352 cm-1处和1594 cm-1处出现了标识石墨烯的D带和G带[36],进一步表明MnO2@graphene为MnO2和graphene的复合产物[37]。
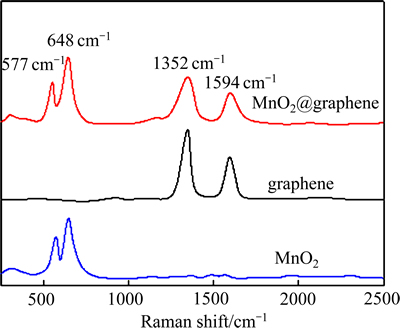
图3 样品的拉曼光谱
Fig. 3 Raman spectra of samples
图4所示为MnO2@graphene复合材料中Mn元素的XPS谱。图4(a)中在642.1 eV和653.8 eV处分别出现了两个强度很大的谱峰,两峰之间的自旋能量间隔为11.7 eV,分别对应Mn4+中Mn(2p3/2)和Mn(2p1/2)的能峰,与文献[38-39]报道的MnO2的XPS谱一致,说明MnO2确实存在于MnO2@graphene复合材料中。对XPS谱进行分峰分析可以知道,图4(a)中除了标识Mn4+的能峰外,还出现了标识Mn5+和Mn3+的相对微弱的能峰,表明MnO2@graphene复合材料Mn的氧化态以+4为主。然而,由于在结合能较低时,谱峰的宽化、拖尾等效应的存在,仅仅从Mn2p谱还不能确切地确定Mn的氧化态,这时可采用Mn 3s分裂能来确定Mn的氧化态[40]。一般而言,Mn2+、Mn3+和Mn4+的Mn 3s分裂能分别约为6.5 eV、5.5和4.5 V[41]。为了进一步说明,也对MnO2@graphene复合材料中Mn 3s的XPS进行了分析(见图4(b))。从图4(b)可知,MnO2@graphene复合材料中Mn 3s分裂能为4.79 eV。由于Mn 3s分裂能与Mn的氧化态之间为近似线性关系[42],可以推算出在MnO2@graphene复合材料中Mn的平均化合态为+3.87,表明MnO2@graphene复合材料中Mn主要以MnO2的形态存在。
图5所示为石墨烯、MnO2 和MnO2@graphene的SEM像。从图5可知,石墨烯像起皱的纸片,而MnO2则由颗粒尺寸约为400 nm的类球形粒子组成。从MnO2@graphene的SEM像发现,尽管MnO2粒子之间有些团聚,但由于graphene 表面具有很多悬键,MnO2可能通过某种化学作用而实现了与graphene的紧密结合[43],与前面采用光谱分析得到的结果一致。同时,由于粒度较小的MnO2粒子弥散在石墨烯的表面,可望大大增加MnO2@graphene的比表面积,提高其催化活性。
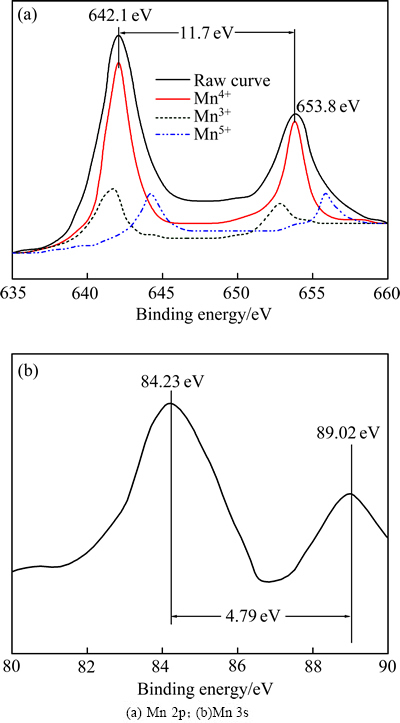
图4 MnO2@graphene的Mn元素的XPS谱
Fig. 4 XPS spectra of Mn in MnO2@graphene
2.2 MnO2@graphene对MFC阴极氧还原反应的电催化活性
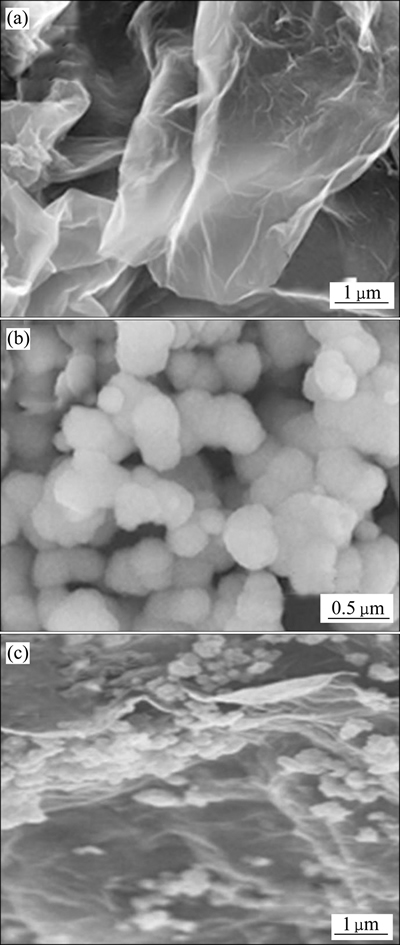
图5 石墨烯、MnO2和MnO2@graphene的SEM像
Fig. 5 SEM images of grapheme(a), MnO2(b) and MnO2@graphene(c)
循环伏安是研究催化材料氧还原催化活性最权威、最有效和最常使用的方法。通常,在循环伏安中采用起峰电位(Onset potential)、峰电位(Peak potentail)和峰电流密度(Peak current density)等参数来评价材料的氧还原催化性能[44]。起峰电位反映了阴极氧还原反应的灵敏度,起峰电位越正,表示氧的电化学还原反应越强烈。峰电位则是阴极氧还原反应的实际电位,峰电位越正,表示氧还原反应越容易。峰电流密度是指在峰电位时通过电极的电流密度,反映了通过电极进行电子传递的有效性。峰电流密度越大,电子传输越快,材料的氧还原催化性能越佳。采用三电极体系研究不同催化材料的循环伏安曲线(见图6)。从图6可知,虽然MnO2@graphene催化电极的起峰电位(约-0.280 V)较Pt/C催化电极的起峰电位(约-0.232 V)稍负,但其峰电位(-0.440 V)却与Pt/C催化电极的峰电位(-0.434 V)比较接近,表明在MnO2@graphene催化电极进行氧还原反应时极化作用较小。值得注意的是,MnO2@graphene催化电极的峰电流密度为12.03 mA/cm2,约为Pt/C催化电极峰电流密度(8.51 mA/cm2)的1.4倍,说明电子在MnO2@graphene催化电极上的传输比在Pt/C电极上的传输更顺畅,MnO2@graphene具有比Pt/C更好的氧还原催化活性。这可能是由于颗粒较小的MnO2粒子弥散在graphene表面后大大增加了MnO2@graphene催化电极的比表面积,导致MnO2@graphene催化电极可以承受更大的电流密度而不产生大的极化作用[45],从而保证MnO2@graphene具有良好的氧还原催化活性。
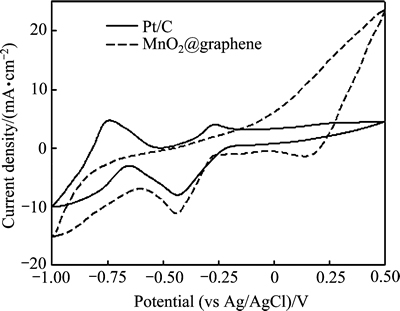
图6 不同催化电极的循环伏安曲线
Fig. 6 Cyclic voltammograms of electrodes with different catalysts at scan rate of 5 mV/s
为了研究不同催化电极的循环稳定性,对不同循环次数后的循环伏安曲线进行了分析。表1所列为经1000次、2000次、5000次和10000次循环后MnO2@graphene催化电极与Pt/C催化电极的起峰电位和峰电流密度。很明显,随着循环次数的增加,Pt/C催化电极的起峰电位基本保持在-0.232 V左右不变,而MnO2@graphene催化电极的起峰电位逐渐负移,100000次循环后的起峰电位较首次循环起峰电位负约0.06 V。同时,随着循环的进行,两种催化电极的峰电流密度均变小,但MnO2@graphene催化电极的峰电流密度随着循环的进行变化很小,10000次循环后的峰电流密度只比首次循环峰电流密度下降1.8%;而随着循环次数的增加,Pt/C催化电极的峰电流密度从首次循环的8.51 mA/cm2降低至10000次循环的4.17 mA/cm2,降幅高达51.4%。分析结果充分表明MnO2@graphene催化剂比Pt/C催化剂具有更稳定的催化活性和更长的循环寿命。
表1 经不同循环次数后MnO2@graphene催化电极与Pt/C催化电极的起峰电位和峰电流密度
Table 1 Initial peak potential and peak current density of electrode with MnO2@graphene or Pt/C catalyst after cycling for different times at scan rate of 30 mV/s and pH=7.0
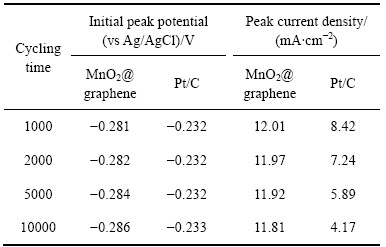
图7所示为负载MnO2@graphene的空气阴极在不同扫描速率下的循环伏安曲线。
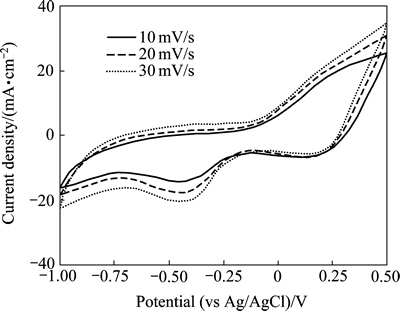
图7 不同扫描速率下MnO2@graphene 催化电极的循环伏安曲线
Fig. 7 Cyclic voltammograms of electrode with MnO2@ graphene catalyst at different scan rates
从图7可以知道,当扫描速率为10 mV/s 时,在-0.44 V处出现了一个明显的氧还原反应峰,大大高于纯MnO2催化电极的电压(-0.71 V)[19],接近Pt/C催化电极的电压(-0.434 V)。而当MnO2@graphene催化电极相应的峰电流达到14.6 mA/cm2,相当于纯MnO2催化电极的4.3倍(3.39 mA/cm2)和Pt/C催化电极的1.2倍 (12.2 mA/cm2)左右。峰电位的正移和峰电流的增加表明MnO2@graphene比纯MnO2和Pt/C具有更好的氧还原反应催化活性。实验表明,MnO2@graphene催化剂的良好催化活性在扫描速率更高时更能得到体现。这可能与MnO2@graphene中高度分散于石墨烯表面的MnO2的粒度较小、比表面积较大、催化活性位置较多有关。
表2列出了不同电极材料的比表面积。从表2可知,单纯的MnO2和graphene的比表面积分别只有36 m2/g、67 m2/g,但当二者复合时,由于颗粒较小的MnO2弥散在石墨烯的表面,使MnO2@graphene的比表面积迅速增加到163 m2/g,分别达到MnO2和graphene比表面积的4.53和2.43倍。正是由于MnO2@graphene复合材料巨大的比表面积,使得其氧还原催化催化活性大大改善,与从XRD和SEM得到的结论一致。WEI等[38]在研究MnO2/三维还原氧化石墨烯复合材料是也得到了类似的结论。
表2 不同电极材料的比表面积
Table 2 Specific area of various electrode materials

图8所示为不同扫描速率下循环伏安曲线的峰电流与扫描速率平方根之间的关系。从图8可以发现,峰电流与扫描速率平方根呈现很好的线性关系,相关系数R2达到0.9989,说明MFC阴极的氧还原催化反应属于扩散控制过程[19]。在氧还原反应研究中,常用电化学阻抗谱分析电催化材料的界面与结构特征、双电层效应、O2的扩散以及腐蚀缓蚀机理等[44],达到揭示氧还原反应的动力学过程的目的。
不同催化电极的电化学阻抗谱如图9所示。从图9可知,3种催化电极的阻抗谱都由一个高频区的半圆和一条低频区的直接组成。其中,高频区的半圆说明了体系的电极反应动力学控制过程,而低频区的直线说明了电极反应的反应物或产物的扩散控制过程。从高频区半圆的直径可以得到各催化电极的电荷转移阻抗(Rct)的大小[19],如表3所列。Rct的大小直接关系到催化剂与反应物或电解质溶液界面反应情况。Rct越小,表明催化剂与反应物或电解质溶液之间的电荷转移越容易。从表3可以知道,3种催化电极中,MnO2@graphene催化电极的Rct最小(12.6 Ω),而纯MnO2催化电极的Rct最大(19.1 Ω),说明电荷在MnO2@graphene催化电极表面的转移最快,在纯MnO2催化电极表面的转移最慢。电荷在MnO2@graphene催化电极表面的快速转移将导致氧还原速率显著增加,大大促进阴极氧还原反应,从而表现出较高的氧还原峰电流密度,与从循环伏安测试(见图6和表1)得到的结论相一致。同时,较小的Rct值,也将导致MnO2@graphene催化电极具有较小的极化作用,保持稳定的电催化活性。电化学阻抗谱中低频区直线的斜率可以用来评价电化学过程的控制扩散步骤[46]。斜率越大,说明扩散阻抗越小,扩散速率大。从图9可知,在3种催化电极中,MnO2@graphene催化电极的斜率最小,说明采用该电极催化的氧还原反应的扩散阻抗最小,这可能与催化剂中graphene特殊的二维层状结构有关[26]。
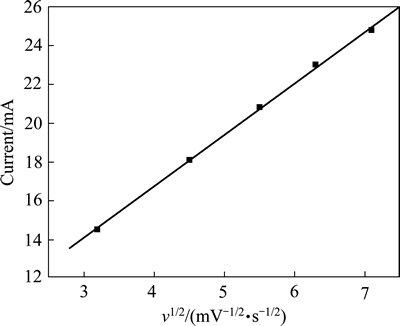
图8 不同扫描速率下循环伏安曲线的峰电流与扫描速率平方根之间的关系
Fig. 8 Relationship between peak current (I) and square root of scan rates (v1/2) at different scan rates
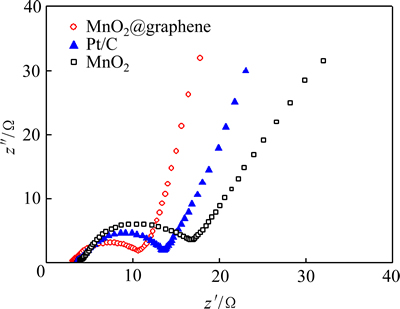
图9 MnO2@graphene 催化电极的电化学阻抗谱
Fig. 9 EIS of electrode with MnO2@graphene catalyst
表3 各催化电极的电荷转移阻抗
Table 3 Charge transfer resistance (Rct) of different catalytic electrodes

MnO2@graphene催化电极良好的催化性能可归因于3个方面:1) MnO2@graphene复合材料中MnO2较小的颗粒尺寸导致复合材料具有较高的比表面积,从而可产生更多的活性位点;2) MnO2@graphene复合材料中graphene特殊的平面结构,可以作为催化活性物质MnO2颗粒有效支撑,使得电极颗粒既能良好的接触又不致团聚;3) MnO2@graphene复合材料中graphene具有良好的导电性和特殊的π共轭结构,使得电子在整个电极体系快速传递,从而促进氧还原反应快速发生。
3 结论
1) 以高锰酸钾、草酸锰、石墨烯为原料,通过化学共沉淀法制备MnO2@graphene复合材料。
2) 由于MnO2@graphene中高度分散于石墨烯表面的MnO2的粒度较小,比表面积较大,催化活性位置较多,MnO2@graphene催化电极具有与Pt/C电极接近的氧还原反应峰电位-0.440 V (vs Ag/AgCl)、更大的峰电流密度、更小的电荷转移阻抗,显示出更优异的催化活性和更长的循环寿命。
REFERENCES
[1] PARK D H, ZEIKUS J G. Improved fuel cell and electrode designs for production electricity from microbial degradation[J]. Biotechnology and Bioengineering, 2003, 81(3): 348-355.
[2] LOGAN B E, REGAN J M. Feature article: Microbial fuel cells- challenges and applications[J]. Environmental Science & Technology, 2006, 40(17): 5172-5180.
[3] LOGAN B E, AELTERMAN P, HAMELERS P, ROZENDAL R, SCHROEDER U, KELLER J, FREGUIAC S, VERSTRAETE W, RABAEY K. Microbial fuel cells: Methodology and technology[J]. Environmental Science & Technology, 2006, 40(17): 5181-5192.
[4] YOU S J, ZHAO Q L, ZHANG J N, JIANG J Q, ZHAO S Q. A microbial fuel cell using permanganate as the cathodic electron acceptor[J]. Journal of Power Sources, 2006, 162(2): 1409-1415.
[5] FRANKS A E, NEVIN K P. Microbial fuel cells, a current review[J]. Energies, 2010, 3(5): 899-919.
[6] 袁浩然, 邓丽芳, 黄宏宇, 小林敬幸, 陈 勇. MnO2为阴极催化剂的微生物燃料电池处理城市垃圾渗滤液研究[J]. 太阳能学报, 2014, 35(9): 1715-1722.
YUAN Hao-yuan, DENG Li-fang, HUANG Hong-yu, KOBAYASHI N, CHEN Yong. Municipal solid waste (MSW) leachate treatment using MnO2-catalyzed microbial fuel cells[J]. Acta Energiae Solaris Sinica, 2014, 35(9): 1715-1722.
[7] LI Y, LU A H, DING H R, WANG X, WANG C Q, ZENG C P, YAN Y H. Microbial fuel cells using natural pyrrhotite as the cathodic heterogeneous Fenton catalyst towards the degradation of biorefractory organics in landfill leachate[J]. Electrochemistry Communications, 2010, 12(7): 944-947.
[8] 费讲驰.高效产电菌的筛选及其在微生物燃料电池中的应用研究[D]. 吉首: 吉首大学, 2014.
FEI Jiang-chi. Screening of efficient electricigens and their basic research in MFC[D]. Jishou: Jishou University, 2014.
[9] 范 平, 支银芳, 吴夏芫, 周楚新. 微生物燃料电池中阳极产电微生物的研究进展[J]. 生物学通报, 2011, 41(10): 6-8.
FAN Ping, ZHI Yin-fang, WU Xia-yuan, ZHOU Chu-xin. Research progress of anode electricigens in microbial fuel cells[J]. Bulletin of Biology, 2011, 41(10): 6-8.
[10] 尤世界, 赵庆良, 姜珺秋. 电极构型对空气阴极生物燃料电池发电性能的影响[J]. 环境科学, 2006, 27(11): 2159-2163.
YOU Shi-jie, ZHAO Qing-liang, JIANG Jun-qiu. Electrode configuration as a factor affecting electricity generation in air cathode microbial fuel cell[J]. Environmental Science, 2006, 27(11): 2159-2163.
[11] PUIG S, SERRA M, COMA M, CABRE M, BALAGUER M D, COLPRIUM J. Microbial fuel cell application in landfill leachate treatment[J]. Journal of Hazardous Materials, 2011, 185(2/3): 763-767.
[12] PHAM H, JANG J K, CHANG I S, KIM B H. Improvement of cathode reaction of a mediatorless microbial fuel cell[J]. Journal of Microbiology and Biotechnology, 2004, 14 (2): 324-329.
[13] YUAN Y, ZHAO B, JEON Y, ZHONG S, ZHOU S, KIM S. Iron phthalocyanine supported on amino-functionalized multi-walled carbon nanotube as an alternative cathodic oxygen catalyst in microbial fuel cells[J]. Bioresource Technology, 2011, 102(10): 5849-5854.
[14] KIM J R, KIM J Y, HAN S B, PARK K W, SARATALE G D, OH S E. Application of Co-naphthalocyanine (CoNPc) as alternative cathode catalyst and support structure for microbial fuel cells[J]. Bioresource Technology, 2011, 102(1): 342-347.
[15] LI X, HU B, SUIB S, LEI Y, LI B. Manganese dioxide as a new cathode catalyst in microbial fuel cells[J]. Journal of Power Sources, 2010, 195(9): 2586-2591.
[16] ROCHE I, SCOTT K. Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (orr) in neutral solution[J]. Journal of Applied Electrochemistry, 2009, 39(2): 197-204.
[17] ZHANG L, LIU C, ZHUANG L, LI W, ZHOU S, ZHANG J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells[J]. Biosensors and Bioelectronics, 2009, 24(9): 2825-2829.
[18] FISCHER A E, PETTIGREW K A, ROLISON D R, STROUD R M, LONG J W. Incorporation of homogeneous, nanoscale MnO2 within ultraporous carbonstructures via self-limiting electroless deposition:Implications for electrochem-ical capacitors[J]. Nano Letters, 2007, 7(2): 281-286.
[19] WEN Q, WANG S Y, YAN J, CONG L J, PAN Z C, REN Y M, FAN Z J. MnO2-graphene hybrid as an alternative cathodic catalyst to platinum in microbial fuel cells[J]. Journal of Power Sources, 2012, 216: 187-191.
[20] 钱晓峰, 褚有群, 李照华, 马淳安. MnOx/CNT的制备及其对氧还原反应的电催化性能研究[J]. 浙江工业大学学报, 2012, 40(4): 365-368.
QIAN Xiao-feng, CHU You-qun, LI Zhao-hua, MA Chun-an. Preparation of MnOx/CNT and its electrocatalytic performance for oxygen reduction reation[J]. Journal of Zhejiang University of Technology, 2012, 40(4): 365-368.
[21] MA S B, LEE Y H, AHN K Y, KIM C M, OH K H, KIM K B. Spontaneously deposited manganese oxide on acetylene black in an aqueous potassium permanganate solution[J]. Journal of the Electrochemical Society, 2006, 153(1): 27-32.
[22] KAWAOKA H, HIBINO M, ZHOU H, HONMA I. Sonochemical synthesis of amorphous manganese oxide coated on carbon and application to high power battery[J]. Journal of Power Sources, 2004, 125(1): 85-89.
[23] HUANG X, YUE H, ATTIA A, YANG Y. Preparation and properties of manganese oxide/carbon composites by reduction of potassium permanganate with acetylene black[J]. Journal of the Electrochemical Society, 2007, 154(1): 26-33.
[24] 曾双双, 郑明森, 董全峰. 直接还原高锰酸钾制备CNT/MnO2复合材料[J]. 电池, 2010, 40(3): 121-123.
ZENG Shuang-xhuang, ZHENG Ming-sen, DONG Quan-feng. Preparing CNT/MnO2 composite by direct reducing potassium permanganate[J]. Battery, 2010, 40(3): 121-123.
[25] ZHANG Y, HU Y, LI S, SUN J, HOU B. Manganese dioxide-coated carbon nanotubes as an improved cathodic catalyst for oxygen reduction in a microbial fuel cell[J]. Journal of Power Sources, 2011, 196(22): 9284-9289.
[26] GEIM A K. Graphene: Status and prospects[J]. Science, 2009, 324(5934): 1530-1534.
[27] 赵东江. 石墨烯在氧还原反应催化剂中应用的研究进展[J]. 绥化学院学报, 2013, 33(9): 153-160.
ZHAO Dong-jiang. The research progress of application of graphene to catalysts for oxygen reduction reaction[J]. Journal of Suihua University, 2013, 33(9): 153-160.
[28] 彭 三, 郭慧林, 亢晓峰. 氮掺杂石墨烯的制备及其对氧还原反应的电催化性能[J]. 物理化学学报, 2014, 30(9): 1778-1786.
PENG San, GUO Hui-lin, KANG Xiao-feng. Preparation of nitrogen-doped graphene and its electrocatalystic activity for oxygen reduction reaction[J]. Acta Phys-Chim Sin, 2014, 30(9): 1778-1786.
[29] 许学飞. 高分散性Pt/C催化剂的制备与研究[D]. 杭州: 浙江大学, 2012.
XU Xue-fei. Synthesis and property of high-dispersed Pt/C catalysts[D]. Hangzhou: Zhejiang University, 2012.
[30] 陈 仲, 李建玲, 陈 宇. 微波水热法制备二氧化锰/石墨烯复合材料[J]. 电池, 2013, 43(1): 15-17.
CHEN Zhong, LI Jian-ling, CHEN Yu. Preparating manganese dioxide/graphene composite by microwave-hydrothermal method[J]. Battery Bimonthly, 2013, 43(1): 15-17.
[31] 徐 晓, 田艳红, 张学军. 石墨烯/二氧化锰复合材料的电化学性能[J]. 硅酸盐学报, 2013, 41(1): 38-43.
XU Xiao, TIAN Yan-hong, ZHANG Xue-jun. Electrochemical properties of graphene/MnO2 composite[J]. Journal of the Chinese Ceramic Society, 2013, 41(1): 38-43.
[32] CAO Y L, YANG H X, AI X P, XIAO L F. The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution[J]. Journal of Electroanalytical Chemistry, 2003, 557(12): 127-134.
[33] ZOU Q, ZAI J T, LIU P, QIAN X F. Synthesis and lithium storage properties of hollow Fe2O3/graphene nanocomposites[J]. Chemical Journal of Chinese Universities, 2011, 32(3): 630-634.
[34] 姚振华, 胡茂从. 二氧化锰/石墨烯臭氧催化氧化甲苯反应性能[J]. 广东化工, 2015, 43(2): 34-36.
YAO Zhen-hua, HU Mao-cong. Reaction performance of MnO2/graphene for ozone catalytic oxidation of toluene[J]. Guangdong Huagong, 2015, 43(2): 34-36.
[35] GAO T, GLERUP M, KRUMEICH F, NESPER R, FJELLVAG H, NORBY P. Microstructures and spectroscopic properties of cryptomelane-type manganese dioxide nanofibers[J]. The Journal of Physical Chemistry C, 2008, 112(34): 13134-13140.
[36] RAO C N R, SOOD A K, SUBRAHMANYAM K S, GOVINDARAJ A. Graphene: The new two-dimensional nanomaterial[J]. Angewandte Chemie International Edition, 2009, 48(42): 7752-7757.
[37] PERERA S D, MARIANO R G, VU K, NIJEM N, SEITZ O, BALKUS K J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity[J]. ACS Catalysis, 2012, 2(6): 949-956.
[38] WEI B, WANG L, MIAO Q, YUAN Y, DONG P, VAJTAI R, FEI W. Fabrication of manganese oxide/three-dimensional reduced graphene oxide composites as the supercapacitors by a reverse microemulsion method[J]. Carbon, 2014, 85: 249-260.
[39] MATHIEU T, THIERRY B, DANIEL B. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor[J]. Chemistry of Materials, 2004, 16(6): 3184-3190.
[40] GU Y, CAI J W, HE M Z, KANG L P, LEI Z B, LIU Z H. Preparation and capacitance behavior of manganese oxide hollow structures with different morphologies via template-engaged redox etching[J]. Journal of Power Sources, 2013, 239: 347-355.
[41] CHIGANE M, ISHIKAWA M. Manganese oxide thin film preparation by potentiostatic electrolyses and electrochromism[J]. Journal of Electrochemistry Society, 2000, 147(6): 2246-2251.
[42] TOUPIN M, BROUSSE T,  D. Influence of microstucture on the charge storage properties of chemically synthesized manganese dioxide[J]. Chemistry of Materials, 2002, 14 (9): 3946-3952.
D. Influence of microstucture on the charge storage properties of chemically synthesized manganese dioxide[J]. Chemistry of Materials, 2002, 14 (9): 3946-3952.
[43] PANG M, LONG G, JIANG S, JI Y, HAN W, WANG B, LIU X, XI Y. Rapid synthesis of graphene/amorphous-MnO2composite withenhanced electrochemical performance for electrochemical capacitor[J]. Materials Science and Engineering B, 2015, 194: 41-47.
[44] SUN M, LIU H, LIU Y, QU J, LI J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction[J]. Nanoscale, 2015, 7(4): 1250-1269.
[45] WU J, ZHANG D, WANG Y, WAN Y. Manganese oxide–graphene composite as an efficient catalyst for 4-electron reduction of oxygen in alkaline media[J]. Electrochimica Acta, 2012, 75: 305-310.
[46] BARD A J, FAULKNER L R. Electrochemical methods: fundamentals and applications[M]. 2nd ed. New York: John Wiley & Sons, 2001.
Preparation and catalytic activity for cathodic oxygen reduction reaction in microbial fuel cell of MnO2@graphene composites
WANG Ya-guang1, 2, HE Ze-qiang1, 2, LONG Qiu-ping1, 2, XIONG Li-zhi1, 2
(1. College of Biology and Environmental Sciences, Jishou University, Jishou 416000, China;
2. The Collaborative Innovation Center of Manganese-Zinc-Vanadium Industrial Technology (the 2011 Plan of Hunan Province), Jishou 416000, China)
Abstract: MnO2@graphene composites were prepared by a chemical co-precipitation method using KMnO4, MnC2O4·2H2O and graphene as raw materials.The microstructure, morphology of the prepared composites were analyzed using X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectrum, Raman spectroscopy, X-ray photoelectron spectroscopy(XPS), scanning electron microscopy (SEM) and specific area measurements. The electrochemical performances of MnO2@graphene composites as catalysts for cathodic oxygen reduction reaction in microbial fuel cell were analyzed using cyclic voltammetry, electrochemical impedance spectrometry (EIS). The results show that spherical MnO2 with uniform particle size of 400 nm is tightly formed on the surface of paper-like graphene by electrostatic interaction. Moreover, the peak potential of MnO2@graphene electrode (-0.440 V) is very close to that for Pt/C electrode (-0.434 V), though the initial peak potential of MnO2@graphene electrode is 0.048 V negative than that for Pt/C electrode. With the increase of cycling times, the initial peak potential of MnO2@graphene electrode decreases, while there is only a small decline for the peak current density of MnO2@graphene electrode, indicating that the MnO2@graphene composites have better catalytic activity and cycling stability for cathodic oxygen reduction reaction in microbial fuel cell than that for Pt/C catalysts. EIS results show that the electron-transfer resistance of MnO2@graphene is only 12.6 Ω, which is smaller than that for Pt/C catalysts and MnO2 catalysts, suggesting MnO2@graphene catalysts promote the cathodic oxygen reduction reaction by decareasing the electron-transfer resistance and accelerating the charge transfer due the introduction of the excellent conductive graphene.
Key words: MnO2; graphene; oxygen reduction reaction; catalytic activity; microbial fuel cell
Foundation item: Projects(51364009, 51262008, 51472107) supported by the National Natural Science Foundation of China; Project(JSU0713) supported by the Construct Program of the Key Discipline in Hunan Province, China; Project supported by Aid program (Environment and Energy Materials and Deep Processing of Mineral Resources in Wuling Mountain) for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, China; Project(JGY201641) supported by the Research Foundation of Jishou University, Hunan Province, China; Project([2015]20) supported by Program of Innovation Base for Graduate Students of Jishou University, Hunan Province, China
Received date: 2015-10-19; Accepted date: 2016-05-08
Corresponding author: XIONG Li-zhi; Tel: +86-743-8564416; E-mail: kmpanda99111@163.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51364009,51262008,51472107);湖南省重点学科建设项目(JSU0713);湖南省高校科技创新团队支持计划“环境能源材料与武陵山区矿产资源精深加工”项目;吉首大学校级项目(JGY201641); 吉首大学研究生培养创新基地项目([2015]20号)
收稿日期:2015-10-19;修订日期:2016-05-08
通信作者:熊利芝,副教授,博士;电话:0743-8564416;E-mail: kmpanda99111@163.com
摘 要:以高锰酸钾、草酸锰、石墨烯为原料,采用化学共沉淀法制备MnO2@graphene 复合材料,用X射线衍射、红外光谱、拉曼光谱、扫描电镜、比表面测定等对其进行表征。以MnO2@graphene为MFC阴极氧还原反应催化剂,采用循环伏安法和电化学阻抗法研究MnO2@graphene催化电极对氧还原反应的催化活性。结果表明:粒度为400 nm左右的MnO2颗粒通过静电相互作用均匀而牢固地分散在纸片状graphene表面,形成MnO2@graphene复合材料。循环伏安测试结果表明:当扫描速率为5 mV/s时,虽然MnO2@graphene催化电极在pH为7.0的磷酸盐缓冲体系(PBS)的氧还原反应起峰电位比Pt/C催化电极负0.048V,但其峰电位(-0.440 V)与Pt/C催化电极的起峰电位(-0.434 V)接近。随着循环次数的增加,MnO2@graphene催化电极的起峰电位稍有下降,但峰电流密度下降很小,表明MnO2@graphene催化剂具有更好的氧还原催化活性和更优秀的循环稳定性。电化学阻抗实验发现:MnO2@graphene催化电极的电荷转移阻抗为12.6 Ω,比同条件下Pt/C催化电极和MnO2催化电极的低,表明由于graphene增加MnO2的导电性,降低催化电极电荷转移阻抗,加快电子的转移速率,促进阴极氧还原反应。
[5] FRANKS A E, NEVIN K P. Microbial fuel cells, a current review[J]. Energies, 2010, 3(5): 899-919.
[6] 袁浩然, 邓丽芳, 黄宏宇, 小林敬幸, 陈 勇. MnO2为阴极催化剂的微生物燃料电池处理城市垃圾渗滤液研究[J]. 太阳能学报, 2014, 35(9): 1715-1722.
[8] 费讲驰.高效产电菌的筛选及其在微生物燃料电池中的应用研究[D]. 吉首: 吉首大学, 2014.
[9] 范 平, 支银芳, 吴夏芫, 周楚新. 微生物燃料电池中阳极产电微生物的研究进展[J]. 生物学通报, 2011, 41(10): 6-8.
[10] 尤世界, 赵庆良, 姜珺秋. 电极构型对空气阴极生物燃料电池发电性能的影响[J]. 环境科学, 2006, 27(11): 2159-2163.
[20] 钱晓峰, 褚有群, 李照华, 马淳安. MnOx/CNT的制备及其对氧还原反应的电催化性能研究[J]. 浙江工业大学学报, 2012, 40(4): 365-368.
[24] 曾双双, 郑明森, 董全峰. 直接还原高锰酸钾制备CNT/MnO2复合材料[J]. 电池, 2010, 40(3): 121-123.
[26] GEIM A K. Graphene: Status and prospects[J]. Science, 2009, 324(5934): 1530-1534.
[27] 赵东江. 石墨烯在氧还原反应催化剂中应用的研究进展[J]. 绥化学院学报, 2013, 33(9): 153-160.
[28] 彭 三, 郭慧林, 亢晓峰. 氮掺杂石墨烯的制备及其对氧还原反应的电催化性能[J]. 物理化学学报, 2014, 30(9): 1778-1786.
[29] 许学飞. 高分散性Pt/C催化剂的制备与研究[D]. 杭州: 浙江大学, 2012.
[30] 陈 仲, 李建玲, 陈 宇. 微波水热法制备二氧化锰/石墨烯复合材料[J]. 电池, 2013, 43(1): 15-17.
[31] 徐 晓, 田艳红, 张学军. 石墨烯/二氧化锰复合材料的电化学性能[J]. 硅酸盐学报, 2013, 41(1): 38-43.
[34] 姚振华, 胡茂从. 二氧化锰/石墨烯臭氧催化氧化甲苯反应性能[J]. 广东化工, 2015, 43(2): 34-36.


