- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discus...▲
- 3.1 Comparison of cryogen water mass transfer strengthened by ultrasound and electric heating rod with same power
- 3.2 Distance effect between ultrasonic transducer and mass transfer interface on strengthening transfer of cryogen water
- 3.3 Effects of different ultrasonic powers on mass transfer enhancement of cryogen water
- 4 Conclusions▲
- References
- Figure
- Fig. 1 Schematic diagram of experimental unit
- Fig. 2 Changes of LiBr solution content with heating temperature and time
- Fig. 3 Mass flux of cryogen water versus LiBr solution content under driving heater temperature of 80 °C
- Fig. 4 LiBr solution mass flux with content of 53% versus heating temperature
- Fig. 5 LiBr solution mass flux with content of 55% versus heating temperature
- Fig. 6 LiBr solution temperature versus testing time under heating temperature of 80 °C
- Fig. 7 LiBr solution content versus heating time and H under heating temperature of 80 °C
- Fig. 8 Mass flux of cryogen water versus LiBr solution content and H at heating temperature of 80 °C
- Fig. 9 LiBr solution content versus heating time and ultrasonic power at heating temperature of 80 °C
- Fig. 10 Mass flux of cryogen water versus LiBr solution content and ultrasonic power at heating temperature of 80 °C
J. Cent. South Univ. (2016) 23: 405-412
DOI: 10.1007/s11771-016-3085-1
Mass transfer enhancement for LiBr solution using ultrasonic wave
HAN Xiao-dong(韩晓东), ZHANG Shi-wei(张仕伟), TANG Yong(汤勇), YUAN Wei(袁伟), LI Bin(李斌)
Key Laboratory of Surface Functional Structure Manufacturing of Guangdong Higher Education Institutes
(South China University of Technology), Guangzhou 510640, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
The methods were studied to improve the cooling performance of the absorption refrigeration system (ARS) driven by low-grade solar energy with ultrasonic wave, while the mechanism of ultrasonic wave strengthening boiling mass transfer in LiBr solution was also analyzed with experiment. The experimental results indicate that, under the driving heat source of 60–100 °C and the ultrasonic power of 20–60 W, the mass flux of cryogen water in LiBr solution is higher after the application of ultrasonic wave than auxiliary heating with electric rod of the same power, so the ultrasonic application effectively enhances the heat utilization efficiency. The distance H from ultrasonic transducer to vapor/liquid interface significantly affects mass transfer enhancement, so an optimal Hopt corresponding to certain ultrasonic power is beneficial to reaching the best strengthening effect for ultrasonic mass transfer. When the ultrasonic power increases, the mass transfer obviously speeds up in the cryogen water; however, as the power increases to a certain extent, the flux reaches a plateau without obvious increment. Moreover, the ultrasound-enhanced mass transfer technology can reduce the minimum temperature of driving heat source required by ARS and promote the application of solar energy during absorption refrigeration.
Key words:
mass transfer enhancement; LiBr solution; ultrasonic wave; solar absorption refrigeration system;
1 Introduction
As the global climate grows warmer, the electricity consumption gradually increases for air conditioning in summer, which greatly exacerbates the shortage of power resources. Compared with electric comprehensive refrigeration air conditioner, solar absorption products with the working medium of LiBr solution take solar hot water as the driving energy. The latter not only boasts superior seasonal design but also produces no greenhouse gas as its work medium has no pollution to the environment [1]. Currently, many demonstrative and application projects of solar air conditioning have been put into operation [2–6] and these refrigeration systems produce certain social and economic benefits.
As solar energy is unevenly distributed in time and space, solar thermal collectors show a low output temperature in the regions with inadequate light or under poor weather conditions. Traditional single-effect ARS cannot operate effectively when being driven by heat resource below 80 °C because of low coefficient of performance (COP) [7–8]. In such cases, the majority of commercial absorption refrigeration products fail to run solely driven by solar hot water and require auxiliary heat source (gas or electricity) to improve water temperature [9]. However, the application of such sources leads to a larger ratio of the non-solar part to the driving energy of solar ARS, reduces the environment-friendly performance, and goes against the original design of solar air conditioning.
Therefore, to realize the effective application of low-grade solar hot water is the highlight for developing and promoting solar ARS. During the mass transfer of commercial ARS with the working medium of LiBr solution, when the driving heat source shows high temperature, the bottleneck of mass transfer appears mainly in the steam absorption process of LiBr solution. Many studies were made on improving absorption for mass transfer, for instance, to improve mass absorption rate by agitation [10] and spraying [11], to apply super-hydrophobic nanofibrous structures [12], binary nanofluid [13] and to add surface active agent [14–16] to enhance solution absorption capacity. When the driving heat source shows low temperature, evaporation of cryogen water in LiBr solution of generator is the main bottleneck for mass transfer.
The popular method for mass transfer enhancement in the generator is to boost the driving force of mass transfer by raising the energy conversion efficiency during the boiling and mass transfer processes of cryogen water with the help of various enhanced heat transfer tubes [17–18]. KULANKARA and HEROLD [19] researched the method of adding surface active agent in LiBr solution to enhance heat and mass transfer while the agent not only reduced the surface tension of LiBr solution but also raised the steam mass flux. SURESH and MANI [20] used R134a-DMF solution as the working medium for absorption refrigeration to get high heat and mass transfer efficiency during driving operation by low-grade heat source. SHI et al [21] investigated the performance of falling film generator compared to the traditional immersed tube generator and found the former was more suitable for low-grade heat driven solar ARS. Some researchers [22–25] studied desorption of aqueous LiBr solution by vacuum membrane distillation process. Vacuum membrane distillation technology can obtain better performance compared to other methods, and be advantageous to the miniaturization of the LiBr ARS. But, the vacuum membrane distillation usually needed a large pressure drop [25], and immensely increased the generator design complexity.
Aiming at the shortcomings of the above methods, some researchers tried to strengthen the transfer during distillation and desorption using ultrasonic wave. ZHU and LIU [26] got the conclusion from the distillation research with ultrasonic air gap membrane: ultrasonic excitation increased the mass flux for membrane distillation and the value was increased as the ultrasonic power became larger. LIU et al [27] applied ultrasound to strengthen the mass transfer of dissolved oxygen desorption with vacuum membrane. And the results indicated that ultrasound enhanced membrane mass transfer performances. Under the same vacuum degree, the transfer coefficient increased with ultrasonic intensity, and the bigger the vacuum degree, the greater the increasing rate. Because the separation and mass transfer of cryogen water in the ARS is the distillation process under high vacuum degree, it is feasible to enhance mass transfer of cryogen water with ultrasound.
Compared with other methods, such as increasing the driving force of cryogen water with enhanced heat transfer tubes or reducing the resistance with surface active agent, ultrasound can not only strengthen the heat transfer of LiBr solution and improve the driving force of mass transfer, but also reduce the transfer resistance in the solution and effectively enhance the mass transfer capacity of cryogen water. In addition, the solar ARS with ultrasonic technology does not need to change existing generator structure and can be maintained easily. In this work, ultrasonic technology was used in ARS driven by low-grade solar hot water, and the strengthening mechanism of evaporation mass transfer in cryogen water was studied with ultrasound through experiment. It is intended to provide useful data for ultrasonic strengthening in solar ARS.
2 Experimental
2.1 Experimental setup
A single-effect absorption refrigeration cycle contains two main processes of phase transition and mass transfer: the evaporation and mass transfer of cryogen water in the generator where it is separated from LiBr solution, and LiBr solution in the absorber absorbing vapor from the evaporator. This experiment focused on the former process, so the experimental unit was designed with two key components of the generator and condenser reserved for mass transfer in the generator. The schematic diagram of experimental unit is shown in Fig. 1.
The ultrasonic power of 20–60 W and the frequency of 1.7 MHz as well as the vacuum pump of Leybold D16C were used in the experiment. The temperature sensor applied was DS18B20 with the temperature range from –55 °C to 125 °C and the precision of ±0.5 °C. And the pressure transmitter was Mik-P300G of diffused silicon high-temperature type with the measurement range of 0–2 MPa and the precision of ±1000 Pa.
A constant-temperature circulating water tank with adjustable output temperature of 30 °C–100 °C, heating power of 1 kW and precision of ±0.1 °C was used in the experiment to simulate the hot water output of solar thermal collector. The custom-tailored glass container was used as generator which contained a double-wall interlayer and hot water ran through silicon tube with surface cotton insulation into the interlayer to heat the generator. The upper section of generator was equipped with four connections: the three side ones were connected respectively to addition funnel, pressure transmitter, vacuum pump and condensation tube; meanwhile, the overhead one was used to install ultrasonic transducer and electric heating rod. And this connection used a special seal cover to guide temperature sensor and power cord into generator.
2.2 Experimental procedure
The working medium was LiBr solution with the mass fraction of 50% in experiment. And the experiment was divided into three groups in total:
1) Perform contrast experiment of cryogen water mass transfer under ultrasonic strengthening and auxiliary heating by electric heating rod with the same power.
Install ultrasonic transducer and electric heating rod with the same power (20 W), respectively, in glass generators and perform mass transfer experiment of cryogen water under different water temperatures for heating.
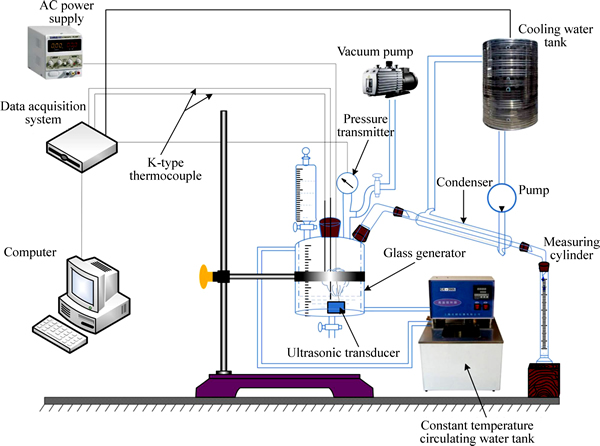
Fig. 1 Schematic diagram of experimental unit
2) Perform ultrasonic mass transfer enhancement under different distances between ultrasonic transducer and vapor-liquid interface.
Transducer support with variable height was used to adjust the distance H between ultrasonic transducer and vapor-liquid interface, so that the mass transfer experiment was carried out under different H. The values of H were 5, 15, 30 and 45 mm. In this group of experiment, the heating temperature was 80 °C and the ultrasonic power was 20 W.
3) Perform ultrasonic mass transfer enhancement experiment under different powers.
The ultrasonic powers chosen were 20, 40 and 60 W under the same height H of 30 mm and the heating-used hot water of 80 °C.
The rest experimental operation processes are basically the same for the foregoing three groups. And the specific steps are as follows.
Gas tightness test was carried out for the experimental unit before the experiment. And after the test, the air in the generator was evacuated until “0” indication of the pressure transmitter. 450 mL of LiBr solution was added with addition funnel to the generator. The water temperature in the constant-temperature water tank was adjusted to that of the solar hot water simulated and then LiBr solution of the generator was heated by turning on hot water circulating switch. Meanwhile, cooling water tank was open. Finally, the digit record program was run on the computer to begin timing and collect experimental data.
The cooling water temperature was around 30 °C in the experiment. The measurement was conducted for three times per each group under the same conditions to ensure the reliability of results and then averaging of all data gained was performed and the final measurement results were obtained.
After the completion of experiment, mass content C of LiBr solution was calculated with
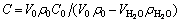 (1)
(1)
where V0 is the initial volume, ρ0 is the initial density and C0 is the initial content of the LiBr solution. For cryogen water collected, 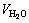 is the volume and
is the volume and 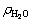 is its density. The content of LiBr solution can be calculated corresponding to the measurement of cryogen water collected at different time.
is its density. The content of LiBr solution can be calculated corresponding to the measurement of cryogen water collected at different time.
Similarly, the average mass flux of cryogen water in a certain period was calculated with
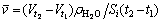 (2)
(2)
where Si indicates the area of vapor-liquid interface and 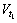 and
and 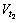 are the amounts of cryogen water collected at t1 and t2 respectively. Mass flux depends on the evaporation rate of cryogen water in LiBr solution. For calculation of mass flux with Eq.(2), the interval
are the amounts of cryogen water collected at t1 and t2 respectively. Mass flux depends on the evaporation rate of cryogen water in LiBr solution. For calculation of mass flux with Eq.(2), the interval 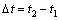 chosen should be small enough to better reflect the instant flux.
chosen should be small enough to better reflect the instant flux.
For boiling mass transfer, the following equation can also be used to calculate the mass flux of liquid phase transition:
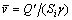 (3)
(3)
where Q′is the heat transfer rate, and γ is the latent heat of vaporization. Mass flux of liquid phase transition varies proportionally with heat transfer capacity.
3 Results and discussion
3.1 Comparison of cryogen water mass transfer strengthened by ultrasound and electric heating rod with same power
The research indicates that ultrasonic wave can effectively strengthen the mass transfer capacity of cryogen water in LiBr solution, but it undoubtedly increases energy input. An electric heating rod with the same power as ultrasonic waves is used as auxiliary heat source to strengthen mass transfer of cryogen water in LiBr solution, so that it is possible to better compare the strengthening results with ultrasonic waves. Figure 2 shows the changes of LiBr solution mass fractions with time and heat source temperature when the mass transfers are strengthened by auxiliary heating with the electric rod and sole ultrasonic waves. For the solution fraction under the same heating temperature, the contraction rate with ultrasound mass transfer enhancement is faster than that with auxiliary heating of electric rod. Therefore, the strengthening effect of boiling mass transfer by ultrasonic waves is not equal to heat effect.
From Fig. 2, the solution content and the temperatures of driving heat sources both show similarlinear relationship no matter ultrasonic wave or electric heating rod is used, under the same heating time for LiBr solution. However, the strengthening effect is related to the ratio of heat produced by the rod to the total driving energy when the electric heating rod is taken as auxiliary heater. The larger the ratio is, the better the strengthening effect is. When ultrasonic waves are applied to strengthening mass transfer of LiBr solution, the transfer increment is relatively stable for a certain period of time and the influence of heating temperature on strengthening effects is not as obvious as that under auxiliary heater. But, the overall effects are better than those under auxiliary heat source with the same power.

Fig. 2 Changes of LiBr solution content with heating temperature and time
Boiling mass transfer of LiBr solution is closely related to solution content: higher content goes with bigger transfer resistance, leading to more evaporation difficulties for cryogen water, so driving heat source with higher temperature is required to improve mass transfer rate of cryogen water. When the source temperature is low, the evaporation mass flux of cryogen water in the generator is lower than that of absorber, so it is very important to enhance the evaporation mass transfer in the generator for ARS driven by low-grade heat source.
For cryogen water under driving heat source of 80 °C, the changes of evaporation mass flux are shown in Fig. 3 and the ascending part of the curve indicates the solution transition from sub-cooled status to saturation boiling. When the solution content reaches approximately 52%, the solution enters saturation boiling status. Meanwhile, the lower the solution content is, the larger the mass flux is; in addition, as concentration increases, the flux gradually decreases. The flux of cryogen water with ultrasonic waves is higher than that with auxiliary heater of the same power under the same solution content. Therefore, it can be concluded that as the LiBr solution with the same content runs into the generator, the one under ultrasonic waves shows higher content at the outlet for the same solution circulation. The waves can not only increase the mass flux of cryogen water, but also raise the content of LiBr solution entering absorber. The solution absorption capacity for steam becomes stronger as LiBr solution content goes higher in the absorber. Therefore, the increase of the content mentioned above also results in better absorption capacity to steam of the absorber.

Fig. 3 Mass flux of cryogen water versus LiBr solution content under driving heater temperature of 80 °C
As the content of LiBr solution is constant, the mass flux of cryogen water goes up with heat source temperature increasing. Figures 4 and 5 show the changes of such flux with the temperature mentioned above under LiBr solution content of 53% and 55%, respectively. Under these two contents, the strengthening effects of mass transfer by ultrasonic waves are always better than those by auxiliary heater. And the former is obviously superior to the latter under a higher content.

Fig. 4 LiBr solution mass flux with content of 53% versus heating temperature

Fig. 5 LiBr solution mass flux with content of 55% versus heating temperature
Figure 6 shows the internal temperature changes of LiBr solution with heating time under the constant heating temperature of 80 °C. The solution temperature with ultrasonic waves is slightly higher than that of other cases, because the micro disturbance of solution by ultrasonic waves speeds up the heat transfer in the solution.
From the foregoing, the mass transfer enhancement by ultrasonic waves completely differs from auxiliary heating with electric heating rod at mechanism. For its reason, the major factor is not its heat effect but the combination of micro disturbance, cavitation, acoustic streaming effects etc produced by the waves during mass transfer enhancement using ultrasonic waves.
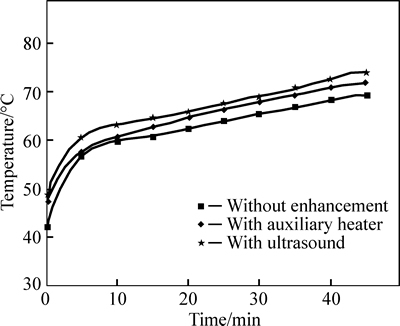
Fig. 6 LiBr solution temperature versus testing time under heating temperature of 80 °C
3.2 Distance effect between ultrasonic transducer and mass transfer interface on strengthening transfer of cryogen water
The distance H between ultrasonic transducer and mass transfer interface is a key factor for strengthening the mass transfer of LiBr solution by ultrasonic waves. The content of LiBr solution changes under different H for the transducer when the heat source temperature is 80 °C, as shown in Fig. 7. The contraction rate is the highest for LiBr solution content if H=30 mm. But the rate slows down if H decreases (H=5, 15 mm) or increases (H=45 mm). The mass flux of cryogen water changes with LiBr solution content under different H, as shown in Fig. 8. When H=30 mm, the flux is the largest during evaporation of cryogen water and the ultrasonic strengthening effects are the best. When H>30 mm or H<30 mm, the flux is inferior to that with H=30 mm.For mass transfer enhancement of cryogen water, the effect of H at other driving heat source temperatures shows a similar trend to that under 80 °C.

Fig. 7 LiBr solution content versus heating time and H under heating temperature of 80 °C
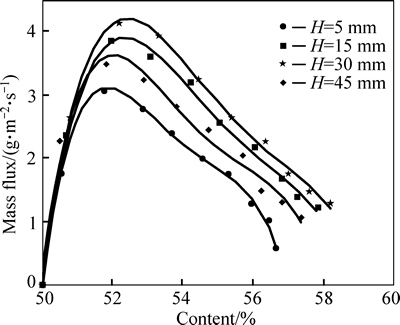
Fig. 8 Mass flux of cryogen water versus LiBr solution content and H at heating temperature of 80 °C
The inherent mechanism of ultrasonic mass transfer enhancement is the reason why the strengthening effect is the best with the transducer placed at certain H and the effect declines if the actual height is higher or lower than the certain H.
As known from double-film theory, the mass transfer of solution phase transition takes place at the vapor/liquid interface, where the transfer film consists of a thin vapor/liquid film and the transfer film produces the resistance during mass transfer. JANI et al [28] discovered that the larger the solution viscosity was, the thicker the liquid film was, and the mass flux was inversely proportional to film thickness, so the maximum flux was located at the film with the minimum thickness. Analyzed from the overall mass transfer, ultrasonic waves strengthen such transfer mainly through the following two actions.
1) Micro disturbance of ultrasonic waves speeds up the heat and mass transfer in the solution, so the heat and mass exchanges between the main solution and the liquid layer at transfer interface are both accelerated. In addition, it can effectively supplement the water molecules evaporated away from mass transfer interface.
2) The ultrasonic cavitation effect reduces the solution viscosity at the vapor/liquid interface leading to a decrease in liquid membrane thickness, and further reduces the transfer resistance at the interface. So, the water molecules go easily through liquid membrane into vapor phase in this case.
The foregoing two actions reflect the mechanism of ultrasonic mass transfer enhancement. As H decreases, ultrasonic waves produce larger effects on liquid membrane thickness at the vapor/liquid interface with smaller transfer resistance. But the effects of action 1) decline, because the liquid area influenced by ultrasonic waves decreases and so does the strengthening effect of heat and mass transfer in the solution. However, as H increases, the effects of action 2) decline, but that of action 1) gets enhanced. So, the best comprehensive effect of the two actions exists at a certain H with the largest mass flux of cryogen water.
3.3 Effects of different ultrasonic powers on mass transfer enhancement of cryogen water
Ultrasonic power under different heating temperatures shows similar influences on mass transfer, so the heating temperature of 80 °C is chosen for analyzing the effects of ultrasonic power on mass transfer of cryogen water. It is found in Fig. 9 that, when the power increases, the content increasing rate of LiBr solution speeds up, but the net increment of content decreases. The change of mass flux in Fig. 10 also indicates a similar law, that is, as the ultrasonic power becomes stronger under the same content, the flux increases but the rate slows down.
As the ultrasonic power increases, its effect of mass transfer is enhanced, but the increasing rate of mass flux decreases as the power rises. When the power reaches a certain value, the strengthening rate of mass transfer no longer increases. Because only part of ultrasonic wave is absorbed by the solution and used to strengthen the mass transfer of the solution and the other part not absorbed by the solution directly runs through solution and dissipates.
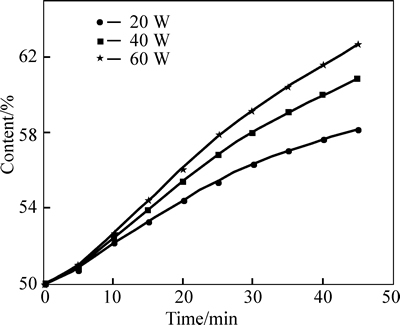
Fig. 9 LiBr solution content versus heating time and ultrasonic power at heating temperature of 80 °C

Fig. 10 Mass flux of cryogen water versus LiBr solution content and ultrasonic power at heating temperature of 80 °C
As the ultrasonic power increases, the ultrasonic energy absorbed by the solution gradually tends to the saturation status and the redundant of ultrasonic energy is not absorbed by the solution, so the effective utilization ratio of ultrasonic wave decreases and the strengthening effect gradually tends to be stable during solution mass transfer.
4 Conclusions
1) Ultrasonic waves can effectively strengthen the evaporation and mass transfer of cryogen water in LiBr solution. The strengthening effect of mass transfer by ultrasonic waves is stronger than that with auxiliary heating with electric rod of the same power, especially for small ultrasonic power. The distance between ultrasonic transducer and mass transfer interface makes obvious effects on mass transfer enhancement of cryogen water and the optimal distance (Hopt) corresponding to a certain ultrasonic power boasts best strengthening effect. Meanwhile, Hopt is related to the selection of heating temperature, ultrasonic power and frequency. As ultrasonic power increases, the mass transfer strengthening effect of cryogen water is enhanced with ultrasonic waves, but the increasing rate gradually decreases as the power increases and tends to reach a fixed value. Ultrasonic mass transfer enhancement can be effectively applied to ARS driven by low-grade heat sources, such as solar energy, to lower the minimum driving heat-source temperature required by ARS and improve the refrigeration performance.
2) As the ultrasonic transducer has a fixed- frequency in the experiment, the author failed to study the impact of ultrasonic frequencies on the mass transfer enhancement for LiBr solution. Therefore, future research will be made on the effect of ultrasonic frequencies on the mass transfer enhancement of cryogen water.
References
[1] XU Z Y, WANG R Z, XIA Z Z. A novel variable effect LiBr-water absorption refrigeration cycle [J]. Energy, 2013, 60: 457–463.
[2] ZHAI X Q, WANG R Z. A review for absorption and adsorption solar cooling systems in China [J]. Renewable and Sustainable Energy Reviews, 2009, 13: 1523–1531.
[3] BALARAS C A, GROSSMAN G, HENNING H M, FERREIRA C A I, PODESSER E, WANG L, WIEMKEN E. Solar air conditioning in Europe–An overview [J]. Renewable & Sustainable Energy Reviews, 2007, 2: 299–314.
[4]  F, SERRA L. Monitoring and simulation of an existing solar powered absorption cooling system in Zaragoza (Spain) [J]. Applied Thermal Engineering, 2011, 31: 28–35.
F, SERRA L. Monitoring and simulation of an existing solar powered absorption cooling system in Zaragoza (Spain) [J]. Applied Thermal Engineering, 2011, 31: 28–35.
[5] PRAENE J P, MARC O, LUCAS F, MIRANVILLE F. Simulation and experimental investigation of solar absorption cooling system in Reunion Island [J]. Applied Energy, 2011, 88: 831–839.
[6] FONG K F, CHOW T T, LIN Z, CHAN L S. Simulation– optimization of solar-assisted desiccant cooling system for subtropical Hong Kong [J]. Applied Thermal Engineering, 2010, 30: 220–228.
[7] VARGAS J V C, ORDONEZ J C, DILAY E, PARISE J A R. Modeling, simulation and optimization of a solar collector driven water heating and absorption cooling plant [J]. Solar Energy, 2009, 83: 1232–1244.
[8]  F, GUALLAR J. Stationary analysis of a solar LiBr-H2O absorption refrigeration system [J]. International Journal of Refrigeration, 2011, 34: 518–526.
F, GUALLAR J. Stationary analysis of a solar LiBr-H2O absorption refrigeration system [J]. International Journal of Refrigeration, 2011, 34: 518–526.
[9] DARKWA J, FRASER S, CHOW D H C. Theoretical and practical analysis of an integrated solar hot water-powered absorption cooling system [J]. Energy, 2012, 39: 395–402.
[10] TSAI B B, BLANCO H P. Limits of mass transfer enhancement in lithium bromide-water absorbers by active techniques [J]. International Journal of Heat and Mass Transfer, 1998, 41: 2409–2416.
[11] SU Feng-min, MA Hong-bin, GAO Hong-tao. Characteristic analysis of adiabatic spray absorption process in aqueous lithium bromide solution [J]. International Communications in Heat and Mass Transfer, 2011, 38: 425–428.
[12] ISFAHANI R N, MOGHADDAM S. Absorption characteristics of lithium bromide (LiBr) solution constrained by superhydrophobic nanofibrous structures [J]. International Journal of Heat and Mass Transfer, 2013, 63: 82–90.
[13] MA Xue-hu, SU Feng-min, CHEN Jia-bin, TAO Bai, HAN Zhen- xing. Enhancement of bubble absorption process using a CNTs- ammonia binary nanofluid [J]. International Communications in Heat and Mass Transfer, 2009, 36: 657–660.
[14] PARK S B, LEE H. Heat and mass transfer of the new LiBr-based working fluids for absorption heat pump [J]. Industrial & Engineering Chemistry Research, 2002, 41: 1378–1385.
[15] NAKORYAKOV V E, GRIGORYEVA N I, BUFETOV N S, DEKHTYAR R A. Heat and mass transfer intensification at steam absorption by surfactant additives [J]. International Journal of Heat and Mass Transfer, 2008, 51: 5175–5181.
[16] KENJI I, YASUHIKO H M. Surface tension of aqueous lithium bromide solutions containing 1-octanol as a “heat-transfer additive” [J]. International Communications in Heat and Mass Transfer, 1996, 23: 907–915.
[17] LEE L, CHO K. Effect of the geometry of flattened tube on the thermal performance of a high temperature generator [J]. International Journal of Refrigeration, 2009, 32: 667–674.
[18] MARCOS J D, IZQUIERDO M, LIZARTE R, PALACIOS E, INFANTE F C A. Experimental boiling heat transfer coefficients in the high temperature generator of a double effect absorption machine for the lithium bromide/water mixture [J]. International Journal of Refrigeration, 2009, 32: 627–637.
[19] KULANKARA S, HEROLD K E. Surface tension of aqueous lithium bromide with heat/mass transfer enhancement additives: The effect of additive vapor transport [J]. International Journal of Refrigeration, 2002, 25: 383–389.
[20] SURESH M, MANI A. Heat and mass transfer studies on a compact bubble absorber in R134a-DMF solution based vapour absorption refrigeration system [J]. International Journal of Refrigeration, 2013, 36: 1004–1014.
[21] SHI Cheng-ming, CHEN Qing-hua, JEN T C, YANG Wang. Heat transfer performance of lithium bromide solution in falling film generator [J]. International Journal of Heat and Mass Transfer, 2010, 53: 3372–3376.
[22] SUDOH M, TAKUWA K, IIZUKA H, NAGAMATSUY K. Effects of thermal and concentration boundary layers on vapor permeation in membrane distillation of aqueous lithium bromide solution [J]. Journal of Membrane Science, 1997, 131: 1–7.
[23] RIFFAT S B, WU S, BOL B. Pervaporation membrane process for vapour absorption system [J]. International Journal of Refrigeration 2004, 27: 604–611.
[24] WANG Zan-she, GU Zhao-lin, FENG Shi-yu, LI Yun. Application of vacuum membrane distillation to lithium bromide absorption refrigeration system [J]. International Journal of Refrigeration, 2009, 32: 1587–1596.
[25] ALI A H H. Design of a compact absorber with a hydrophobic membrane contactor at the liquid–vapor interface for lithium bromide–water absorption chillers [J]. Applied Energy, 2010, 87: 1112–1121.
[26] ZHU Chao, LIU Guang-liang. Modeling of ultrasonic enhancement on membrane distillation [J]. Journal of Membrane Science, 2000, 176: 31–41.
[27] LIU Li-ying, DING Zhong-wei, CHANG Li-jing, MA Run-yu, YANG Zu-rong. Ultrasonic enhancement of membrane based deoxygenation and simultaneous influence on polymeric hollow fiber membrane [J]. Separation and Purification Technology, 2007, 56: 133–142.
[28] JANI S, SAIDI M H, MOZAFARI A A, HEYDARI A. Modeling of heat and mass transfer in falling film absorption generators [J]. Scientia Iranica, 2004, 11: 81–91.
(Edited by YANG Bing)
Foundation item: Project(51275180) supported by the National Natural Science Foundation of China; Project(S201304416899) supported by the Natural Science Foundation of Guangdong Province, China; Project(sybzzxm201213) supported by Doctorate Dissertation Funds of Guangdong Province, China
Received date: 2014-12-04; Accepted date: 2015-06-08
Corresponding author: TANG Yong, Professor, PhD; Tel: +86–13570161623; E-mail: ytang@scut.edu.cn
Abstract: The methods were studied to improve the cooling performance of the absorption refrigeration system (ARS) driven by low-grade solar energy with ultrasonic wave, while the mechanism of ultrasonic wave strengthening boiling mass transfer in LiBr solution was also analyzed with experiment. The experimental results indicate that, under the driving heat source of 60–100 °C and the ultrasonic power of 20–60 W, the mass flux of cryogen water in LiBr solution is higher after the application of ultrasonic wave than auxiliary heating with electric rod of the same power, so the ultrasonic application effectively enhances the heat utilization efficiency. The distance H from ultrasonic transducer to vapor/liquid interface significantly affects mass transfer enhancement, so an optimal Hopt corresponding to certain ultrasonic power is beneficial to reaching the best strengthening effect for ultrasonic mass transfer. When the ultrasonic power increases, the mass transfer obviously speeds up in the cryogen water; however, as the power increases to a certain extent, the flux reaches a plateau without obvious increment. Moreover, the ultrasound-enhanced mass transfer technology can reduce the minimum temperature of driving heat source required by ARS and promote the application of solar energy during absorption refrigeration.
- Mass transfer enhancement for LiBr solution using ultrasonic wave


