Recovery of gallium from phosphorus industry flue
XU Ke(许 可), DENG Tong(邓 彤), DAI Yu-jie(戴玉杰), WANG Jing(王 静)
(Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China)
Abstract:
The flue dust generated during electric furnace production of elemental phosphorus was investigated for the recovery of gallium. Then the flue dust was slurried with water and blended with concentrated sulfuric acid, followed by ageing. The gallium in the dust was thereby converted to soluble sulfate. The factors affecting the dust curing were investigated to understand the process chemistry of the pretreatment. The optimal curing conditions are determined as follows: the mass ratio of dust to water and acid is 1∶1∶1, ageing temperature and time are 200℃ and 2h, respectively. Almost all the gallium available to acid dissolution in the dust, about 90% gallium, can be extracted by leaching the cured dust at 80℃ for 1h.
Key words:
gallium; flue dust; phosphorus; extraction CLC number: TF843.1;
Document code: A
1 INTRODUCTION
Gallium is a strategically important metal used extensively in high-tech industries[1-3]. Although gallium is relatively abundant, it is widely disseminated, except in some rare instances[4, 5]. It is therefore not economical to mine any minerals for merely recovering gallium. Gallium is recovered traditionally as by-product, basically from the production of alumina, and to a lesser zinc[6-12]. The increasing demand for gallium promoted a search for new sources and extraction processes for this metal. Gallium was found to be concentrated in flue dust generated during electric furnace smelting of phosphate to produce elemental phosphorus. Therefore, an attempt has been made to develop a new process for recovering gallium from flue dust in our laboratory[13]. In the proposed process flue dust was subjected to the curing with strong sulfuric acid as pretreatment followed by hot water leaching. Gallium in the leaching liquor can be recovered using solvent extraction[14-16]. In this paper the processing of the flue dust using the new method are discussed.
2 EXPERIMENTAL
2.1 Materials and reagents
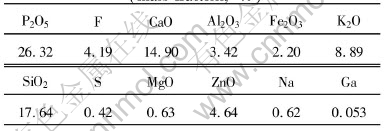
The flue dust used in this study was obtained from electrostatic precipitators in the production of elemental phosphorus by electric furnace reduction. Dust agglomerates consisted of particles of several micrometers and were ground and screened to a proper size for optimal surface contact with reagents before test. The particle sizes of flue dust used in this study were smaller than 0.417mm (35 mesh). The major phases of the flue dust were unreacted apatite, quartz, amorphous silicate and small amount of coke, and the compositions of the flue dust are listed in Table 1. The gallium-containing phase was not identified because of its very small content, but it was believed that gallium distributed evenly in the flue dust, as described in the previous paper[11]. The preliminary tests showed that 90% of the gallium in the dust was available to acid dissolution. Deionized water and analytical grade chemicals were used in this study.
Table 1 Compositions of flue dust(mass fraction, %)
2.2 Procedure
2.2.1 Dust curing
50g ground flue dust were slurried with a small volume of water. Concentrated (preferably undiluted) sulfuric acid was then introduced rapidly into the dust slurry while stirring, and the temperature of the dust-acid-water mixture rose sharply as strong exothermal chemical reactions took place vigorously. The treated mixture was kept at a desired temperature for sufficient duration by insulating or heating in oven if necessary.
2.2.2 Leaching
Measured volume of water was charged into a 500mL glass round bottom flask with three necks, which was immersed in a water bath and electrically heated. The temperature change of the water bath was kept within about 0.5℃ by a temperature controller. When the temperature reached the required value the pretreated dust was transferred into the glass flask. Mechanical agitation was provided immediately after the dust addition and timing began. Samples were withdrawn at a certain interval for tracing gallium leaching. The content of the reactor was poured out and filtrated. The filtrate and the dried cake were analyzed for gallium, and the leaching rate was calculated.
2.2.3 Gallium analysis
Analysis of gallium was made using Rhodamine B spectrophotometric method.
2.2.4 SEM and XRD analysis
SEM analysis of samples were made using Cambridge S250MK3 scanning electron microscope equipped with Link AN10000 energy dispersive X-ray analyzer. XRD analysis of samples were carried out on Rigaku D/Max-2400R X-Ray diffractometer.
3 RESULTS AND DISCUSSION
3.1 Pretreatment process
In the pretreatment process the metal values in the flue dust were converted to sulfates amenable to leaching by concentrated sulfuric acid. Effects of pretreatment parameters on gallium extraction were evaluated with the recovery of gallium in 2h leaching of the pretreated dust at the ratio of liquid to solid 4∶1, 80℃ and 800r/min.
3.1.1 Effect of sulfuric acid amount on gallium recovery
An increase of sulfuric acid addition during acid digestion enhanced the gallium recovery, as shown in Fig.1. The maximum recovery of gallium appeared at mass ratio of sulfuric acid to dust near 1∶1, and little benefit to gallium recovery is observed as addition of sulfuric acid increases further.
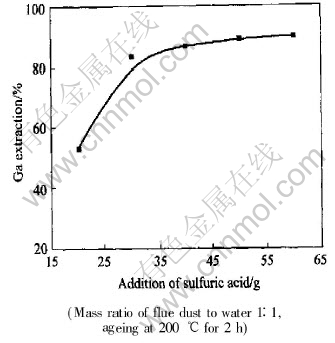
Fig.1 Effect of sulfuric acid addition on gallium extraction
It is preferable, on the other hand, to limit the free acid concentration in the subsequent aqueous leaching to alleviate the corrosion problem and to favour the gallium recovery from the leaching liquor. It was therefore recommended the mass ratio of sulfuric acid to flue dust 1∶1 in the acid digestion.
3.1.2 Effect of moisture in sulfuric acid digestion
The gallium extraction was improved as water was added for slurrying the flue dust before sulfuric acid digestion, and the maximum gallium extraction appeared at mass ratio of water to dust of 1∶1, as shown in Fig.2. Too low moisture seems not to ensure the necessary fluidity of the dust for the homogeneous mixing of sulfuric acid and flue dust. In addition, the moisture gives rise to the dilution heat when concentrated sulfuric acid is added to the dust slurry, which prompts the reaction of flue dust with sulfuric acid and hence enhances the gallium extraction.
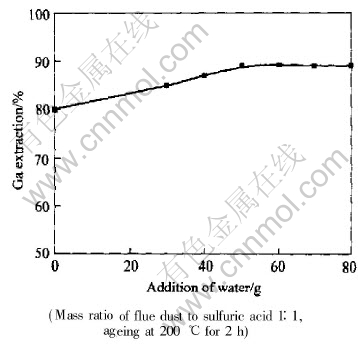
Fig.2 Effect of water addition on gallium extraction
3.1.3 Effects of ageing temperature and time on gallium extraction
The flue dust blended with sulfuric acid is preferably allowed to age for a period to increase the degree of conversion of metal values to water-soluble sulfates. Effects of ageing time and temperature on gallium extraction are shown in Figs.3 and 4, respectively. The gallium extraction from the dust blended with sulfuric acid was only 75% if not aged, but it increased to 90% after 2h ageing.
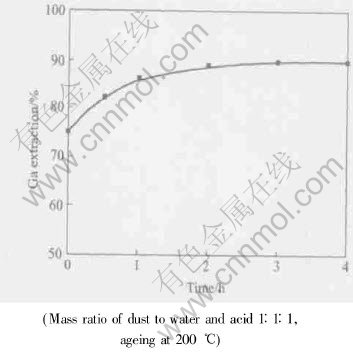
Fig.3 Effect of ageing time on Ga extraction
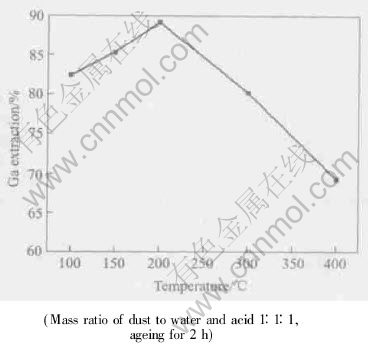
Fig.4 Effect of ageing temperature on Ga extraction
The optimal ageing temperature seems to be around 200℃.
Fig.5 demonstrates that in the cross-sectional backscattering graph of the flue dust treated with sulfuric acid but without ageing, there are some darker color areas, where no sulfur is observed, as seen in the sulfur element distribution graph, implying sulfuric acid does not enter into those areas. This indicates the present of intact particles in the treated flue dust. On the other hand, sulfur distributes homogeneously in the whole cross-section of the dust, blended with sulfuric acid and aged at 200℃ for 2h, as shown in Fig.6, which confirms that the ageing of the dust blended with sulfuric acid makes the reactions of dust and acid more complete. Besides, the properly elevated ageing temperature alleviates the formation of gelatinous materials.
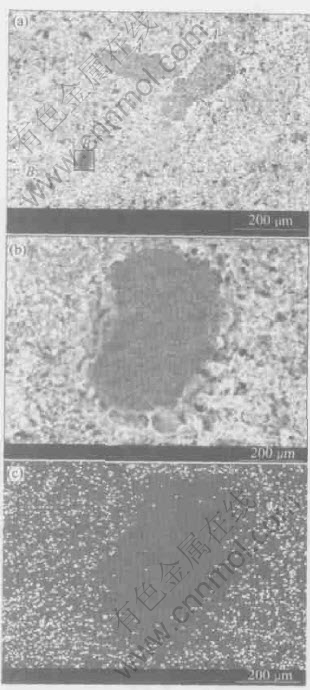
Fig.5 Cross-sectional backscattering graph(a) of flue dust blended with sulfuric acid without ageing, backscattering graph of area B(b) and corresponding sulfur elemental distribution graph(c)
There appear new peaks for KAl0.5Fe0.5P2O7 in the XRD pattern (Fig.7) of the residue from leaching of the treated flue dust with concentrated sulfuric acid and ageing at 400℃, which explains why the extraction of gallium from the flue dust aged beyond 200℃ decreases sharply. A new compound KAl0.5Fe0.5P2O7 was formed during ageing at elevated temperatures and some gallium was probably enwrapped in this insoluble substance and could not be leached by water.
3.2 Leaching of cured flue dusts
The samples used in these leaching tests were the flue dust treated under optimal curing conditions determined above, i.e. 50g flue dust was slurried with 50g water, then blended with 50g sulfuric acid and aged at 200℃ for 2h. The effects of leaching variables were studied as follows.

Fig.6 Cross-sectional backscattering graph(a) and sulfur elemental distribution graph(b) of flue dust blended with sulfuric acid and aged at 200℃ for 2h
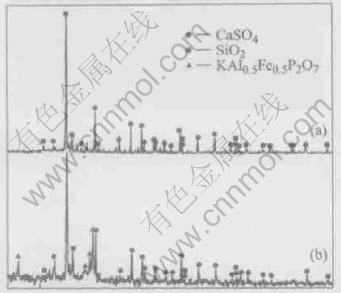
Fig.7 XRD patterns of leaching residues of flue dust aged at 200℃(a) and 400℃(b)
3.2.1 Effect of stirring speed on gallium extraction
From Fig.8 it can be seen that, gallium extraction increases quickly with the increase of stirring speed up to 600r/min, and remains almost constant above this stirring speed. In all the following leaching tests the stirring speed is therefore kept at 800r/min to eliminate the interference with mass transfer through the liquid boundary film around solid particles.
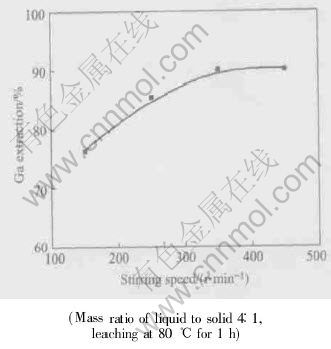
Fig.8 Effect of stirring speed on gallium extraction
3.2.2 Effect of leaching temperature and time on gallium extraction
Effect of leaching temperature and time on gallium extraction is shown in Fig.9. It can be seen that gallium extraction increases with the increase of leach temperature and leach time. All the gallium available to acid dissolution (to be 90% as determined by chemical phase analysis) in the flue dust was leached at 80℃ for 1h.
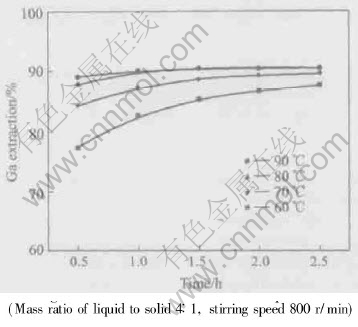
Fig.9 Effect of leaching temperature and time on gallium extraction at different temperatures
4 CONCLUSIONS
1) Gallium is concentrated in the flue dust generated during electric furnace smelting of phosphate to produce elemental phosphorus. This dust could become a new source of gallium and worthwhile to study its processing for gallium recovery.
2) The gallium in the flue dust can be converted to soluble sulfate by concentrated sulfuric acid curing. The optimal curing conditions are determined as follows: the flue dust is slurried with water at mass ratio of 1∶1 and then blended with equivalent amount of sulfuric acid, followed by ageing at 200℃ for 2h.
3) Proper moisture provides desirable fluidity of the flue dust for curing and thereby promotes the conversion of metal values in the dust.
4) Ageing of the flue dust blended with sulfuric acid at suitable temperature favors adequate sulfation as well as alleviates the formation of gelatinous materials. However, the gallium extraction declines above ageing temperature of 200℃ due to the formation of insoluble compound KAl0.5- Fe0.5P2O7 that may enwrap gallium to prevent it from dissolution.
5) Almost all the acid-soluble gallium, i.e. about 90% gallium, can be extracted when the cured flue dust is leached for 1h at 80℃, mass ratio of liquid to solid 4∶1 and stirring speed 800r/min.
REFERENCES
[1]Moskalyk R R. Gallium: the backbone of the electronics industry [J]. Minerals Engineering, 2003, 16(10): 921-929.
[2]Schoenung J M, Clark J P. Gallium demand for electronic devices [J]. JOM, 1987, 39(6): 36-39.
[3]Katrak F E, Agarwal J C. Gallium: Long-run supply [J]. JOM, 1986, 38(9): 33-36.
[4]Metz S, Trefry J H. Chemical and mineralogical influences on concentrations of trace metals in hydrothermal fluids[J]. Geochimica et Cosmochimica Acta, 2000, 64(13): 2267-2279.
[5]Pearson A, Cochran C N. Gallium [A]. Hart L D. Alumina Chemicals Science and Technology Handbook [C]. Westerville, Ohio: The American Ceramic Society Inc, 1990. 185-189.
[6]Torma A E, Jiang H. Extraction processes for gallium and germanium [J]. Miner Process Extr Met Review, 1991, 7(3-4): 235-258.
[7]Bautista R G. Gallium metal recovery [J]. Journal of Metals, 1989, 41(6): 30-31.
[8]Hoffmann J E. Advances in the extractive metallurgy of selected rare and precious metals [J]. JOM, 1991, 48(4): 18-23.
[9]Olsen T M, Voelker D E, Smith R A. Gallium and germanium extraction from the Apex mine ore [A]. Torma A E, Gundiler I H. Precious and Rare Metal Technologies [C]. Amsterdam: Elsevier, 1989. 531-545.
[10]Dutrizac J E, Chen T T. The behaviour of gallium during jarosite precipitation [J]. Canadian Metallurgical Quarterly, 2000, 39(1): 1-14.
[11]Xu K, Deng T, Chen J Y, et al. Pretreatment of phosphorus industry flue dust for gallium extraction [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1025-1030.(in Chinese)
[12]Tian R C, Zou J Y, Zhu L Z. New technology for indium, germanium and gallium recovery in an electric zinc plant [A]. Jones M J, Gill P. Mineral Processing and Extractive Metallurgy [C]. London: Institution of Mining and Metallurgy, 1984. 615-624.
[13]Deng T, Huang L J, Fu L. Synergistic of gallium from sulfate solution [J]. Chinese Journal of Chemical Engineering, 2002, 10(1): 112-115.
[14]Mihaylov I, Distin P A. Gallium solvent extraction in hydrometallurgy [J]. Hydrometallurgy, 1992, 28(1): 13-27.
[15]Zhou T L, Zhong X, Zheng L A. Recovering In, Ge and Ga from zinc residues [J]. JOM, 1989, 41(6): 36-40.
[16]Bradbury J A, Coleman M E, Roberts S D. Solvent Extraction of Gallium from Acidic Solutions Containing Phosphorus [P]. US 5221525, 1993.
Foundation item: Project(20076048) supported by the National Natural Science Foundation of China
Received date: 2004-04-19; Accepted date: 2004-06-06
Correspondence: DENG Tong, Professor, PhD; Tel: +86-10-82627082; Fax: +86-10-62561822; E-mail: dengt@126.com


