J. Cent. South Univ. (2016) 23: 757-764
DOI: 10.1007/s11771-016-3121-1
Influence factors on stress corrosion cracking of P110 tubing steel under CO2 injection well annulus environment
LIU Zhi-yong(刘智勇)1, 2, ZHAO Tian-liang(赵天亮)1, 2, LIU Ran-ke(刘然克)1, 2,
JIA Jing-huan(贾静焕)1, 2, DU Cui-wei(杜翠薇)1, 2, LI Xiao-gang(李晓刚)1, 2
1. Corrosion and Protection Center, University of Science and Technology Beijing, Beijing 100083, China;
2. Key Laboratory of Corrosion and Prevention of Ministry of Education, Beijing 100083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Stress corrosion cracking (SCC) behavior of P110 tubing steel in simulated CO2 injection well annulus environments was investigated through three-point bent tests, potentiodynamic polarization and EIS measurements. The results demonstrate that SCC of P110 tubing steel could occur in acidulous simulated environment, and the sensitivity of SCC increases with the decrease of pH, as well as increase of sulfide concentration and total environmental pressure. Both anodic dissolution and hydrogen embrittlement make contributions to the SCC. Adequate concentration of corrosion inhibitor can inhibit the occurrence of SCC on account of the inhibition of localized anodic dissolution and cathodic hydrogen evolution.
Key words:
P110 tubing steel; stress corrosion cracking; annulus environment; CO2 flooding;
1 Introduction
As an environmentally friendly and efficient extraction method, CO2 flooding can seal the greenhouse gas CO2 and improve recovery of oil and gas fields; thus, with the exploration of heavy and low-permeability reservoir oil, the process of CO2 flooding has exhibited good development [1]. However, in practical application, this technique encounters severe corrosion problems. Serious brittle fracture incidents of oil tubing in CO2 injection wells during CO2 flooding have occurred in several oilfields in China. Failure analysis reveals that all ruptures occur within 200 m to 600 m underground at 0 °C to 15 °C and are caused by stress corrosion cracking (SCC) that originate in the outer pipe wall. Substantial sulfide components have been detected on the tubing wall, and high concentrations of CO2,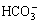 ,
,  and Cl-, as well as small amounts of H2S, have been tested in the annulus medium. The findings provide good conditions for sulfide stress corrosion cracking (SSCC) [2]. During the CO2 injection process, CO2 leaks to the annulus because of high injection pressure [3], after which it could dissolve in water to form carbonic acid [4]. As a result, the pH of the medium will greatly decrease. Meanwhile, the annulus is a closed anaerobic environment with high concentration of
and Cl-, as well as small amounts of H2S, have been tested in the annulus medium. The findings provide good conditions for sulfide stress corrosion cracking (SSCC) [2]. During the CO2 injection process, CO2 leaks to the annulus because of high injection pressure [3], after which it could dissolve in water to form carbonic acid [4]. As a result, the pH of the medium will greatly decrease. Meanwhile, the annulus is a closed anaerobic environment with high concentration of 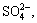 so sulfate-reducing bacteria will produce sufficient quantities of H2S at an appropriate temperature [5]. Within the temperature range of 16 °C to 30 °C, the low pH environment induced by the hydrolysis of CO2 will promote the activity of hydrogen evolution reaction of H2S on the metal surface, as well as reinforce hydrogen embrittlement (HE) and hydrogen induced cracking (HIC) risks, resulting in a pronounced increase in SSCC sensitivity [6]. Most studies on SCC in CO2/H2S coexistence environment focus on room temperature and high temperature/high pressure conditions [7–10], whereas studies on stress corrosion behavior and its key influencing factors in such annulus environments of CO2 injection wells characterized by low-temperature (0 °C to 15 °C) and high-pressure CO2/H2S coexistence environment are still limited.
so sulfate-reducing bacteria will produce sufficient quantities of H2S at an appropriate temperature [5]. Within the temperature range of 16 °C to 30 °C, the low pH environment induced by the hydrolysis of CO2 will promote the activity of hydrogen evolution reaction of H2S on the metal surface, as well as reinforce hydrogen embrittlement (HE) and hydrogen induced cracking (HIC) risks, resulting in a pronounced increase in SSCC sensitivity [6]. Most studies on SCC in CO2/H2S coexistence environment focus on room temperature and high temperature/high pressure conditions [7–10], whereas studies on stress corrosion behavior and its key influencing factors in such annulus environments of CO2 injection wells characterized by low-temperature (0 °C to 15 °C) and high-pressure CO2/H2S coexistence environment are still limited.
In this work, P110 tubing steel SCC behavior and its influence factors were investigated under the simulated CO2 injection well annulus working conditions to provide reference for a comprehensive understanding of the law of typical tubing steel corrosion behavior in CO2 injection well annulus environment and establish appropriate protection methods.
2 Materials and experimental procedures
The chemical composition and mechanical properties of P110 tubing steel used in this work are shown in Table 1 and Table 2, respectively, which meets the requirements of the standard API 5CT [11]. The microstructure of the steel is homogeneous, comprising martensite plus part ferrite, and has no obvious rolling texture features, as shown in Fig. 1.
Table 1 Chemical composition of P110 tubing steel (mass fraction, %)
Table 2 Mechanical properties of P110 tubing steel

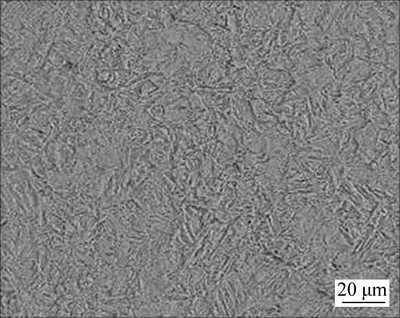
Fig. 1 Metallurgical structure of P110 tubing steel
Laboratory-simulated solution consisted of 13.50 g/L Na2S, 2.71 g/L NaHCO3, 6.15 g/L NaCl and 0.33 g/L Na2SO4, which was configured based on the component analysis of the CO2 injection well annulus media data. Considering the actual working conditions, inhibitor (imidazoline) is usually added to slow down corrosion of materials. Therefore, this work was carried out with different concentrations of the same inhibitor and Na2S. Furthermore, 0.5% of CH3COOH solution and NaOH solution was used to adjust the pH, which aimed to simulate the changes in corrosion inhibitor, sulfide concentration, and other factors. Before the tests, the solution was deaerated for 1 h by high-purity N2 with a rate of 500 mL/min.
For the stress corrosion testing, three-point bend specimens were employed. The load was controlled by fixing the deflection of the specimen to set value. The sample surfaces were then degreased with acetone [12]. Samples for electrochemical measurements with size of 10 mm×10 mm×3 mm were sealed with epoxy resin, and only the working surface was exposed to the medium. All the samples’ working surfaces were abraded to a mirror finish with 1500 SiC paper. The polished direction of three-point bend specimen was consistent with its tensile stress direction. Subsequently, these samples were cleaned with distilled water and acetone.
Stress corrosion testing and electrochemical measurements were performed in a sealed autoclave. Before the tests, a sufficient amount of high-purity nitrogen gas was input into the autoclave to replace oxygen and simulate annulus hypoxic environment. Subsequently, the autoclave was pressurized with CO2 and N2 to the desired CO2 partial pressure and total pressure. After the stress corrosion testing, a Cambridge S-360 scanning electron microscope was used to observe the corrosion morphology.
Electrochemical measurements used a conventional three-electrode system consisting of a working electrode of the sample, a counter electrode of platinum, and an Ag/AgCl reference electrode in saturated KCl connected via a salt bridge filled with the test solution. To obtain a stable open circuit potential, the experiment system was kept for 30 min after the pressurization, and then polarization curves and electrochemical impedance spectroscopy were measured at a PARSTAT-2273 electrochemical workstation. Potentiodynamic polarization curves were obtained by changing the electrode potential automatically from cathode branch to anode branch in a range from –500 mV to 800 mV (versus OCP) at a rate of 0.5 mV/s. The EIS experiments were conducted over the frequency range 100 kHz to 0.01 Hz with 10 mV amplitude signal.
3 Results
3.1 Stress corrosion testing
Figure 2 shows the stress corrosion morphology of the sample surfaces in the solution with pH of 4.0 under different experimental conditions. Ulcer-like corrosion occurs on the surface of the specimen without inhibitor addition (Figs. 2(a) and (b)). Small cracks are observed at the local area, which demonstrates that P110 tubing steel has certain susceptibility to stress corrosion under such conditions. However, the cracks are still stuck on a relatively small scale after 720 h, that is, the stress corrosion is relatively slow because of the similar rate between uniform corrosion and cracking growth. When 10 g/L inhibitors (Figs. 2(c) and (d)) are added, the specimen surface corrodes much more lightly and localized corrosion susceptibility increases, characterized by the presence of a large number of pitting. Meanwhile, the cracks grow rapidly to visible macroscopic SCC and even lead to specimen fracture (Table 3). When the amount of inhibitor reaches 20 g/L (Figs. 2(e) and (f)),localized corrosion is restrained. Pitting is significantly reduced and SCC does not emerge even after 720 h of immersion test. The results indicate that a sufficient amount of inhibitor can hinder or delay the occurrence of stress corrosion. Moreover, from the results in Fig. 2, raising the stress level can promote crack growth (Figs. 2(c) and (d)).
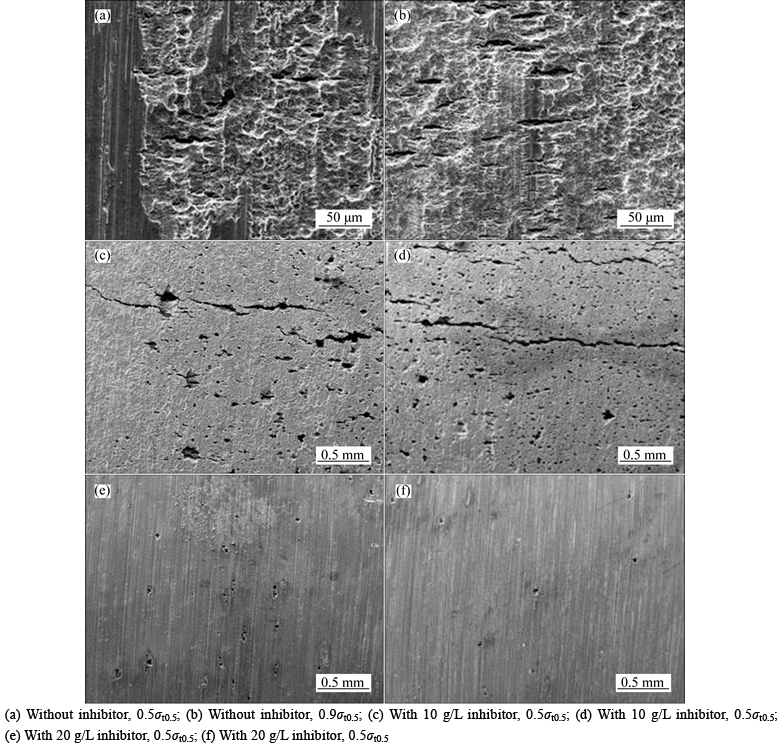
Fig. 2 Stress corrosion morphology of sample surfaces in solution with pH of 4.0 under different experimental conditions:
Table 3 Stress corrosion test results of P110 tubing steel
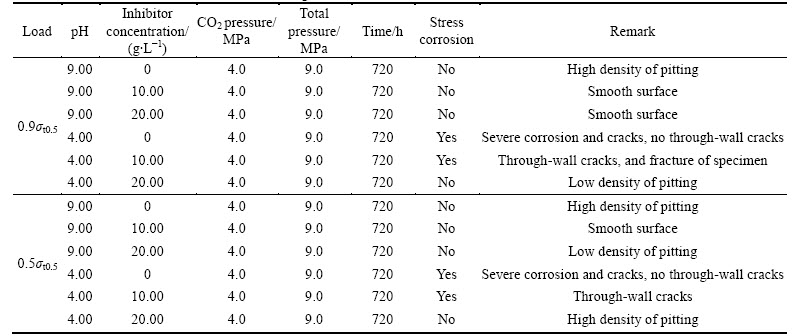
Table 3 presents the statistics of P110 tubing steel stress corrosion under different experimental conditions. Based on the table, SCC occurs only at pH of 4.0, and is not observed when the pH value and inhibitor amount increase. No obvious corrosion features on the sample surface are observed, part of the specimens remain bright, and some specimens exhibit obvious pitting. When no inhibitor is added, severe uniform corrosion occurs on the surface of the sample, and abundant black iron sulfide precipitate in the test solution. These phenomena confirm that the metal corrodes quickly in this case and only SCC can nucleate (and only small cracks), resulting in lower susceptibility to SCC.
3.2 Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization curves of P110 steel at different pH values are displayed in Fig. 3. As shown in Fig. 3(a), when the pH values are 4.0, 6.5, and 9.0, EIS capacitive arc radius increases successively, which indicates that electrode reaction can be inhibited sharply by elevating pH, leading to the decrease of P110 steel corrosion rate. In Fig. 3(b), when the pH value reaches above 6.5, the polarization curve moves to the left and the corrosion current density is reduced significantly. Furthermore, increase of pH debases the cathode and the anode reaction rate simultaneously, which not only leads to the decreased corrosion rate of steel P110, but also reduces the amount of hydrogen in the steel. Accordingly, hydrogen embrittlement effect can be suppressed, thus lowering the SCC susceptibility.
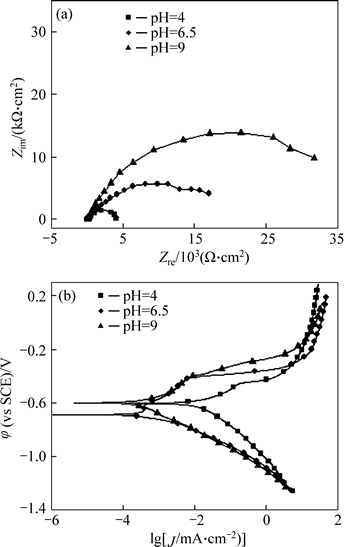
Fig. 3 Electrochemical impedance spectroscopy (EIS) (a) and potentiodynamic polarization curves (b) of P110 steel at different pH values (ptotal=9 MPa, 
When the spectrum data are fitted by using an equivalent circuit (Fig. 4), the testing curve agrees well with the fitting curve, where Rs represents the solution resistance, Qf is the constant phase angle element reflecting corrosion product layer information, Rf is the corrosion product resistance reflecting inhibition of corrosion products on the surface during the electrode process, Qdl is the constant phase element accounting for the electric double-layer capacitor, and Rt represents the charge transfer resistance. Rf and Rt obtained from Fig. 4 at different pH values are compared as shown in Fig. 5.
Figure 5 shows that the charge transfer resistance at pH of 6.5 is much greater than that at pH of 4.0. At the same time, because of the formation of more compact corrosion product films (increased Rf), the reaction process is hindered, making the corrosion rate and cathodic hydrogen evolution rate at pH of 6.5 significantly lower compared with that of pH of 4.0. At pH of 9.0, although the corrosion product film resistance decreases, the charge transfer resistance increases substantially, which enhances the difficulty of the reaction process. Therefore, pH reaching 6.5 or more will reduce the penetration of the hydrogen atom into the surface of the steel substrate and depress the anodic dissolution process of the active site, thereby reducing the susceptibility to SCC.
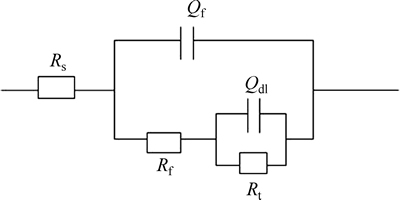
Fig. 4 Equivalent circuit for fitting of EIS data
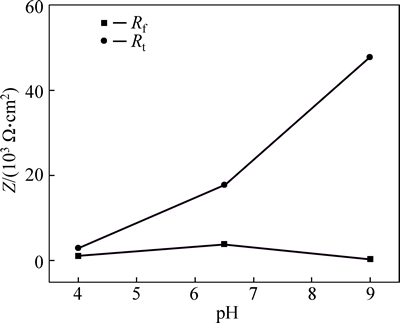
Fig. 5 Rf and Rt obtained at different pH values
EIS and polarization curves of P110 steel in the simulated solution of different concentrations of sulfide are shown in Fig. 6. Figure 6(a) shows that when the sulfide concentration is above 1650 mg/L, the effect of changes in the sulfide concentration on the EIS capacitive arc radius is not evident. However, when the sulfur concentration is reduced to 625 mg/L, the radius of the impedance arc increases remarkably and the electrode process is inhibited. It can be seen from Fig. 6(b) that the density of the corrosion current decreases with the decline of sulfide concentration. When the sulfide concentration is reduced to 1650 mg/L or less, the corrosion current density decreases slightly. However, compared with concentration of sulfide 5×10-3, the corrosion potential increases notably, and the cathode and anode processes are both inhibited. Therefore, reducing the sulfide concentration significantly will depress the anode and cathode processes, which do not only debase the corrosion rate of P110 steel significantly, but also reduce the amount of hydrogen into the steel. Consequently, the effect of hydrogen embrittlement on SCC is lowered.
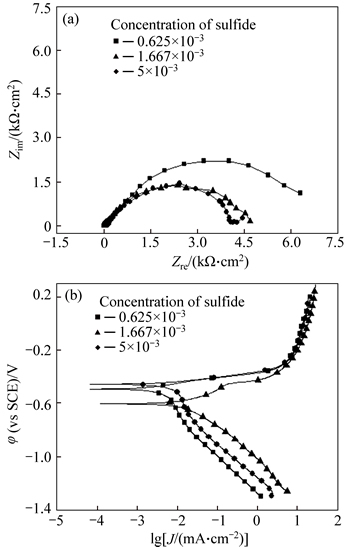
Fig. 6 Electrochemical impedance spectroscopy (EIS) (a) and polarization curves (b) of P110 steel in simulated solution of different concentrations of sulfide (ptotal=9 MPa, 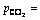 4 MPa)
4 MPa)
The electrochemical behaviors of P110 steel exhibited in Fig. 7 are evaluated under different annulus pressures. Figure 7(a) shows that when the annular pressure (ptotal) is less than 10 MPa, the increase in pressure causes a decrease in polarization resistance, indicating that the electrode reaction is accelerated. As shown in Fig. 7(b), when ptotal exceeds 10 MPa, the polarization resistance increases slightly. This phenomenon may be caused by further increase in the area of cathode region induced by the increase in ptotal. From Fig. 7(b), the corrosion current density increases remarkably with the increase in ptotal. When ptotal is less than 10 MPa, its increase induces the polarization curves to move parallel to the right, thus promoting the cathode and anode processes. When ptotal reaches 14 MPa, the cathodic process is further promoted, which results in the enhancement of the corrosion potential and corrosion current density. Based on the results above, increasing pressure will facilitate the precipitation of hydrogen and its permeation in the steel, and may promote local anodicdissolution of the active points, resulting in an increase of sensitivity to the SCC of the steel.
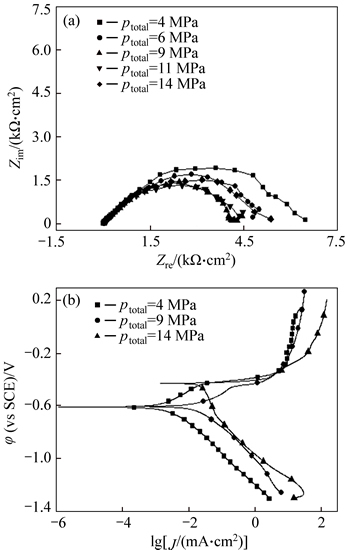
Fig. 7 Electrochemical impedance spectroscopy (EIS) (a) and polarization curves (b) of P110 steel under different annulus pressures (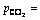 4 MPa, pH=4.0)
4 MPa, pH=4.0)
Figure 8 shows the EIS and polarization curves of P110 steel in the annulus-simulated solution with different inhibitor levels. The second capacitance arc of EIS is much more apparent with the inhibitor concentration increasing from 10 g/L to 20 g/L (Fig. 8(a)). The polarization resistance is also higher, which suggests that increasing the adsorption rate of the inhibitor on P110 steel surface will enhance the resistance for corrosive media passing through the inhibitor film. The fitting circuit is shown in Fig. 4. As shown in Fig. 8(b), with increasing amount of corrosion inhibitor, the polarization curve moves to the left and the corrosion potential increases. It indicates that adding inhibitor mainly suppress the anodic processes, thus reducing the corrosion rate and the rate of hydrogen evolution. Therefore, an increase in inhibitor concentration can reduce stress corrosion sensitivity of P110 steel.
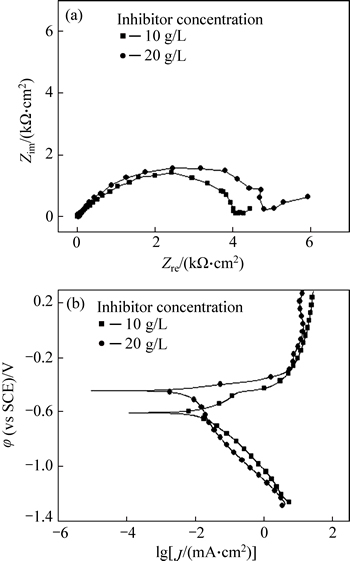
Fig. 8 Electrochemical impedance spectroscopy (EIS) (a) and polarization curves (b) of P110 steel in annulus-simulated solution with different inhibitor levels (ptotal=9 MPa, 
4 Discussion
The electrochemical corrosion mechanism of CO2-H2S in aqueous solution is generally considered to be a reduction of hydrogen ions and production of iron sulfides and carbonates. The CO2 corrosion mechanism is as follows [13-16]:
Anodic process:
Fe→Fe2++2e (1)
Fe2++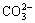 →FeCO3 (2)
→FeCO3 (2)
Cathodic process:
CO2+H2O→H2CO3 (3)
H2CO3→H++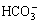 →2H++
→2H++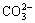 (4)
(4)
H2CO3 +e-→H+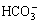 (5)
(5)
H+H→H2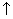 (6)
(6)
H2S corrosion mechanism is illustrated below [17-18]:
Anodic process:
Fe→Fe2++2e (7)
Fe2++S2-→FeS (8)
Fe2++HS-→FeS+H+ (9)
Fe+HS-→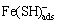 (10)
(10)
Fe(SH) →
→ +2e (11)
+2e (11)
Fe(SH) +HS-→FeS+H2S (12)
+HS-→FeS+H2S (12)
Cathodic process:
H2S→H++HS-→2H++S2- (13)
H2S+e→HS-+H (14)
HS-+e→S2-+H (15)
H++e→H (16)
In CO2/H2S medium, stability and permeability of corrosion product films on the steel surface have significant influence on the corrosion characteristics of steel [19]. From the above corrosion mechanism, the product films are mainly composed of different substances of FeCO3, Fe3O4, FeS and metal oxides. These materials are mixed and in different proportions under the influence of different factors (including pH, pressure, H2S and temperature). These product films of different nature are very different to use in hindering hydrogen penetration, so the SCC susceptibility of P110 steel under different conditions are not the same.
SCC behavior of P110 tubing steel is affected by pH. Figure 3(b) shows that cathodic hydrogen evolution reaction is suppressed by an elevated pH, leading to the reduction of both corrosion current density and current density for hydrogen evolution. This phenomenon is ascribed to the condition of pH of 4.0, wherein a large number of H2CO3 and H2S molecules exist in the solution, which cannot easily form FeS and FeCO3 with Fe2 + directly. As a result, the electrode reaction process is not hindered because of the formed loose product film. Both corrosion current density and current density for the hydrogen evolution are relatively high. When the pH is elevated to 6.5 and the concentrations of 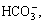 HS-,
HS-, 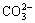 and S2- generated by ionization of H2CO3 and H2S in the solution are increased, the chance of those irons reacting with Fe2+ to form a dense product film becomes larger. Once the product film is formed, it can prevent the transfer of charge and absorption of hydrogen to a certain extent, resulting in lower corrosion current density and current density for hydrogen evolution. Naturally, at pH of 9.0,
and S2- generated by ionization of H2CO3 and H2S in the solution are increased, the chance of those irons reacting with Fe2+ to form a dense product film becomes larger. Once the product film is formed, it can prevent the transfer of charge and absorption of hydrogen to a certain extent, resulting in lower corrosion current density and current density for hydrogen evolution. Naturally, at pH of 9.0,  HS-, and S2- are mainly found in the solution, making the generation of FeCO3 and FeS easier. As a result, the consisting product film is denser, so the transfer of charge and absorption of hydrogen is hindered strongly and the corrosion current density and current density for hydrogen evolution are further decreased. Therefore, with the increase of pH and with reduced corrosion current density and cathodic current density of hydrogen evolution, the adsorbed hydrogen atoms are decreased significantly, resulting in a substantial reduction in the amount of hydrogen atoms into the steel that micrifies P110 tubing steel hydrogen- induced cracking and SCC sensitivity.
HS-, and S2- are mainly found in the solution, making the generation of FeCO3 and FeS easier. As a result, the consisting product film is denser, so the transfer of charge and absorption of hydrogen is hindered strongly and the corrosion current density and current density for hydrogen evolution are further decreased. Therefore, with the increase of pH and with reduced corrosion current density and cathodic current density of hydrogen evolution, the adsorbed hydrogen atoms are decreased significantly, resulting in a substantial reduction in the amount of hydrogen atoms into the steel that micrifies P110 tubing steel hydrogen- induced cracking and SCC sensitivity.
Moreover, sulfide concentration has a significant influence on the SCC behavior of P110 tubing steel. The material corrosion rate is significantly decreased only in the condition that sulfide concentration is reduced to a certain extent. At higher concentrations of sulfur, the degree of inhibition of various concentrations on the reaction changes slightly (Fig. 6) because when the sulfide concentration is lower, CO2 corrosion dominates the reaction, leading to the presence of dense and stable product film FeCO3 generated on the surface of the material [20], which can prevent the permeation of hydrogen atoms to the substrate effectively and reduce the risk of SCC. However, if the sulfide concentration becomes higher, the proportion of FexS in the corrosion product film increases, which can hinder the generation of the more protective FeCO3 film [21]. In addition, because of the poisonous effect of S2-, FexS film can promote the penetration of hydrogen into steel and aggravate the embrittlement effect [18]. The abovementioned reasons increase the SCC sensitivity of P110 tubing steel at higher sulfide concentrations (Table 3).
Environmental pressure also notably affects P110 steel SCC behavior. The influence of increased pressure on the reaction process involves three aspects: accelerating the reaction process by facilitating transfer of ions and charges [22]; increasing the likelihood of hydrogen-induced cracking by making the generated hydrogen atoms penetrate the surface of the steel substrate more easily [23]; and suppressing the electrode reaction by improving the density of corrosion products. Given the competition and synergistic effect of these three aspects, the influence of pressure on the SCC becomes complicated. When ptotal is less than 10 MPa, the increase in pressure is mainly to promote the electrode process (Fig. 7). Both anodic reaction and cathodic process are accelerated, thus increasing the susceptibility to SCC (Table 3). When ptotal>10 MPa, the increase in pressure is not only to promote the cathodic process (Fig. 7(b)), but also to make the corrosion product film become denser, which is characterized by the slight increase in the impedance of ptotal=14 MPa (Fig. 7(a)). Accordingly, at higher pressures, the competitive effects of the promotion of increased pressure on the cathodic process and inhibition of dense corrosion product film on the reaction determine the SCC susceptibility of the materials.
In addition, the work also considers the effect of the corrosion inhibitor on P110 steel SCC. The inhibitor used in the experiment is imidazoline, which can form an adsorption layer with strong binding force on the surface of the tubing steel. It is mutually embedded with the corrosion film, making the product film more compact. Simultaneously, Fe ions in the corrosion product layer and the hybrid atom of the inhibitor can form coordination bonds, enhancing the adhesion of molecules in the corrosion product film [24]. As a result, the mechanical properties of the corrosion product films can be improved substantially, effectively suppressing the anode and cathode reactions (Fig. 8). Without inhibitors, the corrosion product film consists only of FeCO3, FexS, and other substances, and they cannot effectively mitigate uniform corrosion. Thus, SCC initiation and development of uniform corrosion compete with each other, making it difficult for SCC to extend and cause destruction (Fig. 2 and Table 3). If a moderate concentration of inhibitor (10 g/L) is added, the protection of the surface layer is enhanced because of the strengthening role of the corrosion inhibitor; it significantly inhibits uniform corrosion. The local weakening of the film is prone to pitting, and the SCC easily initiates and expands (Fig. 2(b)). When the inhibitor concentration is higher (20 g/L), the surface of P110 tubing steel has a better protective film whose defective sites are significantly reduced. Only a small amount of pitting occurs, and SCC initiates with difficulty.
5 Conclusions
SCC of P110 steel occurs in annulus environments. It contains small amounts of corrosion inhibitors H2S and CO2. The pH can reach as low as 4. Both anodic dissolution and hydrogen embrittlement make contributions to the SCC. Higher pressure, lower inhibitor concentration, decrease of pH, and increase of sulfide concentration facilitate cathodic hydrogen evolution, leading to increased sensitivity to SCC. When a sufficient amount of inhibitor is added to the simulated solution and the pH is adjusted to 6.5 or greater, P110 tubing steel susceptibility to SCC is reduced significantly.
References
[1] WANG Tong-lei, SONG Yong-chen, ZHAO Yue-Chao, LIU Yu, ZHU Ning-jun. Measurement of immiscible CO2 flooding processes and permeability reduction due to asphaltene precipitation by X-ray CT imaging [J]. Energy Procedia, 2013, 37: 6920-6927.
[2] Ziaei S M R, Kokabi A H, Nasr-Esfehani M. Sulfide stress corrosion cracking and hydrogen induced cracking of A216-WCC wellhead flow control valve body [J]. Case Studies in Engineering Failure Analysis, 2013, 1: 223-234.
[3] Yevtushenko O, Bettge D, Bohraus S, Pfenning A, Kranzmann A. Corrosion behavior of steels for CO2 injection [J]. Process Safety Environmental Protection, 2014, 92: 108-118.
[4] Zhang G A, Zeng Y, Guo X P, Jiang F, Shi D Y, Chen Z Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment [J]. Corrosion Science, 2012, 65: 37-47.
[5] Guan J, Xia L P, Wang L Y, Liu J F, Gu J D, Mu B Z. Diversity and distribution of sulfate-reducing bacteria in four petroleum reservoirs detected by using 16S rRNA and dsrAB genes [J]. International Biodeterioration Biodegradation, 2013, 76: 58-66.
[6] Joseph A P, Keller J, Bustamante H, Bond P L. Surface neutralization and H2S oxidation at early stages of sewer corrosion: Influence of temperature, relative humidity and H2S concentration [J]. Water Research, 2012, 46: 4235-4245.
[7] Dong S J, Zhou G S, Li X X, Ouyang S, An H F. Comparison of corrosion scales formed on KO80SS and N80 steels in CO2/H2S environment [J]. Corrosion Engineering, Science and Technology, 2011, 46(6): 692-696.
[8] Abayarathna D, Naraghi A R, Wang S. The effect of surface films on corrosion of carbon steel in a CO2-H2S-H2O system [C]// Corrosion. Houston: NACE International, 2005: 177-209.
[9] Li Wen-fei, Zhou Yan-jun, Xue Yan. Corrosion behavior of 110S tube steel in environments of high H2S and CO2 content [J]. Journal of Iron & Steel Research International, 2012, 19(12): 59-65.
[10] Yin Z F, Zhao W Z, Bai Z Q, Feng Y R, Zhou W J. Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2 [J]. Electrochimica Acta, 2008, 53(10): 3690-3700.
[11] API/5CT. Specification for casing and tubing, eighth edition (ISO adoption from ISO 11960:2004) [S]. Washington, DC: API, 2005.
[12] GB/T 15970.6-2007. Corrosion of metal and alloys-Stress corrosion testing—Part 6: Preparatation and use of pre-cracked specimens for tests under constant load or constant displacement [S]. (in Chinese)
[13] Wu Y M. Applying process modeling to screen refining equipment for wet hydrogen sulfide service [J]. Corrosion, 1998, 54(2): 169-173.
[14] Heuer J K, Stubbins J F. An XPS characterization of FeCO3 films from CO2 corrosion [J]. Corrosion Science, 1999, 41(7): 1231-1243.
[15] Ma Hou-yi, Cheng Xiao-liang, Li Gui-qiu, Chen Shen-hao, Quan Zhen-lan, Zhao Shi-yong, Lin Niu. The influence of hydrogen sulfide on corrosion of iron under different conditions [J]. Corrosion Science, 2000, 42(10): 1669-1683.
[16] Remita E, Tribollet B, Sutter E, Vivier V, Ropital F, Kittel J. Hydrogen evolution in aqueous solutions containing dissolved CO2: Quantitative contribution of the buffering effect [J]. Corrosion Science, 2008, 50(5): 1433-1440.
[17] Ma H Y, Cheng X L, Li G Q. The influence of hydrogen sulfide on corrosion of iron under different conditions [J]. Corrosion, 2000, 42(10): 1669-1683.
[18] Liu Z Y, Dong C F, Li X G, Zhi Q, Cheng Y F. Stress corrosion cracking of 2205 duplex stainless steel in H2S-CO2 environment [J]. Journal of Materials Science, 2009, 44(16): 4228-4234.
[19] Ding Jin-hui, Zhang Lei, Lu Min-xu, Wang Jing, Wen Zhi-bin, Hao Wen-hui. The electrochemical behaviour of 316L austenitic stainless steel in Cl- containing environment under different H2S partial pressures [J]. Applied Surface Science, 2014, 289(15): 33-41.
[20] Banas J, Lelek-Borkowska U, Mazurkiewicz B, Solarski W. Effect of CO2 and H2S on the composition and stability of passive film on iron alloys in geothermal water [J]. Electrochimica Acta, 2007, 52(18): 5704-5714.
[21] Marcus P. Corrosion mechanism in theory and practice [M]. London: CRC Press, 2002: 287-310.
[22] Bica I, Anitas E M, Bunoiu M, Vatzulik B, Juganaru I. Hybrid magnetorheological elastomer: Influence of magnetic field and compression pressure on its electrical conductivity [J]. Journal of Industrial and Engineering Chemistry, 2014, 20(6): 3994-3999.
[23] Spencer E C, Ross N L, Surbella R G, Cahill C L. The influence of pressure on the structure of a 2D uranium(VI) carboxyphosphonoate compound [J]. Journal of Solid State Chemistry, 2014, 218: 1-5.
[24] Jevremovic I, Singer M, Nesic S, Miskovic-Stankovic V. Inhibition properties of self-assembled corrosion inhibitor talloil diethylenetriamine imidazoline for mild steel corrosion in chloride solution saturated with carbon dioxide [J]. Corrosion Science, 2013, 77: 265-272.
(Edited by FANG Jing-hua)
Foundation item: Project(2012AA040105) supported by the High-tech Research and Development Program of China; Project(2014CB643300) supported by National Basic Research Program of China; Project(51741034) supported by National Natural Science Foundation of China
Received date: 2015-01-23; Accepted date: 2015-09-23
Corresponding author: LIU Zhi-yong, PhD, Associate Professor; Tel: +86-10-62333931; Fax: +86-10-62334005; E-mail: liuzhiyong7804@126.com
Abstract: Stress corrosion cracking (SCC) behavior of P110 tubing steel in simulated CO2 injection well annulus environments was investigated through three-point bent tests, potentiodynamic polarization and EIS measurements. The results demonstrate that SCC of P110 tubing steel could occur in acidulous simulated environment, and the sensitivity of SCC increases with the decrease of pH, as well as increase of sulfide concentration and total environmental pressure. Both anodic dissolution and hydrogen embrittlement make contributions to the SCC. Adequate concentration of corrosion inhibitor can inhibit the occurrence of SCC on account of the inhibition of localized anodic dissolution and cathodic hydrogen evolution.


