杂多酸-钛凝胶-聚乙烯基吡咯烷酮复合材料的制备与性能
李衡峰1, 2,边宏1,张钰琴1
(1. 中南大学 材料科学与工程学院,湖南 长沙,410083
2. 中南大学 粉末冶金国家重点实验室,湖南 长沙,410083)
摘 要:
摘 要:以原位复合溶胶-凝胶新型制备方法,利用不同相对分子质量乙烯基吡咯烷酮预聚物产生的位阻效应阻碍钛凝胶团聚,将具有Keggin结构的磷钨酸和磷钼酸分别嵌入钛凝胶中制备杂多酸-钛凝胶-聚乙烯基吡咯烷酮复合材料。利用傅里叶红外分析仪、X线衍射仪和紫外-可见分光光度计对所制备材料的结构和性能进行分析,结果表明:复合材料中保留着部分Keggin结构,其大部分已经消失,形成新的晶体结构;在紫外光的照射下,复合材料由淡黄色变为蓝色,经过20 min光照后仍未饱和,之后,将材料置于无光的环境中,其很快变回淡黄色。
关键词:
中图分类号:O635 文献标志码:A 文章编号:1672-7207(2010)01-0103-05
Preparation and structure on heteropoly acids- TiO2 colloid-Polyvinylpyrrolidone composites
LI Heng-feng1, 2, BIAN Hong1, ZHANG Yu-qin1
(1. School of Materials Science and Engineering, Central South University, Changsha, 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: Composites of Keggin structured H3WP12O40 or H3PMo12O40, TiO2 colloid, Polyvinylpyrrolidone(PVP) were prepared by in-situ sol-gel method, and PVP with different relative molecular mass were used to prevent the crystallization of TiO2 colloid by its space steric effect. The structure and property of the materials were studied via IR, XRD and Uv-vis spectrophotometer. The results show that part of the Keggin structure is still retained in the composites though most of that have disappeared, and new crystal structure is formed. In addition, the color of the composites changes from light yellow to blue, and doesn’t reach saturation degree after ultraviolet irradiation for 20 min. Meanwhile, when it is moved to the dark environment, the composites return to light yellow soon.
Keywords: polyvinylpyrrolidone; TiO2 colloid; polyacids; photochromism; composite
多酸化合物是一类功能性介于无机和有机分子之间的重要金属氧簇化合物[1-2],具有特殊的化学结构和电子多样性[3-7],已被广泛应用于催化、医药、表面化学及材料等领域[8-11]。目前,由多酸化合物和有机分子形成的无机-有机复合材料不但具有多阴离子和有机分子的性质,而且材料中有机分子和无机分子的相互作用还能产生出奇特的结构和光学现象[12-13]。一些含多酸化合物的有机-无机复合材料如聚丙烯酰 胺[14-15]、聚乙烯醇[16-17]、氧化硅溶胶-凝胶[18]、聚乙烯基吡咯烷酮[19]等与杂多酸复合制备光致变色复合材料,有效地提高了杂多化合物的机械性能和可加工性能。并且材料表现出良好的可逆光致变色性能。但关于将Keggin结构多酸化合物通过原位复合法嵌入具有相近能带的二氧化钛凝胶中,制备新型有机-无机纳米复合材料的研究还未见报道。以往光致变色复合材料主要是由有机-多酸配合物和多酸氧化物杂化的方法制备。为了拓展杂多酸类光致变色材料的研究思路并提高其性能,本文作者采用原位复合溶胶-凝胶的新型制备方法,利用不同相对分子质量乙烯基吡咯烷酮预聚物产生的位阻效应阻碍钛凝胶团聚,将具有Keggin结构的磷钨酸和磷钼酸嵌入钛凝胶中制备杂多酸-钛凝胶-聚乙烯基吡咯烷酮三元复合材料。
1 实验
1.1 实验材料
实验材料为:乙烯基吡咯烷酮(德国BASF公司生产,进口分装,使用前经减压蒸馏提纯,收集0.097 MPa下122 ℃馏分);偶氮二异丁腈(AIBN)(天津开发区海光化学制药厂生产,分析纯,用前用乙醇重结晶);钛酸正丁酯(国药集团化学试剂有限公司生产,分析纯); 浓硝酸(国药集团化学试剂有限公司生产);无水乙醇(国药集团化学试剂有限公司生产,纯度为99%)。
1.2 实验主要仪器
主要仪器有:FT-IR光谱仪(KBr压片) (Avatar360型,美国Nicolet 公司制造)、紫外-可见分光光度计(UV-2401PC,日本Shimadzu公司制造)、X线自动衍射仪(日本Rigaku公司制造)。
1.3 材料的制备
1.3.1 Ti-W-PVP体系样品的制备
将AIBN(0.082 9 g)、乙烯基吡咯烷酮(10 mL)、无水乙醇(15 mL)同时加入充满氩气的单口烧瓶中,不断搅拌并由室温升温至80 ℃后反应1 h,得黏度适宜的乙烯基吡咯烷酮均聚物。缓慢注入水解2 h的钛凝胶(5 mL)并继续反应1 h,注入H3PW12O40(0.149 2 g)的无水乙醇溶液(3 mL),继续反应1 h,得到淡黄色透明聚合物。
1.3.2 Ti-Mo-PVP体系样品的制备
将AIBN(0.082 5 g)、乙烯基吡咯烷酮(10 mL)、无水乙醇(15 mL)同时加入充满氩气的单口烧瓶中,不断搅拌并于室温升温至80 ℃后反应15 min,得乙烯基吡咯烷酮预聚物。缓慢注入水解2 h的钛凝胶(5 mL),待反应15 min后,注入H3PMo12O40(0.156 8 g)的无水乙醇溶液(3 mL),继续反应1 h得到透明复合物。
1.3.3 Ti-W-Mo-PVP体系样品的制备
将AIBN(0.083 6 g)、乙烯基吡咯烷酮(10 mL)、无水乙醇(15 mL)同时加入充满氩气的单口烧瓶中,不断搅拌并于室温升温至80 ℃后反应0.5 h得黏度适宜的乙烯基吡咯烷酮均聚物。缓慢注入水解2 h的钛凝胶(5 mL)并继续反应0.5 h,注入含有H3PMo12O40 (0.150 6 g)和H3PW12O40(0.147 8 g)的无水乙醇溶液(3 mL),继续反应1 h得到透明复合物。
2 结果分析
2.1 红外光谱分析
Keggin结构杂多阴离子红外光谱的特点是:v(P—Oa),v(W—Od),v(W—Ob—W)和v(W—Oc—W)键振动峰均出现在700~1 100 cm-1区域,随杂原子、配原子种类不同,各金属-氧键的反对称伸缩振动频率表现出一定差别。一般认为各金属-氧键的反对称伸缩振动位置:对P—Oa键,W系振动峰在1 079 cm-1处,Mo系振动峰在1 064 cm-1处;对M=Od键,W系振动峰在983 cm-1处,Mo系振动峰在964 cm-1处;对M—Ob—M键,振动峰在890~850 cm-1处;对M—Oc—M键,振动峰在800~760 cm-1处。多酸的聚集状态和表面键接状态都会导致多酸复合材料IR图中的峰发生位移且其积分面积发生变化[20]。
图1和图2所示分别为Ti-W-PVP体系和Ti-W-Mo-PVP体系光照前后的红外光谱。复合物光照前的IR光谱中1 089 cm-1处的峰是磷钨酸多氧阴离子的特征峰,对比标准峰值1 079(P—Oa)和983 cm-1(M=Od)来说峰值红移,且其中980 cm-1(图1)和939 cm-1(图2)处特征峰几乎消失,说明虽然化合物中极少杂多酸仍然保持着原来的Keggin结构,但多酸端氧可能与O—Ti—O发生键合作用并形成新的化学键,导致端氧电子结构发生较大变化。1 043 cm-1处出现Ti—O—C键明显的峰,760 cm-1处出现O— Ti—O极弱的伸缩振动峰,说明杂多酸发生反应生成了新键。从图2可以看出:只有少数峰位置发生较小的位移,说明变色前后复合物粒子结构只发生微小变化。
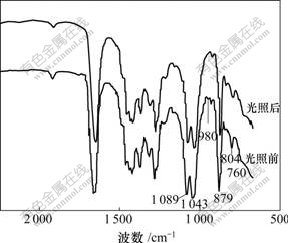
图1 Ti-W-PVP体系光照前后红外光谱
Fig.1 IR spectra of sample Ti-W-PVP before and after photoirradiation
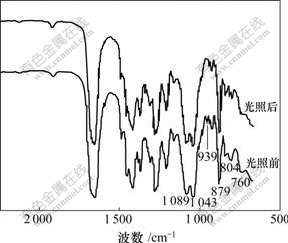
图2 Ti-W-Mo-PVP体系光照前后红外光谱对照
Fig.2 IR spectra of sample Ti-W-Mo-PVP before and after photoirradiation
2.2 X线衍射分析
图3所示为2号Ti-Mo-PVP样品于20 ℃干燥2 h后的X线衍射谱。其中:2θ=28?处出现较明显的尖峰表明该晶体中含有TiO2晶体结构;2θ=45?附近出现的尖峰表明虽然大部分杂多氧化物磷钼酸仍然保持原Keggin结构,但复合体系中的杂多酸含量低于Ti-W-Mo-PVP体系的杂多酸含量,因此,形成的衍射馒头峰也较尖锐,而2θ=60?处的尖峰是新晶体的衍射形成的。
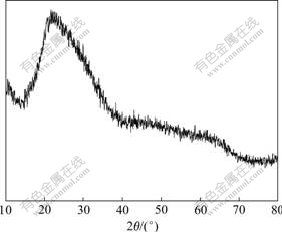
图3 2号Ti-Mo-PVP样品于20 ℃干燥2 h后的X线衍射谱
Fig.3 XRD pattern of sample Ti-Mo-PVP baked at 20 ℃ for 2 h
图4中Ti-W-Mo-PVP体系的X线衍射谱表明:高分子链缠结结构的位阻效应仍然存在并致使TiO2结晶受阻。由于高分子难于完全溶解于无水乙醇中,残存的高分子导致X线衍射谱出现的是馒头峰。图中2θ=12.5?和2θ=28?处馒头峰的消失表明该透明溶液中只有少量TiO2晶体;2θ=58?附近出现的馒头峰表明部分杂多氧化物磷钼酸、磷钨酸仍然保持原Keggin结构,而2θ=28?和2θ=45?处的尖峰是新晶体的衍射形成的。
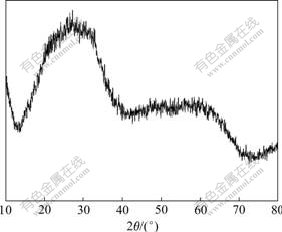
图4 3号Ti-W-Mo-PVP样品于20 ℃干燥2 h后的X线衍射谱
Fig.4 XRD pattern of sample Ti-W-Mo-PVP baked at 20 ℃ for 2 h
2.3 光致变色性质分析
将Ti-W-PVP体系复合物的溶液用自然光进行照射,照射前溶液呈现淡黄色透明状,光照5 min后溶液仍保持淡黄色透明状态,反映在紫外可见吸收光谱(图5)中,其光照前的紫外-可见光吸收谱线与光照后的吸收谱线重叠在一起,且在750 nm处出现吸收峰。而光致变色的TiO2 粒子则在900 nm 左右发生显著吸收,对应的价电子的吸收能量约为1.77 eV。复合体系中生成了O—Ti—O网络。
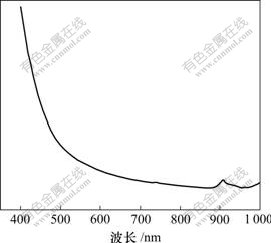
图5 Ti-W-PVP紫外-可见吸收光谱
Fig.5 Uv-vis absorption spectra of sample Ti-W-PVP
图6所示为Ti-Mo-PVP复合物等时间间隔内经自然光照射由无色转变为深蓝色产生的紫外-可见光吸收谱,时间间隔为3 min。经光照后复合物变为蓝色,在730 nm处出现新的特征吸收峰,归属为价层电子(IVCT)。蓝色复合物在室温下、暗处放置3 h左右,薄膜可变为无色,说明复合物具有可逆光致变色性能。将变色后的薄膜置于氩气保护条件下室温暗处放置不发生褪色现象,说明褪色过程与氧气有关。从图6容易看出:随着紫外光照射时间延长,复合物在750 nm处的吸收峰强度逐渐增加,并直至饱和。
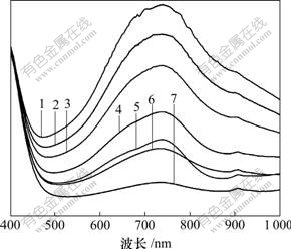
样品吸光时间/min:1—18; 2—15; 3—12; 4—9; 5—6; 6—3; 7—0
图6 Ti-Mo-PVP等时间间隔紫外-可见吸收光谱
Fig.6 Uv-vis absorption spectra of sample Ti-Mo-PVP with the same time interval
图7所示为Ti-W-Mo-PVP复合物等时间间隔内经自然光照射产生的紫外-可见光吸收谱,时间间隔为3 min。经光照后复合物变为蓝色,分别在770 nm和910 nm处出现新的特征吸收峰,可分别归属为价层电子IVCT和金属的d-d跃迁。蓝色复合物在室温下、暗处放置2 h左右薄膜可变为无色,说明该复合物具有可逆光致变色性能。将变色后的薄膜置于氩气保护条件下、室温暗处放置不发生褪色现象,说明褪色过程与氧气有关。从图7中可以看出:随着紫外光照射时间延长,复合物在750 nm处的吸收峰强度逐渐增大,光照20 min仍未饱和,光致变色性能优异。
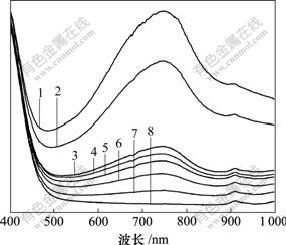
样品吸光时间/min:1—21; 2—18; 3—15; 4—12; 5—9; 6—6; 7—3; 8—0
图7 Ti-W-Mo-PVP等时间间隔紫外-可见吸收光谱
Fig.7 Uv-vis absorption spectra of sample Ti-W-Mo-PVP with the same time interval
3 结论
(1) 通过原位溶胶-凝胶制备方法,制备了Ti-W- PVP,Ti-Mo-PVP和Ti-W-Mo-PVP三元复合薄膜。
(2) 复合薄膜中的杂多酸部分保持着原来的Keggin结构,出现了新的晶体衍射峰,有新的晶体结构形成。
(3) Ti-Mo-PVP和Ti-W-Mo-PVP复合薄膜在光照下由无色变成蓝色,置于无光的条件下,又变回无色,具有光致变色可逆性。其中,Ti-W-Mo-PVP复合薄膜在光照下20 min仍未达到吸收饱和,光致变色性能 优异。
参考文献:
[1] 王恩波, 胡长文, 许林. 多酸化学导论[M]. 北京: 化学工业出版社出版, 1998.
WANG En-bo, HU Chang-wen, XU Lin. Polyacid chemistry introduction[M]. Beijing: Chemical Industry Press, 1998.
[2] 郑汝骊, 王恩波. 钼钨的多酸化学[J]. 化学通报, 1984, 84(9): 12-14.
ZHENG Ru-li, WANG En-bo. Polyacid chemistry of Mo and W[J]. Chemistry, 1984, 84(9): 12-14.
[3] Liu C M, Zhang D Q, Xiong M, et al. A novel two-dimensional mixed molybdenum-vanadium polyoxometalate with two types of cobalt(Ⅱ) complex fragments as bridges[J]. Chem Commun, 2002, 2(13): 1416-1417.
[4] Yuan M, Wang E B, Lu Y, et al. A two-dimensional molybdenum(Ⅴ) phosphate with covalently bonded transition metal coordination complexes: Hydrothermal synthesis and structure characterization of Na2[{Mn(phen)2(H2O)}- {Mn(phen)2}3{MnMoV12O24(H2PO4)2(OH)6}]·4H2O[J]. J Solid State Chem, 2003, 170(1): 192-197.
[5] Zheng S T, Zhang J, Yang G Y. Hydrothermal syntheses and crystal structures of two novel, hybrid materials based on secondary transition-metal-incorporated polyoxovanadate cluster backbones: [Cd(dien)2]2[(dien)CdAs8V13O41(H2O)]·4H2O and [Cd(en)2]2[(en)2Cd2As8V12O40][J]. Inorg Chem, 2005, 44(7): 2426-2430.
[6] Dolbecq A, et al. Hybrid organic-anorganic 1D and 2D frameworks with epsilon-Keggin polyoxomolybdates as building blocks[J]. Chemistry-a European Journal, 2003, 9(12): 2914-2920.
[7] Lei C, et al. A novel organic-inorganic hybrid based on an 8-electron-reduced Keggin polymolybdate capped by tetrahedral, trigonal bipyramidal, and octahedral zinc: Synthesis and crystal structure of (CH3NH3)(H2bipy)[Zn4(bipy)3(H2O)2MoV8MoVI4- O36(PO4)]·4H2O[J]. Inorg Chem, 2004, 43(6): 1964-1968.
[8] Klemper W G, Wall C G. Polyoxoanion chemistry moves toward the future: From solids and solution to surface[J]. Chem Rev, 1998, 98(1): 297-306.
[9] Misono M. Unique acid catalysis of heteropoly compounds (heteropolyoxometalates) in the solid state[J]. Chem Commun, 2001, 13: 1141-1152.
[10] Sadakane M, Steckhan E, Electrochemical properties of polyoxometalates as electrocatalysis[J]. Chem Rev, 1998, 98(1): 219-237.
[11] 李洁, 孙容, 陈启元, 等. [C5H5NH]3[PMo12O40]·4.5H2O 的合成与表征[J]. 中南大学学报: 自然科学版, 2006, 37(1): 58-62.
LI Jie, SUN Rong, CHEN Qi-yuan, et al. Synthesis and properties of charge transfer salt [C5H5NH]3[PMo12O40]·4.5H2O based on molybdophosphoric acid[J]. Journal of Central South University: Science and Technology, 2006, 37(1): 58-62.
[12] Dave B C, Soyez H, Miller J M, et al. Sol-gel materials in electrochemistry[J]. Chem Mater, 1995, 7(8): 1431-1434.
[13] Reetz M T. Entrapment of biocatalysts in hydrophobic sol-gel materials for use in organic chemistry[J]. Adv Mater, 1997, 9(6): 943-948.
[14] Feng W, Zhang T R, Liu Y, et al. Novel hybrid inorganic- organic film based on the PW12-polyacrylamide system: Photochromic behavior and mechanism[J]. Mater Res, 2002(1): 133-136.
[15] Feng W, Zhang T R, Liu Y, et al. Sonochemical preparation of photochromic nanocomposite thin film based on poly- oxometalates well dispersed in polyacrylamide[J]. J Solid State Chem, 2002, 169(1): 1-5.
[16] Yang G C, Pan Y, Gao F M, et al. A novel photochromic PVA fiber aggregates contained H4SiW12O40[J]. Mater Lett, 2005, 59(4): 450-455.
[17] Gao J, Li X D, Shao C L, et al. Photochromic and thermal properties of poly (vinyl alcohol)/H6P2W18O62 hybridmembranes [J]. Mater Chem Phys, 2003, 79(1): 87-93.
[18] Zhang T R, Feng W, Lu R, et al. Synthesis and characterization of polymetalate based photochromic inorganic-organic nanocomposites[J]. Thin Solid Films, 2001, 402(2): 237-241.
[19] 艾丽梅, 李永仙, 冯威, 等. 钨磷酸/聚乙烯基吡咯烷酮纳米复合膜的光致变色性能[J]. 大连海事大学学报, 2007, 33(1): 150-153.
AI Li-mei, LI Yong-xian, FENG Wei, et al. Study on the photochromism of phosphotungstic acid/polyviny pyrrolidone nanocomposite films[J]. Journal of Dalian Maritime University, 2007, 33(1): 150-153.
[20] 何则强, 李新海, 张平民, 等. 新奇的双帽Keggin型磷钒铬杂多化合物的水热合成与表征[J]. 中国有色金属学报, 2002, 12(4): 812-816.
HE Ze-qiang, LI Xin-hai, ZHANG Ping-min, et al. Hydrothermal synthesis and characterization of novel bi-capped Keggin heteropolychromovanadophosphate[J]. The Chinese Journal of Nonferrous Metals, 2002,12(4): 812-816.
收稿日期:2009-02-05;修回日期:2009-05-05
基金项目:国家自然科学基金资助项目(50703048)
通信作者:李衡峰(1972-),男,湖南茶陵人,教授,博士生导师,从事功能高分子材料研究;电话:0731-88877873;E-mail: lihf@mail.csu.edu.cn


